Abstract
Dichloroacetate (DCA) has several therapeutic applications based on its pharmacological property of inhibiting pyruvate dehydrogenase kinase. DCA has been used to treat inherited mitochondrial disorders that result in lactic acidosis, as well as pulmonary hypertension and several different solid tumors, the latter through its ability to reverse the Warburg effect in cancer cells and restore aerobic glycolysis. The main clinically limiting toxicity is reversible peripheral neuropathy. Although administration of high doses to rodents can result in liver cancer, there is no evidence that DCA is a human carcinogen. In all studied species, including humans, DCA has the interesting property of inhibiting its own metabolism upon repeat dosing, resulting in alteration of its pharmacokinetics. The first step in DCA metabolism is conversion to glyoxylate catalyzed by glutathione transferase zeta 1 (GSTZ1), for which DCA is a mechanism-based inactivator. The rate of GSTZ1 inactivation by DCA is influenced by age, GSTZ1 haplotype and cellular concentrations of chloride. The effect of DCA on its own metabolism complicates the selection of an effective dose with minimal side effects.
Keywords: Dichloroacetate, pyruvate dehydrogenase kinase, GSTZ1, pharmacogenetics, pharmacokinetics, inhibition of metabolism
1. Introduction
Dichloroacetate (DCA) is an intriguing small molecule. The pharmacological properties of dichloroacetic acid, its salts and derivatives have been investigated for almost a century. From the 1950s-1960s, diisopropylammonium dichloroacetate (DIPA) and other salts or ionic complexes of DCA were investigated experimentally and clinically for diverse indications, with little insight into how or where they acted (Stacpoole, 1969). Among the more intriguing properties of DIPA was its ability to lower blood glucose levels in rats with chemically-induced diabetes, but not in non-diabetic animals (Lorini & Ciman, 1962), a property subsequently found to be due solely to the DCA anion (Stacpoole & Felts, 1970). During the 1970s-1980s, the metabolic effects of DCA as the sodium salt were studied extensively and its primary sites and mechanisms of action were established (Stacpoole, 1989). The first human pharmacokinetic study of DCA was conducted in the 1980s and showed that DCA elimination was slowed substantially after multiple doses (Curry, et al., 1985). It was subsequently shown that the major pathway of primary metabolism of DCA was conversion to glyoxylate (James, et al., 1998), catalyzed by glutathione transferase zeta 1 (GSTZ1) (Tong, et al., 1998a) and that this enzyme could be irreversibly inactivated by DCA (Anderson, et al., 1999), a property that led to the prolongation of the elimination half-life following multiple doses (Curry, et al., 1985; Schultz, et al., 2002; Stacpoole, et al., 1998a). This article will discuss the pharmacology, therapeutic applications, toxicology, pharmacokinetics and biotransformation of DCA in people and animal models.
2. Mechanism of action of DCA in mitochondria
Although DCA exerts clinically significant effects on lipid and lipoprotein metabolism (Moore, et al., 1979; Stacpoole, et al., 1983c; Stacpoole, et al., 1978), most research has focused on its ability to modulate carbohydrate metabolism at the level of the mitochondrial pyruvate dehydrogenase complex (PDC).
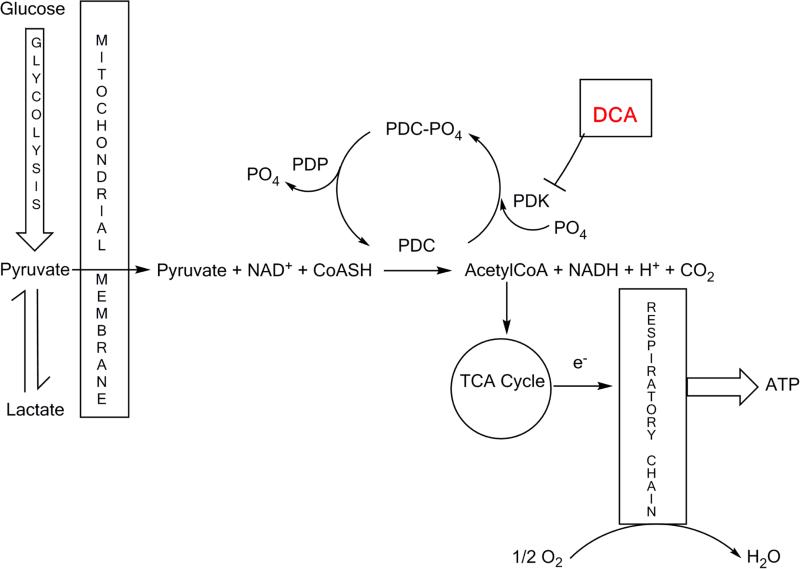
The PDC is a multienzyme complex located in the mitochondrial matrix and performs the gatekeeper role of linking cytoplasmic glycolysis to the mitochondrial tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) as shown in Figure 1. The reaction is rate-limiting for the aerobic oxidation of glucose and pyruvate and for other 3-carbon molecules (alanine, lactate) in equilibrium with pyruvate. Rapid regulation of the nuclear DNA-encoded PDC is mediated post-transcriptionally by reversible phosphorylation of 3 serine residues on the alpha subunit of the E1 (pyruvate dehydrogenase) component of the complex (E1α), in which the phosphorylated form is catalytically inactive (Patel & Korotchkina, 2006). Humans possess 4 isoforms of pyruvate dehydrogenase kinase (PDK 1-4), which inhibit the complex, and 2 isoforms of pyruvate dehydrogenase phosphatase (PDP 1 and 2), which maintain PDC in its unphosphorylated, active state. Both PD kinases and phosphatases are differentially expressed in tissues and are themselves highly regulated proteins (Bowker-Kinley, et al., 1998). In general, PDC undergoes feedforward stimulation and feedback inhibition that is mediated via changes in PDK activity or expression. Thus, accumulation of the PDC reaction products, acetyl CoA and NADH, or the end product of OXPHOS, ATP, increase PDK activity and suppress PDC through phosphorylation at one or more serine residues on E1α. In contrast, the substrates for the PDC reaction inhibit PDKs and maintain or restore PDC activity.
Figure 1.
Role of the pyruvate dehydrogenase complex in intermediary metabolism and site of action of DCA. PDC-PO4 is the inactive phosphorylated form of pyruvate dehydrogenase complex; PDC is the active unphosphorylated form. PDK is pyruvate dehydrogenase kinase; PDP is pyruvate dehydrogenase phosphatase; e− represents transfer of an electron.
DCA, a structural analog of pyruvate, is taken up by cells by the monocarboxylate transporter system (Jackson & Halestrap, 1996) and by the highly efficient sodium-coupled monocarboxylate transport, also called solute carrier family-5 member 8 (SLC5A8) transporter (Babu, et al., 2011) and gains access to the mitochondrial matrix by the mitochondrial pyruvate transporter system (McCommis & Finck, 2015). DCA stimulates PDC activity by binding to the pyruvate binding site in the PDK N-terminal regulatory R domain, according to the co-crystallization structural data obtained for the PDK1-DCA and PDK2-DCA complexes (Kato, et al., 2007; Knoechel, et al., 2006). DCA binding at this site results in inhibition of PDK activity such that PDC remains in the active form. An oral dose of DCA is rapidly absorbed and widely distributed within minutes of administration (Stacpoole, 2011). It readily crosses the blood-brain barrier and can be measured in cerebrospinal fluid. Blood lactate concentrations begin to fall within about 15-30 minutes following oral or parenteral dosing and are a useful biomarker of DCA's action on PDC.
3. Therapeutic uses of DCA
3.1 Diabetes mellitus
Many acquired and genetic diseases, as well as nutritional perturbations, transcriptional factors and xenobiotics can also affect PDC activity by modulating the expression of one or more PDK isoforms. For example, conditions such as starvation, high fat diets and diabetes mellitus elevate circulating levels of free (unesterified) long chain fatty acids. The resultant increase in their mitochondrial beta-oxidation increases intra-mitochondrial ratios of acetyl CoA:CoA and NADH:NAD, leading to increased PDK activity and down-regulation of PDC (Hue & Taegtmeyer, 2009; Randle, et al., 1963). The selective glucose-lowering action of DCA in diabetic, but not in healthy, animals was associated with stimulation of glucose oxidation and inhibition of fatty acid oxidation in peripheral tissues of diabetic rats (Stacpoole & Felts, 1970, 1971) as well as stimulation of PDC (Whitehouse & Randle, 1973). DCA and some other halogenated acetic acid derivatives were found to activate PDC by inhibiting PDK, which was up-regulated by diabetes and other conditions in which fatty acid beta-oxidation was increased (Stacpoole, 1989). By activating PDC, DCA lowers circulating glucose independently of insulin, as illustrated in insulin-deficient streptozotocin- or alloxan-induced diabetic rats, in which DCA reduced both blood glucose and ketone bodies and decreased mortality (Blackshear, et al., 1974; Eichner, et al., 1974). In patients with non-ketotic type 2 diabetes, oral DCA reduced circulating levels of lactate and alanine, which are in equilibrium with pyruvate and glucose, while modestly raising beta-hydroxybutyrate concentrations (Stacpoole, et al., 1978). Administration of DCA under these conditions is reported to inhibit and restore PDC activity, improving glucose tolerance and lowering blood glucose levels in diabetes (Stacpoole, 1989, 2012; Stacpoole, et al., 1978).
3.2 Lipid and lipoprotein disorders
In patients with type 2 diabetes and hyperlipoproteinemia, up to 50 mg/kg oral DCA reduced blood cholesterol, triglycerides and very low density lipoprotein levels (Stacpoole, et al., 1978). DCA inhibits hepatic triglyceride synthesis (Stacpoole, et al., 1983b). The mechanism for this effect is unknown, but may be due to the ability of the drug to lower tissue levels of citrate (Stacpoole & Felts, 1970), a precursor of cellular lipid synthesis. DCA is also a non-competitive inhibitor of hydroxymethylglutaryl coenzyme A reductase, the rate-limiting enzyme of cholesterolgenesis in rat liver (Stacpoole, et al., 1983c) and human monocytes (Stacpoole, et al., 1987), a mechanism accounting for its ability to reduce both total and low-density lipoprotein (LDL) cholesterol in the circulation of two patients with LDL receptor-negative forms of homozygous familial hypercholesterolemia (Moore, et al., 1979).
3.3 Acquired and congenital lactic acidosis
By virtue of its action on the PDC/PDK axis, DCA is the most potent lactate-lowering agent in clinical use, an effect verified in experimentally-induced models of tissue or systemic lactic acidosis, including myocardial or cerebral ischemia, heart failure and sepsis (Stacpoole, 1997). Phase 2 and phase 3 clinical trials of repeated intravenous doses of 50 mg/kg DCA in adult patients with various acquired causes of lactic acidosis have confirmed the ability of the drug to reduce circulating lactate and improve acid-base status (Stacpoole, et al., 1983a; Stacpoole, et al., 1988; Stacpoole, et al., 1992), but its effect on survival is problematic given the dire clinical state of the patients who received the drug (Stacpoole, et al., 1992).
Congenital lactic acidosis (CLA) is a term that encompasses several life-threatening inborn errors of mitochondrial energy metabolism that often present during the neonatal period or infancy (Stacpoole, 2003). Severe loss-of-function mutations in PDC are considered the commonest cause of CLA affecting neonates. DCA has been administered intravenously acutely and chronically by mouth or feeding tube to treat children with PDC deficiency or with one or more defects in nuclear DNA or mitochondrial DNA-encoded proteins of the respiratory chain complexes (Abdelmalak, et al., 2013; Berendzen, et al., 2006; Stacpoole, 1989; Stacpoole, et al., 1997), in whom the drug appears generally well tolerated and effective in reducing both blood and cerebrospinal lactate concentrations and may improve outcome of affected children (Abdelmalak, et al., 2013; Stacpoole, et al., 2008). In contrast, treatment of adults with primary mitochondrial diseases is problematic, owing to the slowing of DCA metabolism with age and the consequent increased risk of peripheral neuropathy (Kaufmann, et al., 2006), see section 11. A randomized controlled trial has been initiated recently in children with PDC deficiency, based on clinical and molecular genetic criteria (NCT02616484, 2016).
3.4 Cancer
Terminally differentiated, quiescent cells typically derive most of their energetic needs through mitochondrial OXPHOS. However, cells stimulated under physiological or pathological conditions undergo rapid proliferation and require both the energy and biomass (e.g. membranes, nucleotides) required for cell growth and division. They achieve this objective by undergoing a so-called “glycolytic shift,” whereby glycolysis is transcriptionally up-regulated and OXPHOS is relatively suppressed, mainly through transcriptional activation of PDKs (Costa & Soares, 2013; Lim, et al., 2013; Loftus & Finlay, 2016; Semenza, 2011). Increased glucose fermentation to lactate in the presence of oxygen is termed aerobic glycolysis (the Warburg effect) and is a hallmark of several pathological conditions, including cancer (Warburg, et al., 1927) and pulmonary arterial hypertension (PAH). A major driver of aerobic glycolysis in both cancer and PAH is hypoxia-inducible factor 1 alpha (HIF1α), a master transcriptional activator of hundreds of genes, including most that encode glycolytic enzymes and all PDKs (Saunier, et al., 2016; Semenza, 2011). Pathological stable up-regulation of HIF1α in cancer is facilitated in part by the accumulation of pyruvate and lactate that occur in aerobic glycolysis, which retard HIF1α degradation (Koukourakis, et al., 2005) and create a positive feedback loop, promoting HIF1α action on glycolysis, independently of ambient oxygen concentration. Many studies have reported an inverse association between up-regulation of HIF1α, one or more PDK isoforms and/or circulating or tumor lactate concentrations and survival in cancer patients (Brocato, et al., 2014; Kankotia & Stacpoole, 2014; Zhang, et al., 2015). DCA reverses the Warburg effect in human tumor cells by inhibiting PDKs, re-activating PDC and OXPHOS and decreasing pyruvate and lactate levels, leading to decreased expression of HIF1α (Kankotia & Stacpoole, 2014). Several studies have demonstrated that, as a consequence of OXPHOS stimulation, DCA increases reactive oxygen species production by the mitochondrial respiratory chain and induces other down-stream changes in mitochondrial function, leading to selective apoptosis of tumor cells and, in rodents harboring human tumor xenografts, to decreased proliferation of cancer cells and increased host survival (Kankotia & Stacpoole, 2014). As discussed in more detail below (sections 9 and 11), it has been hypothesized that DCA treatment could result in induction of NADPH oxidase, as has been demonstrated in GSTZ1−/− mice (Blackburn, et al., 2006), however there is no direct evidence this occurs (Theodoratos, et al., 2009).
Use of DCA to treat solid tumors was proposed based on DCA's efficacy in reversing the Warburg effect (Michelakis, et al., 2008). Four open label phase 1 clinical trials have investigated the chronic safety of oral DCA doses of 12.5-25 mg/kg/day in adults with recurrent malignant brain tumors (Dunbar, et al., 2014; Sutendra, et al., 2010) or other solid tumors (Chu, et al., 2015; Garon, et al., 2014). These clinical trials showed that DCA was generally well tolerated, but induced a peripheral neuropathy in some patients that reversed upon dose reduction or drug withdrawal. As recently reviewed, DCA and DCA derivatives hold promise for tumor treatment (Brocato, et al., 2014; Kankotia & Stacpoole, 2014; Zhang, et al., 2015), however as yet there are few peer-reviewed reports of the effectiveness of DCA in treating cancer in patients (Strum, et al., 2013).
3.5 Pulmonary arterial hypertension (PAH)
PAH is a neoplastic disease in which HIF1α-driven aerobic glycolysis and PDK up-regulation in the endothelial and smooth muscle cells of pulmonary arteries stimulates their proliferation; in turn, these changes cause progressive narrowing of the pulmonary vessels, leading to right heart failure and death (Dromparis, et al., 2010). Thus, the pathophysiology of PAH resembles that of cancer, in which a HIF-driven glycolytic shift results in a pro-proliferative, anti-apoptotic state favoring cell division over cell differentiation and quiescence. In hypoxic, pharmacologically and genetically-induced rodent models of PAH, DCA has reversed aerobic glycolysis and induced caspase-mediated apoptosis of pulmonary artery endothelial and smooth muscle cells, leading to luminal enlargement of vessels and improved heart function and survival (Bonnet, et al., 2006; Guignabert, et al., 2009; McMurtry, et al., 2004; Michelakis, et al., 2002). A phase 1 trial of DCA in adult patients with PAH is in progress (NCT01083524, 2010).
3.6 Other disorders
Aerobic glycolysis and the induction of one or more PDKs has also emerged as potential therapeutic targets for DCA in chronic inflammatory states associated with autoimmune disease (Gerriets, et al., 2016; Gerriets, et al., 2015), obstructive pulmonary disease (Calvert, et al., 2008; Mercken, et al., 2009; Ostroukhova, et al., 2012), coronary artery restenosis (Deuse, et al., 2014) and amyotrophic lateral sclerosis (ALS) (Miquel, et al., 2012; Palamiuc, et al., 2015; Valbuena, et al., 2016). Mouse models of ALS, due to mutations in Cu/Zn superoxide dismutase (SOD), have described the occurrence of a metabolic shift towards aerobic glycolysis, with upregulation of PDK and phosphorylation of PDC. Chronic administration of DCA to mice harboring the ALS-linked SOD (G93A) mutation increased grip strength, decreased astrocyte reactivity and prevented motor neuron loss. Further studies are required to determine the drug's clinical utility in cancer, PAH, coronary artery disease and ALS. Nevertheless, it is clear that DCA represents the prototype of a new class of metabolic modulators acting post-transcriptionally to intervene at a critical site of cellular fuel metabolism.
4. Derivatives of DCA
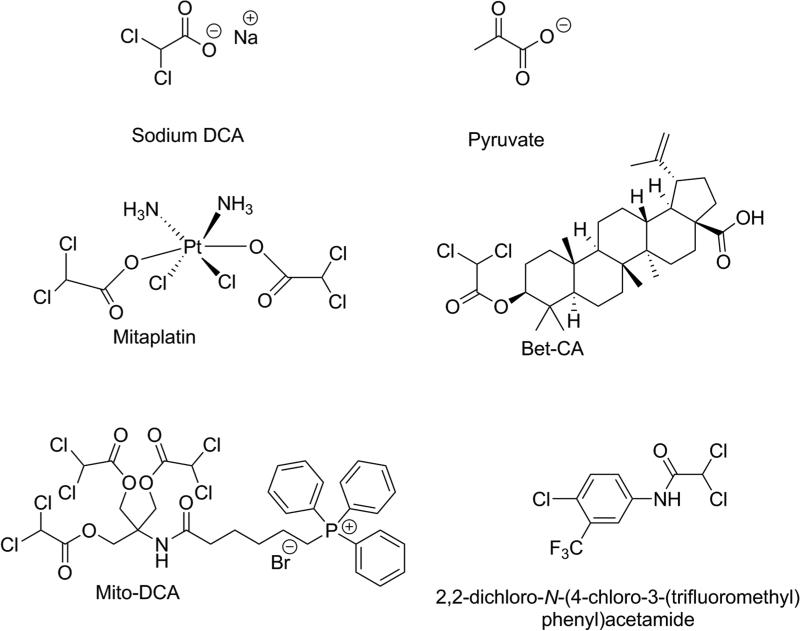
In part because of the unpatentability of DCA as a therapeutic agent, several DCA derivatives have been synthesized recently and proposed as substitutes (Figure 2), based on preclinical testing. One approach has been to couple DCA to other anticancer agents, with the objective of delivering two therapeutic strategies in one molecule. An example is the drug mitaplatin, in which DCA attached through ester linkages to the alkylating agent cisplatin (Dhar & Lippard, 2009). Mitaplatin was found to be effective at killing several tumor cell lines. In most of the studied tumor cell lines, mitaplatin had comparable cell-killing potency to cisplatin, however, unlike cisplatin, mitaplatin did not kill normal cells (Dhar & Lippard, 2009) Recently a formulation of mitaplatin as a nanoparticle encapsulation was found to have improved bioavailability characteristics and to be effective in animal models (Johnstone, et al., 2013). Another example of a DCA co-drug is Bet-CA, an ester of the alcoholic hydroxy group of betulinic acid and the carboxylate of DCA (Saha, et al., 2015). Betulinic acid is a natural product with anticancer activity (Fulda & Kroemer, 2009). Bet-CA was reported to be more potent than either drug alone or a mixture of betulinic acid and DCA in several cancer cell lines, but was non-toxic to normal cells (Saha, et al., 2015). So far, no clinical data have been reported for either of the co-drugs. Another approach has been to prepare derivatives of DCA that might be more readily taken up into the mitochondria of cancer cells, where DCA has its effect. Examples of this approach are the drugs mito-DCA, in which the positively charged triphenylphosphonium group is linked via a substituted alkyl chain and ester bonds with DCA (Pathak, et al., 2014), and several amide derivatives of DCA (Trapella, et al., 2016; Zhang, et al., 2016). Interestingly, one of the amide derivatives shown in Figure 2, 2,2-dichloro-N-(4-chloro-3-(trifluoromethyl)phenyl)acetamide, was shown to bind intact to the ATP-binding pocket of PDK1, thereby activating PDC at a different site than DCA (Zhang, et al., 2016).
Figure 2.
Structures of representative DCA derivatives and co-drugs, with pyruvate and sodium DCA shown for comparison. Bet-CA is an ester of betulinic acid with DCA.
To date, there have been no detailed studies of the in vivo fate of any of these DCA derivatives in animals or humans, so it is not known how quickly DCA is released from the ester or amide linkages or if the derivatives have the same effects on GSTZ1 as DCA itself (see the following section).
5. Biotransformation of DCA, role of GSTZ1
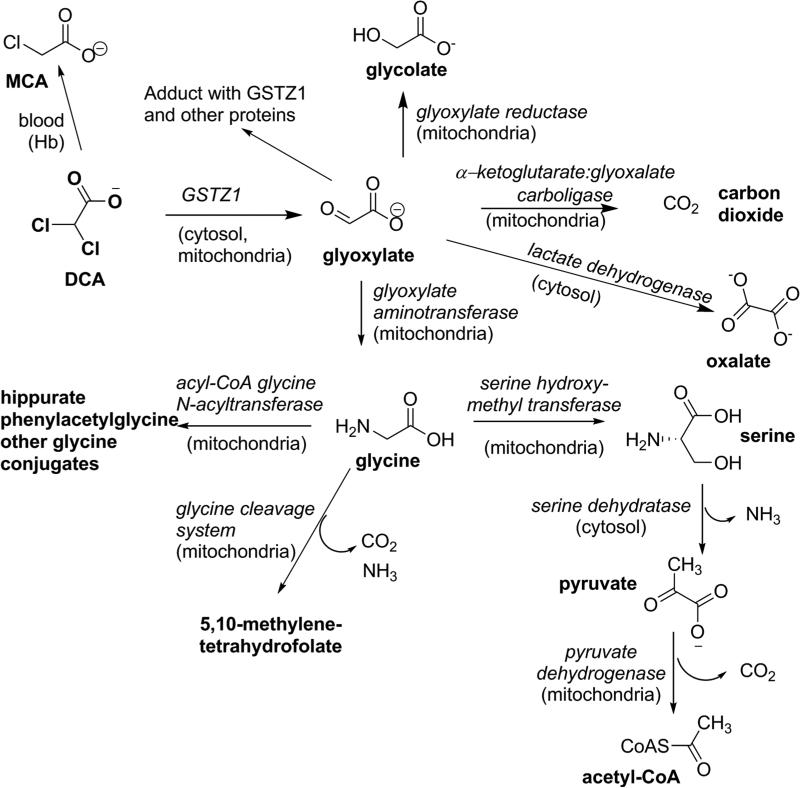
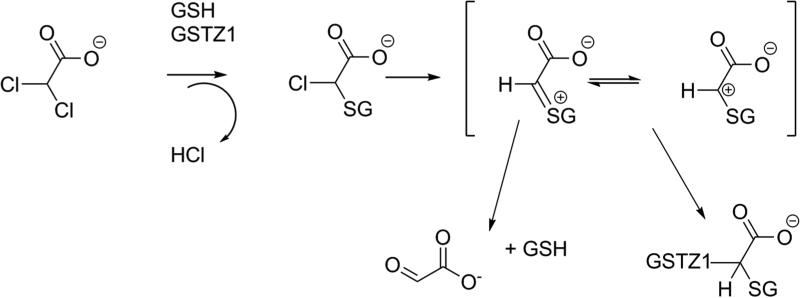
During the early clinical trial use of DCA to lower plasma lactic acid levels in patients (Stacpoole, et al., 1983a) and, subsequently, in healthy adults, (Curry, et al., 1985; Curry, et al., 1991) it was observed that the pharmacokinetics of DCA changed upon repeated doses, such that elimination from plasma after oral or intravenous administration was slowed. Subsequent studies in rats showed that DCA is dechlorinated in a glutathione (GSH)-dependent process in liver cytosol to an inactive metabolite, glyoxylate (Lipscomb, et al., 1995), as shown in Figure 3, and provided the first evidence that DCA treatment reduced the hepatic GSH-dependent dechlorination (James, et al., 1997), consistent with the human pharmacokinetics. In vivo rat studies showed that urinary excretion of unchanged DCA was low after a single dose, but increased with repeated dosing, as would be expected with reduced DCA metabolism (Gonzalez-Leon, et al., 1999; Gonzalez-Leon, et al., 1997). As discussed in detail below, an unusual glutathione transferase, GSTZ1, expressed in liver cytosol and mitochondria, was shown to catalyze the conversion of DCA to glyoxylate in a reaction that required but did not consume GSH (Li, et al., 2011; Tong, et al., 1998a, 1998b). The glyoxylate arising from DCA was converted to secondary metabolites, including glycine, carbon dioxide and oxalate, as documented from studies in rats and mice (Gonzalez-Leon, et al., 1997; James, et al., 1998). Some of the glycine was excreted in urine in the form of hippuric acid, phenylacetylglycine and other glycine conjugates (James, et al., 1998). Most of the enzymes leading to the secondary metabolites of DCA are expressed primarily in the mitochondria, one of the sites of conversion of DCA to glyoxylate by GSTZ1. Although glyoxylate is the major primary metabolite of DCA, treatment of animals with high doses of DCA has resulted in detection of small amounts of monochloroacetate (MCA) in blood and urine (Shroads, et al., 2008). The concentrations were low, however this was of concern because MCA is neurotoxic (Berardi, et al., 1987; Lu, et al., 2015) and toxic to the liver and kidney (Dote, et al., 2003). Further discussion of the mechanism of toxicity is presented in section 11.4. Investigation of the route of formation of MCA from DCA showed this was not a hepatic pathway of metabolism; instead, incubation of relatively high (mM) concentrations of DCA with blood or hemoglobin but not water resulted in the formation of small amounts of MCA (Shroads, et al., 2008). The mechanism of dechlorination has not been studied. The known metabolites of DCA are shown in Figure 3.
Figure 3.
The known metabolites of DCA; the enzymes responsible are shown in italics and the subcellular location is given, where this is known. Abbreviations are: DCA, dichloroacetate; MCA, monochloroacetate; GSTZ1, glutathione transferase zeta 1.
It should be noted that there is a misconception in the literature that DCA exhibits low membrane permeability and does not readily cross into the mitochondria, its site of action (Pathak, et al., 2014; Trapella, et al., 2016). While it is true that biotransformation to the inactive metabolite, glyoxylate, reduces the bioavailability of parent DCA through first-pass metabolism, at least for the first dose (Li, et al., 2008; Saghir & Schultz, 2002), DCA as a small, water- and lipid-soluble molecule readily crosses membranes and is completely absorbed following oral administration (James, et al., 1998). As discussed in section 2, DCA is known to be a substrate for uptake transporters (Babu, et al., 2011; Jackson & Halestrap, 1996; McCommis & Finck, 2015), however further studies are needed to fully describe the intercellular and intracellular transport of DCA.
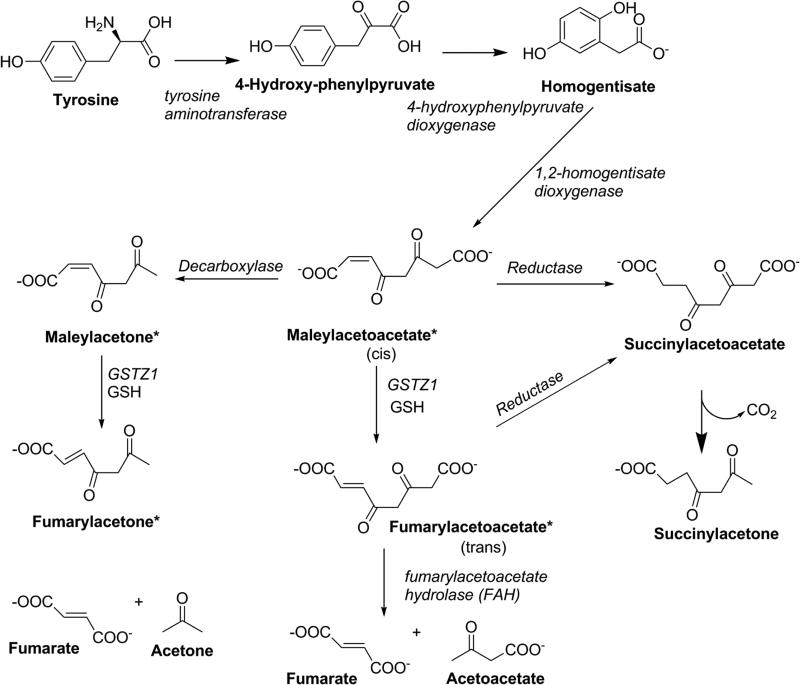
Following the discovery that DCA was converted to glyoxylate by a glutathione transferase, a partial sequence of the rat enzyme revealed that it was identical to GSTZ1 (Tong, et al., 1998b), an enzyme that was shown to have the physiological function of serving as maleylacetoacetate isomerase in the penultimate step of the tyrosine catabolism pathway (Board & Anders, 2011; Fernandez-Canon, et al., 1999). This pathway is depicted in Figure 4. One gene has been identified for GSTZ1 in humans, located on chromosome 14q24.3 (Blackburn, et al., 1998). This gene codes for a polypeptide of 216 residues, with a predicted monomeric molecular weight of 24.2 kDa. The catalytically active enzyme, like most other GSTs, exists as a homodimer.
Figure 4.
Tyrosine catabolism showing the role of GSTZ1 in isomerization of maleylacetoacetate and maleylacetone. Compounds shown with an asterisk are electrophilic, chemically reactive substances.
Two reported crystal structures for human GSTZ1 have been published and have provided important insights into the molecular interactions of this protein (Boone, et al., 2014; Polekhina, et al., 2001). These studies have advanced our understanding of the active site residues involved in the interaction of GSTZ1 with its substrates. Like other cytosolic GSTs, GSTZ1 adopts a canonical fold, with two domains, a GSH-binding site and a substrate-binding site. Unlike other GSTs, the GSH-binding site is found deep within a crevice between the N- and C-terminals, and this site includes a characteristic motif of this enzyme 14-SSC-16 (Blackburn, et al., 1998; Board & Anders, 2005; Board, et al., 2003). The carboxylic acid substrates of GSTZ1, including DCA and maleylacetoacetate, also bind in this crevice, stabilized by formation of a salt bridge between R-175 and the substrate carboxylate group. Site-directed mutagenesis studies showed that S-14 was essential for catalytic function and C-16 was involved in orientation of GSH for binding and catalytic activity (Board, et al., 2003). The salt bridge between R-175 and the carboxylate of DCA appears to facilitate attack of GSH on the α-carbon and halide displacement (Polekhina, et al., 2001). Such features play integral roles for the unusual catalytic activity of GSTZ1, which differs from other GSTs that cannot metabolize DCA.
Mechanistically, the conversion of DCA to glyoxylate is thought to occur by initial displacement of one chloride of DCA with GSH via an SN2–like reaction to form S-(α-chloro-carboxymethyl) glutathione, which can undergo hydrolysis with loss of the second chloride, yielding glyoxylate (Anderson, et al., 2002). Figure 5 shows the likely mechanism, which accounts for the finding that, although GSH is required for the reaction, it is not consumed.
Figure 5.
Mechanism of DCA dechlorination (adapted from Tzeng et al., 2000). Abbreviations are: GSH, glutathione; GSTZ1, glutathione transferase zeta 1.
6. The physiological role and properties of GSTZ1
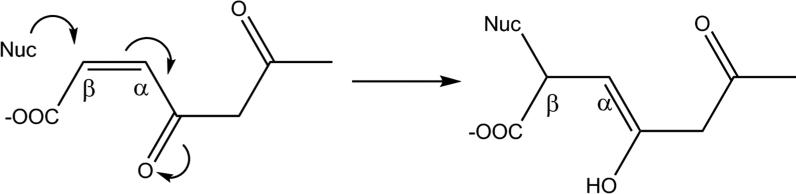
As well as differing from common mammalian GSTs in the amino acid critical for glutathione binding, which is serine in GSTZ1 but tyrosine in GSTs in the Alpha, Mu, Pi and Sigma classes (Board & Menon, 2013), GSTZ1 has an important physiological role. Intermediates in the catabolism of tyrosine, maleylacetoacetate and maleylacetone, are the physiological substrates for GSTZ1. The role of GSTZ1 is to convert these cis substrates to their trans forms, fumarylacetoacetate and fumarylacetone (Figure 4). As with metabolism of DCA, GSH is required but is not consumed during the isomerase reaction. GSTZ1-catalyzed isomerization of maleylacetoacetate and maleylacetone proceeds several orders of magnitude more rapidly than conversion of DCA to glyoxylate, as shown in Table 1 (Board & Anders, 2005). The role of GSTZ1 in isomerization of maleylacetoacetate and maleylacetone to fumarylacetoacetate and fumarylacetone is thought to be important in reducing the potential toxicity of these electrophilic, chemically reactive alpha-beta-unsaturated ketones. If maleylacetoacetate and maleylacetone are not isomerized, these intermediates could form covalent bonds with cellular nucleophiles such as free SH, NH2 or OH groups found in proteins from the amino acids cysteine, lysine, arginine, serine and threonine, or with NH2 groups in DNA bases through Michael addition reactions, as shown in Figure 6: evidence for maleylacetone adducts with protein cysteine residues has been presented (Lantum, et al., 2002b). If critical proteins or sites in DNA form covalent adducts, this can alter their structure and lead to toxicity (Guengerich & MacDonald, 2007). Fumarylacetoacetate and fumarylacetone are also chemically reactive alpha-beta unsaturated ketones, however, fumarylacetoacetate and fumarylacetone (but not their cis isomers) are substrates for fumarylacetoacetate hydrolase (FAH). FAH converts fumarylacetoacetate to fumarate and acetoacetate, non-toxic substances that are building blocks in intermediary metabolism. Evidence for the toxicity of fumarylacetoacetate is that persons with hereditary tyrosinemia, who do not have an active FAH enzyme (Tanguay, et al., 1990), often develop liver cancer, a disorder that has been linked to the build-up of fumarylacetoacetate and fumarylacetone (Angileri, et al., 2015b).
Table 1.
Rates of metabolism of DCA and maleylacetone by expressed recombinant enzymes corresponding to the polymorphic variants of GSTZ1. Rates are μmol/min/mg protein and data are from Blackburn et al. (2001).
| Variant | DCA | Maleylacetone |
|---|---|---|
| GSTZ1A (KRT) | 1.61 | 318 |
| GSTZ1B (KGT) | 0.45 | 1010 |
| GSTZ1C (EGT) | 0.45 | 1856 |
| GSTZ1D (EGM) | 0.3 | 464 |
Figure 6.
Mechanism of nucleophilic attack of a cellular nucleophile (Nuc) to the electrophilic carbon of maleylacetone. The cellular nucleophile could be a free thiol (−SH) group from cysteine present in a protein.
The GSTZ1 enzyme was first identified as a hepatic cytosolic enzyme (Board, et al., 1997; Tong, et al., 1998b), in common with most of the GSTs studied to date (Board & Menon, 2013). It was then shown that the GSTZ1 transcript is present in kidney, testis, heart, brain and other tissues of mice and humans (Fernandez-Canon, et al., 1999). The GSTZ1 protein was shown to be expressed in these tissues in rats (Lantum, et al., 2002a), albeit at lower levels in than in liver. Activities with maleylacetone and chlorofluoroacetic acid in rat extrahepatic tissues were reported to be considerably lower than in liver, however in these studies activity was measured in cell-free homogenate, which may not exhibit the same properties as cytosol in all tissues.
Further studies of human and rat liver showed that GSTZ1 protein expression and activity with DCA were present in the mitochondria, and that in the mitochondria, the enzyme was localized to the matrix (Li, et al., 2011). Considering the total expression of GSTZ1 in rat liver, 86% is in cytosol and 14% is in mitochondria. The properties of the hepatic mitochondrial enzyme are not identical to those of the cytosolic form, in that the apparent Km for GSH is higher in mitochondria than in cytosol in the rat (Li, et al., 2011) and human (Zhong, et al., 2014b). To date, however, no protein sequence differences have been identified in GSTZ1 isolated from each location in either rat or human (Zhong and James, unpublished observations), and the molecular basis for these differences in enzyme kinetics is not yet known. With respect to the ability to metabolize DCA, the presence of GSTZ1 in mitochondria is of interest because, as noted above, the site of action of DCA is in the mitochondria, and there is evidence that DCA can be taken up into mitochondria (Jackson & Halestrap, 1996).
7. Ontogeny of GSTZ1
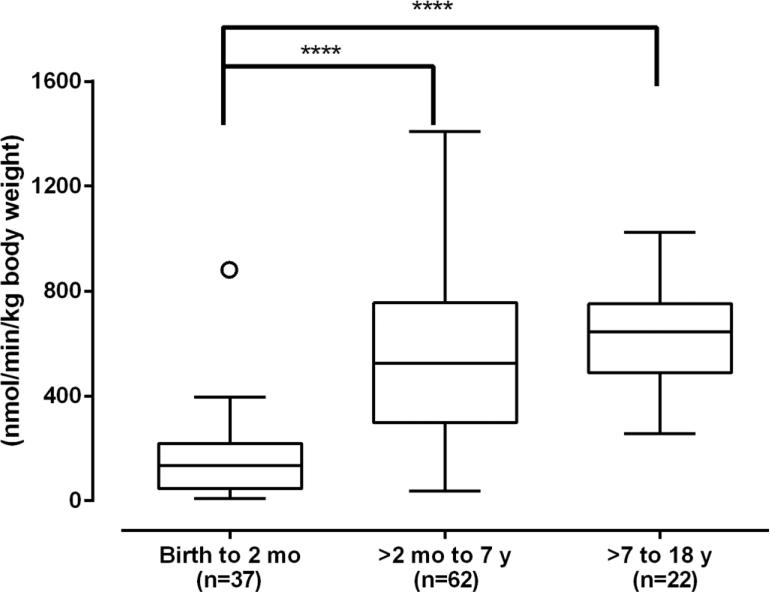
The age-related development of cytosolic GSTZ1 has been studied using a bank of 232 human liver samples from gestational day 42 through to age 74 (Li, et al., 2012). It was found that the expression and activity of cytosolic GSTZ1 with DCA was at or below the limits of detection prenatally and until about 2 months after birth. Thereafter, expression and activity with DCA rose with age up to age 7; above age 7, values were similar to adults through age 74 (Li, et al., 2012). This developmental pattern is common among drug-metabolizing enzymes and has been described as class 3 (Hines, 2013). It was somewhat surprising to find this pattern for GSTZ1, in view of the results from pharmacokinetic studies in children, which showed similar (first dose) or more efficient (chronic treatment) elimination of DCA, compared with adults (Shroads, et al., 2008; Shroads, et al., 2012). However, when the higher liver/body weight ratio of children was taken into consideration, the ability of children above 2 months of age to metabolize DCA was similar to that of subjects older than age 7 years (Figure 7) (Li, et al., 2012). Another reason the class 3 developmental pattern was surprising for GSTZ1 relates to its physiological function in the tyrosine catabolism pathway. The lack of GSTZ1 in the human fetus means there is no ability to convert maleylacetoacetate to fumarylacetoacetate and raises the possibility that this reactive substance could build up in the fetus. There is some evidence that tyrosine is poorly taken up into the fetus (Van den Akker & Van Goudoever, 2010) and that phenylalanine, which is readily transported into the fetus, can be hydroxylated to tyrosine in fetal liver (Bessman, et al., 1977). However, the presence and activities of tyrosine catabolic pathways in the fetus are not known, so it is possible that the fetus does not readily produce maleylacetoacetate.
Figure 7.
Development of GSTZ1 activity adjusted to account for the relatively larger liver size in children. The box and whiskers plot shows the median with the minimum and maximum values. A single outlier in the birth to 2 months of age group is shown separately. The birth to 2 months group had significantly lower activity than the 2 months to 7 years or the 7 to 18 years age groups, which did not differ from each other. Asterisks (****) indicate significant differences, p<0.0001. Adapted from Li et al. (2011).
8. Polymorphisms of GSTZ1 in humans
Many single nucleotide polymorphisms (SNPs) have been identified in the coding and non-coding regions of the GSTZ1 gene. Of these, three non-synonymous SNPs in the coding region (rs7975, rs7972, rs1046428) have been identified in people and give rise to five commonly found haplotypes of GSTZ1, shown in Table 2 (Blackburn, et al., 2001; Shroads, et al., 2012). As recently reviewed (James & Stacpoole, 2016), the incidence of these haplotypes varied somewhat with ethnic group, however in all groups studied to date, GSTZ1C, coding for glutamic acid at position 32, glycine at position 42 and threonine at position 82 of the expressed enzyme protein is the most common. GSTZ1C is also referred to as EGT, highlighting the variant amino acids. Studies of expressed recombinant enzymes corresponding to four of the five common variants showed that the amino acid changes resulted in alterations of activity with DCA and maleylacetone (Table 1). DCA was a better substrate for GSTZ1A (KRT) than the other variants, but maleylacetone was most readily metabolized by GSTZ1C (EGT) and least well metabolized by GSTZ1A (Blackburn, et al., 2001). Although there are differences in rates of metabolism of maleylacetone, and presumably maleylacetoacetate by the GSTZ1 variants, it is notable that the endogenous substrate is much more rapidly metabolized than DCA by all variants, indicating the enzyme structure is optimized for isomerizing the tyrosine catabolites.
Table 2.
Approximate distribution of the five known GSTZ1 haplotypes in US and Australian populations (reviewed in James and Stacpoole, 2016). The percentage of each haplotype varied with ethnic group.
| Haplotype | Nucleotide position | Amino acid position | % in the population | ||||
|---|---|---|---|---|---|---|---|
| 94 | 124 | 245 | 32 | 42 | 82 | ||
| GSTZ1A | A | A | C | K | R | T | 1-10 |
| GSTZ1B | A | G | C | K | G | T | 25-35 |
| GSTZ1C | G | G | C | E | G | T | 45-55 |
| GSTZ1D | G | G | T | E | G | M | 10-20 |
| GSTZ1F | A | G | T | K | G | M | <1 |
Standard amino acid abbreviations are used, viz., K = lysine, G = glycine, T = threonine, E = glutamic acid, M = methionine
As well as coding region SNPs, a SNP in the non-coding region of the GSTZ1 gene has been shown to contribute to individual differences in the expression of this enzyme. It was found that Caucasians who express K (lysine) at protein position 32 as a result of having the A allele from the G>A coding region SNP (rs7975) exhibit lower expression of GSTZ1 protein in liver cytosol than those who do not (Langaee, et al., 2015). Furthermore, this showed linkage disequilibrium with the promoter region G>A -1002 A allele (rs7160195) (Fang, et al., 2006), indicating these SNPs were inherited together in Caucasians. This linkage was not found in livers from African-American donors, who exhibited similar expression of GSTZ1 protein whether or not their DNA coded for a K-containing variant protein. To date, no studies of the effect of the G>A -1002 A allele on GSTZ1 protein expression have been conducted with other ethnicities. Intriguingly, elderly A carriers of the G>A -1002 allele from a Scottish population, who would be expected to have lower expression of GSTZ1 than non-A carriers, showed a small but significant decline in cognitive function compared with non-A carriers (Starr, et al., 2008). Another study showed a strong relationship between cognitive functioning and the presence of 11 SNPs in the GSTZ1 gene in a Swedish population (Schoormans, et al., 2015), however the mechanism was not investigated.
The effects of GSTZ1 haplotype on the pharmacokinetics of DCA have been studied in adult human volunteers and in patients (children and adults) treated with DCA (Shroads, et al., 2015; Shroads, et al., 2012). In vitro studies with liver cytosol showed that individuals with at least one copy of the GSTZ1A variant (KRT) exhibited higher activity with DCA than those without this variant, confirming studies with expressed recombinant GSTZ1A that showed higher activity than the other variants (Blackburn, et al., 2000; Li, et al., 2012). In view of these findings, it might be expected that people with at least one copy of GSTZ1A (KRT) would metabolize DCA more rapidly than those with other variants. There was some evidence that this was the case with a single dose of DCA, a situation that is most similar to the in vitro studies. Individuals with at least one copy of GSTZ1A did not metabolize DCA more rapidly than other haplotypes following multiple doses of DCA, as would occur during therapy with the drug (Shroads, et al., 2012). Reasons for this are discussed in sections 9 and 10. After five or more days taking therapeutic doses of DCA (25 mg/kg/day in one or two doses), markedly reduced elimination was found in all persons. Those with at least one copy of the GSTZ1C (EGT) variant showed a lower reduction in DCA clearance than those who did not have the GSTZ1C variant (Table 3). Similar results were found with children (Shroads, et al., 2015). As recently reviewed (James & Stacpoole, 2016) individuals can be considered to fall into rapid (at least one copy of the GSTZ1C gene) and slow metabolizer (no copy of GSTZ1C) phenotypes with respect to DCA following administration of multiple doses.
Table 3.
Effect of GSTZ1 haplotype on DCA pharmacokinetics. Data are from volunteers who took DCA, 25 mg/kg/day, and patients with genetic mitochondrial diseases administered 12.5 mg/kg/12h, showing plasma clearance after the indicated dosing duration.
| Plasma Clearance, ml/min | Reference | ||
|---|---|---|---|
| GSTZ1C (EGT) carrier | GSTZ1C (EGT) non-carrier | ||
| Volunteers, 1 dose | 10.73 ± 6.88 (7) | 7.08 ± 6.03 (5) | Shroads et al. 2012 |
| Volunteers, 5 days | 2.22 ± 0.72 (7) | 0.73 ± 0.84* (5) | Shroads et al. 2012 |
| Patients, 12 months | 2.16 ± 0.99 (4) | 0.91, 0.17 | Shroads et al. 2012 |
| Patients, 6 months | 1.90 ± 1.13 (11) | 0.53 ± 0.35* (6) | Shroads et al. 2015 |
| Patients, 30 months | 2.08 ± 1.10 (11) | 0.67 ± 0.45* (6) | Shroads et al. 2015 |
Values shown are mean ± S.D. (n) or individual values where n<3.
Significantly different from EGT carriers, p<0.05
9. Inactivation of GSTZ1 by DCA
An explanation of the effect of repeated doses of DCA on its pharmacokinetics in humans and animals was found when it was realized that DCA could inactivate the GSTZ1 enzyme during its conversion to glyoxylate. This inactivation led to the observed prolongation of elimination of DCA.
Not only is DCA a substrate for GSTZ1, it is also a mechanism-based inactivator. Studies using rodents have demonstrated time- and dose-dependent effects on reducing the protein expression levels and activity of GSTZ1 in liver (Anderson, et al., 1999; Cornett, et al., 1999). Recovery times of GSTZ1 expression and activity also were dependent both on the duration and size of the dose of DCA administered. A single oral dose of 50 mg DCA/kg body weight to male Sprague Dawley rats led to more than 60% reduction of GSTZ1 activity and expression 24h after the dose (Cornett et al., 1999). A single intraperitoneal dose of 0.3 mmol DCA/kg (45 mg/kg) to male Fischer-344 rats resulted in rapid loss of GSTZ1 protein and activity with a nadir of less than 40% initial values at 12h: these were not regained until ten to twelve days after the dose (Anderson, et al., 1999). From the recovery time-course, it was calculated that the rate of natural protein turnover of GSTZ1 was 3.3 days (Anderson, et al., 1999). Five days of administering DCA at lower doses (4 mg/kg body weight/day and 12.5 mg/kg body weight/day) resulted in loss of 20% and 52% of GSTZ1 activity and expression respectively 24h after the fifth dose (Cornett, et al., 1999). In another study, male Sprague-Dawley rats were provided 2.5, 250 or 50,000 μg DCA/kg body weight per day in drinking water for 8 weeks at which time GSTZ1 activity was reduced to 80% of that in age-matched controls by the environmentally relevant doses (2.5 and 250 μg/kg) and to less than 5% control values in the rats given 50 mg/kg/day (Guo, et al., 2006). Rats that received 50 mg DCA/kg/day in drinking water had only 60% of control GSTZ1 activity 1 week after cessation of the DCA, compared to complete recovery of GSTZ1 activity in rats given 2.5 or 250 DCA/kg through drinking water (Guo, et al., 2006).
Despite producing changes in GSTZ1 activity and protein expression, DCA did not affect steady state mRNA levels (Ammini, et al., 2003). The loss of function of this enzyme was proposed to occur post-translationally from adduct formation that leads to protein loss (Anderson, et al., 1999; Tzeng, et al., 2000). Experiments conducted in vitro confirmed the ability of DCA to inhibit GSTZ1, in time- and DCA concentration-dependent manners in human, rat, and mouse liver cytosol (Tzeng, et al., 2000). The rate of loss of GSTZ1 activity produced by DCA depended on the species. For rat, mouse, and human, the in vitro inactivation half-lives for GSTZ1 with DCA in the absence of chloride were respectively 5.4 minutes, 6.6 minutes, and 22 minutes, yet the half-maximal inhibitory concentration of DCA (40 μM) did not differ between species. Although this study exhibited differences in the ability of GSTZ1 to biotransform DCA among rat, mouse and human, it does not completely describe DCA-induced GSTZ1 inactivation. A concurrent study showed that 500 μM DCA in the presence of chloride did not inactivate human hepatic cytosol and inactivated only half the enzyme in rat liver cytosol (Cornett, et al., 1999). Subsequent investigations have revealed that chloride has a protective effect on GSTZ1 inactivation (Zhong, et al., 2014a).
Studies with recombinant human (h) GSTZ1C, 14C-labeled DCA and 35S-labeled GSH showed that both molecules covalently bound to the enzyme (Tzeng, et al., 2000). A subsequent study provided mass spectral evidence that an adduct of carboxymethyl glutathione to C16 was formed during incubation of DCA, GSH and hGSTZ1C (Anderson, et al., 2002). This adduct is believed to form between the nucleophilic cysteine-16 residue of the active site SSC motif of GSTZ1 and the unstable carbonium-sulfonium intermediate formed during DCA dechlorination, leading to a covalently modified and inactivated enzyme that presumably undergoes degradation in vivo to cause the observed loss of GSTZ1 protein. Another study with recombinant hGSTZ1C demonstrated a glyoxylate adduct to S14 of GSTZ1C (Dixit, 2005). Further studies are warranted to explore the mechanisms of inactivation and subsequent degradation of the adducted protein.
10. Chloride as a modulator of GSTZ1 inactivation by DCA
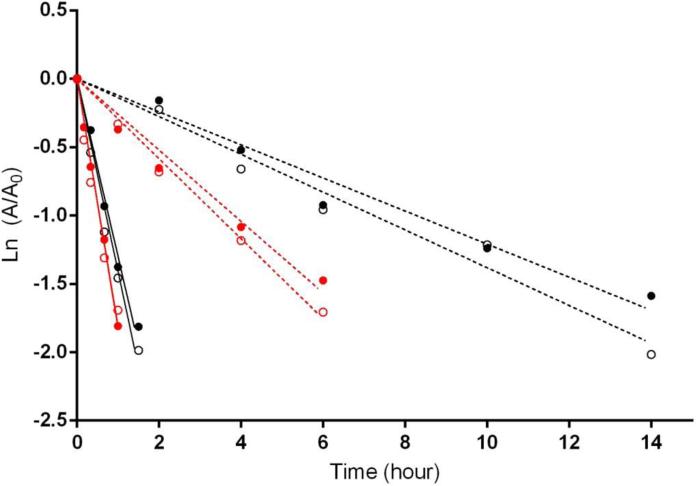
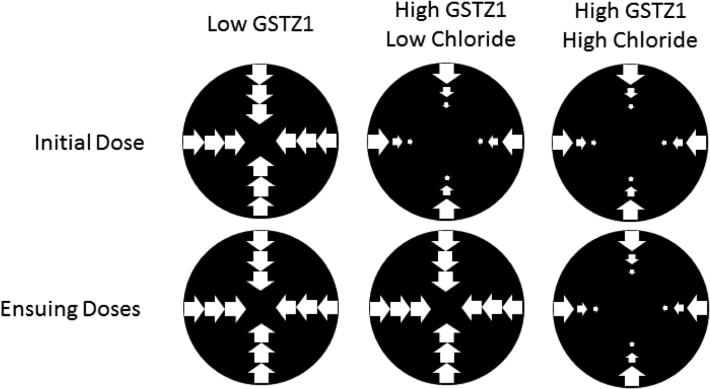
Chloride is a physiologically important anion that is capable of interacting with proteins and modulating enzyme functions (Maurus, et al., 2008; Sinibaldi, et al., 2003). Studies from our laboratory (Zhong, et al., 2014a) demonstrated that in vitro half-lives of GSTZ1 inactivation in human cytosol samples were substantially prolonged by chloride at its reported hepatic physiological concentration of 25-63 mM (Jahn, et al., 2015; Widdowson & Dickerson, 1960). Regardless of which GSTZ1 haplotype the samples exhibited, inactivation half-lives (t½inact) in the absence of chloride were 7-10 fold shorter than those determined with 38 mM of chloride (Figure 8). The protective effect of chloride also showed chloride concentration- and GSTZ1-haplotype-dependence. Protection by chloride was more potent in human liver cytosol samples from EGT homozygotes compared with samples carrying the EGT/KRT haplotype, with average EC50 values of 15.0 and 36.2 mM, respectively. This means that loss of the GST enzyme during therapeutic treatment with DCA is expected to be faster in people who express the KRT variant than those who do not, as physiological chloride concentrations will be less effective in protecting the KRT variant against inactivation. Other anions, including bromide, iodide and sulfite, also attenuated DCA-induced GSTZ1 inactivation in cytosol samples with EGT/EGT haplotype in an anion concentration dependent way. Haplotype dependence was confirmed for bromide as well. Although the EC50 values were in the following order: iodide < bromide < sulfite < chloride (Table 4), we believe that chloride plays the most critical role in modulating GSTZ1 activity. The reported physiological concentration of chloride in blood of 100 mM was far higher than those of other three anions, which were present at μM or lower concentrations (Mitsuhashi, et al., 2004; Sharma & Schwille, 1993; Zhang, et al., 2010). Moreover, there are no data for hepatic concentrations of these anions other than chloride. A recent study in which chloride concentrations were measured in human liver samples from donors aged 1 day to 84 years demonstrated that the chloride level in cytosol was much higher than that in mitochondria (Table 4) (Jahn, et al., 2015). Cytosolic chloride concentration decreased in an age-dependent manner (Jahn, et al., 2015), suggesting a possible explanation for clinical observations seen in patients under chronic treatment of DCA, whereby clearance of DCA was reduced to a greater extent in adults than in children (Shroads, et al., 2008).
Figure 8.
Effect of a physiological concentration of chloride on the time course of GSTZ1 inactivation by DCA, 0.5 mM, in human liver cytosol of EGT/EGT (GSTZ1C/1C, black) and EGT/KRT (GSTZ1C/1A, red). Solid lines show the linear regression for each individual in the absence of chloride; dashed lines show the linear regression in the presence of 38 mM chloride. Each data point is the mean of duplicate determinations with individual cytosol samples. The X axis shows the incubation time and the Y axis shows the natural log of activity remaining at the time point shown (A) divided by control activity (A0, activity at time 0). In the absence of chloride, half-lives of inactivation (t½inact) values were 0.53 and 0.49 h for EGT/EGT (1C/1C) samples, and were 0.38 h for both EGT/KRT (1C/1A) samples. In the presence of 38 mM chloride, t½inact values for EGT/EGT (1C/1C) samples were 5.73 and 5.02 h and for EGT/KRT (1C/1A) samples were 2.66 and 2.37 h (Zhong et al., 2014a).
Table 4.
Human liver chloride concentration and EC50 values of various anions in protecting human cytosolic GSTZ1 with EGT/EGT haplotype from DCA-induced inactivation.
| Anion | EC50 (mM) (n=3) | Hepatic concentration (mM) |
|
|---|---|---|---|
| Cytosol (n=97) | Mitochondria (n=97) | ||
| Chloride | 15.0 ± 3.1A | 105.2 ± 62.4 | 4.2 ± 3.8 |
| Bromide | 1.3 ± 0.3B | - | - |
| Iodide | 0.14 ± 0.06B | - | - |
| Sulfite | 9.6 ± 1.1C | - | - |
Values shown are mean ± S.D.; EC50 is the concentration of anion that protected half the cytosolic GSTZ1 from inactivation following incubation for 2 hours with 0.5 mM DCA. Different superscript letters indicate significant differences in EC50 between anions, p<0.01, calculated by one-way ANOVA analysis of paired samples with Bonferroni's multiple comparison test. EC50 data is from Zhong et al. (2014a) and chloride concentration data from Jahn et al. (2015).
11. Toxicities associated with chronic DCA administration
Use of DCA as a therapeutic drug in people requires a good understanding of its possible side effects and toxicity. Furthermore, small amounts of DCA, as well as other halogenated small organic molecules, are produced as a result of water chlorination, with reported concentrations in drinking water of 0.3 to 100 μg/L (IARC, 2014). The toxicology of DCA has been considered by the US National Toxicology Program (National Toxicology, 2007) and the International Agency for Research on Cancer, a branch of the World Health Organization (IARC, 2004).
11.1 Cancer
Somewhat paradoxically, since DCA has demonstrated anti-cancer activity, the most serious documented effect of DCA exposure is cancer in laboratory rodents. Liver cancer was observed in rats and mice exposed to very high doses of up to 5 g/L in the drinking water for 76 to 104 weeks, an estimated daily dose of 180 to 900 mg/kg body weight/day (DeAngelo, et al., 1999). Doses below 0.5 g/L for 52 weeks did not produce tumors (Bull, et al., 2002). No tumors were found in genetically modified Tg.AC hemizygous and p53 haploinsufficient mice administered DCA at a dose of 500 mg/kg/day for 41 weeks (National Toxicology, 2007). The mouse strains used were selected because they develop cancer rapidly (Pritchard, et al., 2003; Tennant, et al., 1996).
In studies of small numbers of patients, there is no evidence that chronic use of DCA for up to 20 years to treat mitochondrial disorders at therapeutic doses causes cancer (Abdelmalak, et al., 2013) and personal observations by Stacpoole. Environmental exposure of people to DCA occurs together with exposure to many other agents, however the levels to which people are environmentally exposed are in the μg per day range, well below the dosage that produced liver cancer in rodents. Based on the published research available, in 2004 the IARC concluded there was inadequate evidence that DCA was carcinogenic in humans, but sufficient evidence for its carcinogenicity in experimental animals. This conclusion was affirmed in an update published in 2014 (IARC, 2014).
11.2 Liver
Chronic DCA administration to patients can sometimes lead to symptomatic, mild, but reversible elevation of hepatic transaminases (Stacpoole, et al., 2006). However, chronic administration of up to 25 mg/kg/day oral DCA for several years to subjects with primary mitochondrial diseases disclosed no significant effects on any indices of hematological, metabolic, renal or hepatic function, including no consistent effects on serum transaminases (Abdelmalak, et al., 2013). In mice given 0.5 g DCA/kg/day for 26 or 41 weeks there was evidence of hepatocyte vacuolization (National Toxicology, 2007). The mechanisms of effects on liver have not been investigated.
11.3 Peripheral Neuropathy
In patients treated chronically with DCA, the nervous system appears to be most commonly affected. For the central nervous system, a sensation of calmness and drowsiness is seen in some adults, having properties similar to anxiolytics. The most problematic effect from chronic DCA treatment in dogs, rats and humans is reversible peripheral neuropathy and is considered the major limiting factor in clinical DCA use (Stacpoole, et al., 1998b). Peripheral neuropathy is characterized as a tingling sensation in the extremities, muscle weakness in the face, hands, and feet, as well as reduced nerve conduction velocity (Calcutt, et al., 2009). It has been hypothesized that the inactivation of GSTZ1 by DCA plays a role in development of this toxicity, perhaps through perturbation of the phenylalanine and tyrosine catabolic pathway.
11.4 Possible mechanisms of toxicity
As stated in section 5, monodehalogenation of DCA to MCA is quantitatively so low as to be an unlikely cause of DCA toxicity at therapeutic doses in people. However, the catabolism of tyrosine results in formation of chemically reactive and therefore potentially toxic intermediates, particularly maleylacetoacetate and fumarylacetoacetate and their decarboxylation and reduction products (Figure 4). Loss or low activity of FAH has been associated with the human genetic disease of hereditary tyrosinemia (Fernandez-Canon, et al., 2002). The most severe type of metabolic disorder is hereditary tyrosinemia type 1 (HTT1), which is linked to a deficiency and loss of function mutation in the enzyme FAH (Angileri, et al., 2015a). This condition results in build-up of fumarylacetoacetate, fumarylacetone and their reduction products succinylacetoacetate and succinylacetone, as well as the neurotoxin δ-aminolevulinate (δ-ALA) which inhibits peripheral nerve myelination in vitro (Felitsyn, et al., 2004) and untreated patients suffer damage to the hepatic, nervous, and renal systems, including hepatocellular carcinoma and peripheral neuropathy (Phaneuf, et al., 1992). Increases in δ-ALA are thought to arise from inhibition of δ-ALA dehydratase, and it has been shown that maleylacetone and fumarylacetone as well as succinylacetone inhibit this enzyme (Lantum, et al., 2003). It is plausible that long-term use of DCA, which will lead to loss of GSTZ1, will result in build-up of chemically reactive maleylacetoacetate and maleylacetone as well as succinylacetone, which could produce symptoms similar to hereditary tyrosinemia. Indeed, patients and animals treated with DCA have been shown to excrete maleylacetone and δ-ALA in urine (Shroads, et al., 2008; Shroads, et al., 2012). However, the concentrations of δ-ALA in urine of children treated chronically with DCA were much lower than those found in HTT1 patients (Felitsyn, et al., 2004; Shroads, et al., 2012).
As yet, there is no reported human condition arising from loss of the GSTZ1 enzyme. Mice with deletion of the Gstz1 gene have been generated to investigate the likely symptoms and severity of abnormalities that might be associated with loss of the GSTZ1 enzyme (Ammini, et al., 2003; Fernandez-Canon, et al., 2002; Lim, et al., 2004). One study reported that mice with the Gstz1 gene knocked out appeared normal unless stressed with a diet high in homogentisic acid, tyrosine or phenylalanine, which caused renal and hepatic failure thought to be due to build-up of maleylacetoacetate and maleylacetone (Fernandez-Canon, et al., 2002). That study also investigated mice in which both Gstz1 and Fah genes were knocked out. In these mice there was evidence for formation of fumarylacetone in the absence of the FAH enzyme; the authors showed that maleylacetone would convert to fumarylacetone in the presence of >1mM GSH, a physiological concentration in liver (Fernandez-Canon, et al., 2002). Although the rate of GSH-dependent isomerization was slower than the GSTZ1-catalyzed reaction, its occurrence may explain why Gstz1 knockout mice survive unless stressed with a diet high in phenylalanine, tyrosine or homogentisate. In another study, Gstz1 null mice maintained on a standard diet were found to have a variety of symptoms including enlarged livers and kidneys and induction of alpha, mu and pi class GSTs and presence of succinylacetone in serum (Lim, et al., 2004). Exposure of young (<28 days) Gstz1−/− mice to phenylalanine was lethal (Lim, et al., 2004). Additional studies of these mice implicated oxidative stress as the cause of the enlarged organs and induction of other GSTs, and further showed that the Gstz1 null mice had high NAD(P)H: quinone oxidoreductase and low hepatic GSH (Blackburn, et al., 2006). This finding is consistent with build-up of the reactive electrophiles, maleylacetoacetate and maleylacetone, and suggests that chemical knock-down of GSTZ1 by DCA will have similar effects. This hypothesis was explored in a study that compared the effects of high dietary phenylalanine in GSTZ−/− mice with mice that were treated with high dose (200 mg/kg) DCA for 5 days prior to as well as during phenylalanine treatment (Theodoratos, et al., 2009). The GSTZ1 null mice exhibited leucopenia and low blood GSH, consistent with prior results of oxidative stress in these mice, whereas the DCA-treated mice did not develop leucopenia or low blood GSH. The DCA treatment reduced, but did not completely eliminate GSTZ1 from the liver and the authors hypothesized that the low residual GSTZ1 was sufficient to prevent the severe oxidative stress observed in GSTZ1 null mice (Theodoratos, et al., 2009).
DCA pharmacokinetics also have been examined in Gstz1−/− mice; as expected, unchanged DCA was detected at high concentrations in the plasma and urine (Ammini, et al., 2003). The Gstz1 knockout mice excreted high levels of maleylacetone, fumarylacetone and succinylacetone in urine, while wild type mice did not excrete fumarylacetone or succinylacetone, and excreted much lower levels of maleylacetone. These studies demonstrate that DCA treatment causes loss of GSTZ1, which results in accumulation of potentially toxic phenylalanine and tyrosine catabolites that may be causally linked with the observed effects of DCA on peripheral nerve function.
12. GSTZ1 in tumors and response to DCA
The recent increased interest in DCA as a cancer therapeutic has resulted in investigation of the role of GSTZ1 in cancer. A number of studies have examined the link between GSTZ1 polymorphisms and cancer risk. They found no correlation in breast (Andonova, et al., 2010; Saadat, et al., 2012a; Smith, et al., 2001) or gastric (Karakas-Celik, et al., 2014) cancer, while a potential link was identified between the EGM haplotype and disinfectant by-product-associated bladder cancer (Cantor, et al., 2010). Of note, DCA is a disinfectant by-product, and the EGM haplotype has been classified as a slow metabolizer of DCA (Shroads, et al., 2012); however, the significance of the slow metabolizer phenotype is unclear in this context. Interestingly, changes in methylation of the GSTZ1 gene have been associated with the progression of ovarian endometriosis to cancer, although the study does not indicate the direction or magnitude of this change (Ren, et al., 2014). Another study reported that low expression of GSTZ1 in neuroblastoma was associated with a higher probability of survival, though no mechanism was put forward (Yogev, et al., 2016).
Other than one study showing that GSTZ1 polymorphisms do not affect tumor response to traditional chemotherapy (Saadat, et al., 2012b), the effect of GSTZ1 on cancer outcomes has not been well studied. We have recently reported (Jahn, et al., 2016), using meta-analyses, that GSTZ1 is often misregulated in cancers, showing high mRNA expression in some organs in which the basal expression is low, including breast, and low expression in the liver, where GSTZ1 is expressed normally. We found that that low GSTZ1 mRNA expression correlated with poor overall survival in breast cancer for reasons that are not clear (Jahn, et al., 2016).
Expanding on our initial findings, we obtained donor-matched tumor and non-tumor tissue pairs from selected organ sites and examined GSTZ1 protein expression and enzyme activity. Our results mirrored those shown by others at the mRNA level, with dramatic downregulation in hepatocellular carcinoma in the liver, where the bulk of GSTZ1 is normally expressed, and upregulation in a number of breast tumors, where normal breast showed little to no expression. Tumors from the duodenum and kidney showed variable misregulation, with some tumors showing higher expression while others showed lower expression than the surrounding non-tumor tissue (Jahn, et al., 2016).
As noted previously, chloride concentration has a direct impact on the stability of GSTZ1 during exposure to DCA (Zhong, et al., 2014a). The only previous studies examining chloride in cancer utilized cells grown in culture (Habela, et al., 2009; Shiozaki, et al., 2011; Soroceanu, et al., 1999). We measured the chloride concentration in human liver and breast tumors, and found that chloride levels were elevated in hepatocellular carcinoma, compared to the surrounding non-tumor tissue, and showed highly variable regulation in breast tumors (Jahn, et al., 2016). Although the results in the breast tumors were not as consistent as those measured in liver tumors, it is clear that the potential exists for an individual breast tumor to contain elevated levels of both GSTZ1 and chloride, likely making it resistant to DCA treatment.
We confirmed this resistance provided by GSTZ1 expression in both two- and three-dimensional cell culture models (Jahn, et al., 2016). Figure 9 represents our current working model, in which GSTZ1 expression prevents DCA diffusion into the center of a tumor. In tumors with low chloride concentrations, resistance could potentially be overcome by multiple doses of DCA through the degradation of GSTZ1, although high chloride levels could result in a persistently resistant tumor. Further studies are needed to examine this phenomenon in vivo.
Figure 9.
Proposed effect of chloride concentration and GSTZ1 expression on the efficacy of DCA in penetrating the tumor (adapted from Jahn et al., 2016).
13. Summary
As a therapeutic agent, DCA may be effective in treating several conditions related to dysfunction of mitochondrial energy metabolism, although the occurrence of a reversible peripheral neuropathy associated with chronic administration has somewhat limited its clinical utility, especially in adults. An improved understanding of the factors that affect the rate of biotransformation of DCA under conditions of chronic dosing can be expected to lead to more rational treatment of patients with appropriate doses of the drug to reduce side effects, by taking into account variables such as patient age and GSTZ1 haplotype. It is unusual that only a single enzyme, GSTZ1, can metabolize DCA in the liver and to a lesser extent in extrahepatic tissues, in which metabolism of the drug requires further investigation.
Acknowledgements
The authors’ work cited in this review was funded in part by a grant from the US Public Health Service, 1-RO1 GM099871.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
MOJ, SCJ, GZ, MGS and ZH declare they have no conflicts of interest. PWS has a patent pending involving DCA.
References
- Abdelmalak M, Lew A, Ramezani R, Shroads AL, Coats BS, Langaee T, Shankar MN, Neiberger RE, Subramony SH, Stacpoole PW. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab. 2013;109:139–143. doi: 10.1016/j.ymgme.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammini CV, Fernandez-Canon J, Shroads AL, Cornett R, Cheung J, James MO, Henderson GN, Grompe M, Stacpoole PW. Pharmacologic or genetic ablation of maleylacetoacetate isomerase increases levels of toxic tyrosine catabolites in rodents. Biochem Pharmacol. 2003;66:2029–2038. doi: 10.1016/j.bcp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Anderson WB, Board PG, Gargano B, Anders MW. Inactivation of glutathione transferase zeta by dichloroacetic acid and other fluorine-lacking alpha-haloalkanoic acids. Chem Res Toxicol. 1999;12:1144–1149. doi: 10.1021/tx990085l. [DOI] [PubMed] [Google Scholar]
- Anderson WB, Liebler DC, Board PG, Anders MW. Mass spectral characterization of dichloroacetic acid-modified human glutathione transferase zeta. Chem Res Toxicol. 2002;15:1387–1397. doi: 10.1021/tx025553x. [DOI] [PubMed] [Google Scholar]
- Andonova IE, Justenhoven C, Winter S, Hamann U, Baisch C, Rabstein S, Spickenheuer A, Harth V, Pesch B, Bruning T, Ko YD, Ganev V, Brauch H. No evidence for glutathione S-transferases GSTA2, GSTM2, GSTO1, GSTO2, and GSTZ1 in breast cancer risk. Breast Cancer Res Treat. 2010;121:497–502. doi: 10.1007/s10549-009-0589-5. [DOI] [PubMed] [Google Scholar]
- Angileri F, Bergeron A, Morrow G, Lettre F, Gray G, Hutchin T, Ball S, Tanguay RM. Geographical and Ethnic Distribution of Mutations of the Fumarylacetoacetate Hydrolase Gene in Hereditary Tyrosinemia Type 1. JIMD Rep. 2015a;19:43–58. doi: 10.1007/8904_2014_363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angileri F, Roy V, Morrow G, Scoazec JY, Gadot N, Orejuela D, Tanguay RM. Molecular changes associated with chronic liver damage and neoplastic lesions in a murine model of hereditary tyrosinemia type 1. Biochim Biophys Acta. 2015b;1852:2603–2617. doi: 10.1016/j.bbadis.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Babu E, Ramachandran S, CoothanKandaswamy V, Elangovan S, Prasad PD, Ganapathy V, Thangaraju M. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene. 2011;30:4026–4037. doi: 10.1038/onc.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi MR, Snyder R, Waritz RS, Cooper KR. Monochloroacetic acid toxicity in the mouse associated with blood-brain barrier damage. Fundam Appl Toxicol. 1987;9:469–479. doi: 10.1016/0272-0590(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Berendzen K, Theriaque DW, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Wapnir RA, Towell ME. Development of liver phenylalanine hydroxylase and brain aromatic hydroxylases in human fetuses. Biochem Med. 1977;17:1–7. doi: 10.1016/0006-2944(77)90002-3. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Coggan M, Tzeng HF, Lantum H, Polekhina G, Parker MW, Anders MW, Board PG. GSTZ1d: a new allele of glutathione transferase zeta and maleylacetoacetate isomerase. Pharmacogenetics. 2001;11:671–678. doi: 10.1097/00008571-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Matthaei KI, Lim C, Taylor MC, Cappello JY, Hayes JD, Anders MW, Board PG. Deficiency of glutathione transferase zeta causes oxidative stress and activation of antioxidant response pathways. Mol Pharmacol. 2006;69:650–657. doi: 10.1124/mol.105.018911. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Tzeng HF, Anders MW, Board PG. Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics. 2000;10:49–57. doi: 10.1097/00008571-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Woollatt E, Sutherland GR, Board PG. Characterization and chromosome location of the gene GSTZ1 encoding the human Zeta class glutathione transferase and maleylacetoacetate isomerase. Cytogenet Cell Genet. 1998;83:109–114. doi: 10.1159/000015145. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Holloway PA, Alberti KG. The metabolic effects of sodium dichloroacetate in the starved rat. Biochem J. 1974;142:279–286. doi: 10.1042/bj1420279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board PG, Anders MW. Human glutathione transferase zeta. Methods Enzymol. 2005;401:61–77. doi: 10.1016/S0076-6879(05)01004-9. [DOI] [PubMed] [Google Scholar]
- Board PG, Anders MW. Glutathione transferase zeta: discovery, polymorphic variants, catalysis, inactivation, and properties of Gstz1−/− mice. Drug Metab Rev. 2011;43:215–225. doi: 10.3109/03602532.2010.549132. [DOI] [PubMed] [Google Scholar]
- Board PG, Baker RT, Chelvanayagam G, Jermiin LS. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem J. 1997;328(Pt 3):929–935. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta. 2013;1830:3267–3288. doi: 10.1016/j.bbagen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Board PG, Taylor MC, Coggan M, Parker MW, Lantum HB, Anders MW. Clarification of the role of key active site residues of glutathione transferase zeta/maleylacetoacetate isomerase by a new spectrophotometric technique. Biochem J. 2003;374:731–737. doi: 10.1042/BJ20030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- Boone CD, Zhong G, Smeltz M, James MO, McKenna R. Preliminary X-ray crystallographic analysis of glutathione transferase zeta 1 (GSTZ1a-1a). Acta Crystallogr F Struct Biol Commun. 2014;70:187–189. doi: 10.1107/S2053230X13033591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocato J, Chervona Y, Costa M. Molecular responses to hypoxia-inducible factor 1alpha and beyond. Mol Pharmacol. 2014;85:651–657. doi: 10.1124/mol.113.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RJ, Orner GA, Cheng RS, Stillwell L, Stauber AJ, Sasser LB, Lingohr MK, Thrall BD. Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol Appl Pharmacol. 2002;182:55–65. doi: 10.1006/taap.2002.9427. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Lopez VL, Bautista AD, Mizisin LM, Torres BR, Shroads AL, Mizisin AP, Stacpoole PW. Peripheral neuropathy in rats exposed to dichloroacetate. J Neuropathol Exp Neurol. 2009;68:985–993. doi: 10.1097/NEN.0b013e3181b40217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert LD, Shelley R, Singh SJ, Greenhaff PL, Bankart J, Morgan MD, Steiner MC. Dichloroacetate enhances performance and reduces blood lactate during maximal cycle exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1090–1094. doi: 10.1164/rccm.200707-1032OC. [DOI] [PubMed] [Google Scholar]
- Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, Malats N, Chanock S, Yeager M, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castano-Vinyals G, Samanic C, Rothman N, Kogevinas M. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ Health Perspect. 2010;118:1545–1550. doi: 10.1289/ehp.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33:603–610. doi: 10.1007/s10637-015-0221-y. [DOI] [PubMed] [Google Scholar]
- Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione S-transferase zeta and tyrosine metabolism by dichloroacetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem Biophys Res Commun. 1999;262:752–756. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–1045. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Curry SH, Chu PI, Baumgartner TG, Stacpoole PW. Plasma concentrations and metabolic effects of intravenous sodium dichloroacetate. Clin Pharmacol Ther. 1985;37:89–93. doi: 10.1038/clpt.1985.17. [DOI] [PubMed] [Google Scholar]
- Curry SH, Lorenz A, Chu PI, Limacher M, Stacpoole PW. Disposition and pharmacodynamics of dichloroacetate (DCA) and oxalate following oral DCA doses. Biopharm Drug Dispos. 1991;12:375–390. doi: 10.1002/bdd.2510120507. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, George MH, House DE. Hepatocarcinogenicity in the male B6C3F1 mouse following a lifetime exposure to dichloroacetic acid in the drinking water: dose-response determination and modes of action. J Toxicol Environ Health A. 1999;58:485–507. doi: 10.1080/009841099157115. [DOI] [PubMed] [Google Scholar]
- Deuse T, Hua X, Wang D, Maegdefessel L, Heeren J, Scheja L, Bolanos JP, Rakovic A, Spin JM, Stubbendorff M, Ikeno F, Langer F, Zeller T, Schulte-Uentrop L, Stoehr A, Itagaki R, Haddad F, Eschenhagen T, Blankenberg S, Kiefmann R, Reichenspurner H, Velden J, Klein C, Yeung A, Robbins RC, Tsao PS, Schrepfer S. Dichloroacetate prevents restenosis in preclinical animal models of vessel injury. Nature. 2014;509:641–644. doi: 10.1038/nature13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Lippard SJ. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc Natl Acad Sci U S A. 2009;106:22199–22204. doi: 10.1073/pnas.0912276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. Inactivation of Glutathione S Transferase Zeta by Dichloroacetic Acid. University of Florida; Gainesville: 2005. [Google Scholar]
- Dote T, Kono K, Usuda K, Shimizu H, Tanimoto Y, Dote E, Hayashi S. Systemic effects and skin injury after experimental dermal exposure to monochloroacetic acid. Toxicol Ind Health. 2003;19:165–169. doi: 10.1191/0748233703th191oa. [DOI] [PubMed] [Google Scholar]
- Dromparis P, Sutendra G, Michelakis ED. The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 2010;88:1003–1010. doi: 10.1007/s00109-010-0670-x. [DOI] [PubMed] [Google Scholar]
- Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32:452–464. doi: 10.1007/s10637-013-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner HL, Stacpoole PW, Forsham PH. Treatment of streptozotocin diabetes with diisopropylammonium dichloroacetate (DIPA). Diabetes. 1974;23:179–182. doi: 10.2337/diab.23.3.179. [DOI] [PubMed] [Google Scholar]
- Fang YY, Kashkarov U, Anders MW, Board PG. Polymorphisms in the human glutathione transferase zeta promoter. Pharmacogenet Genomics. 2006;16:307–313. doi: 10.1097/01.fpc.0000205000.07054.b3. [DOI] [PubMed] [Google Scholar]
- Felitsyn NM, Henderson GN, James MO, Stacpoole PW. Liquid chromatography-tandem mass spectrometry method for the simultaneous determination of delta-ALA, tyrosine and creatinine in biological fluids. Clin Chim Acta. 2004;350:219–230. doi: 10.1016/j.cccn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Fernandez-Canon JM, Baetscher MW, Finegold M, Burlingame T, Gibson KM, Grompe M. Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism. Mol Cell Biol. 2002;22:4943–4951. doi: 10.1128/MCB.22.13.4943-4951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Canon JM, Hejna J, Reifsteck C, Olson S, Grompe M. Gene structure, chromosomal location, and expression pattern of maleylacetoacetate isomerase. Genomics. 1999;58:263–269. doi: 10.1006/geno.1999.5832. [DOI] [PubMed] [Google Scholar]
- Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–890. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F, Patel C, von Euw E, Black A, Michelakis ED, Dubinett SM, Slamon DJ. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140:443–452. doi: 10.1007/s00432-014-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, Shinohara ML, MacIver NJ. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016 doi: 10.1002/eji.201545861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Leon A, Merdink JL, Bull RJ, Schultz IR. Effect of pre-treatment with dichloroacetic or trichloroacetic acid in drinking water on the pharmacokinetics of a subsequent challenge dose in B6C3F1 mice. Chem Biol Interact. 1999;123:239–253. doi: 10.1016/s0009-2797(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Leon A, Schultz IR, Xu G, Bull RJ. Pharmacokinetics and metabolism of dichloroacetate in the F344 rat after prior administration in drinking water. Toxicol Appl Pharmacol. 1997;146:189–195. doi: 10.1006/taap.1997.8232. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, MacDonald JS. Applying mechanisms of chemical toxicity to predict drug safety. Chem Res Toxicol. 2007;20:344–369. doi: 10.1021/tx600260a. [DOI] [PubMed] [Google Scholar]
- Guignabert C, Tu L, Izikki M, Dewachter L, Zadigue P, Humbert M, Adnot S, Fadel E, Eddahibi S. Dichloroacetate treatment partially regresses established pulmonary hypertension in mice with SM22alpha-targeted overexpression of the serotonin transporter. FASEB J. 2009;23:4135–4147. doi: 10.1096/fj.09-131664. [DOI] [PubMed] [Google Scholar]
- Guo X, Dixit V, Liu H, Shroads AL, Henderson GN, James MO, Stacpoole PW. Inhibition and recovery of rat hepatic glutathione S-transferase zeta and alteration of tyrosine metabolism following dichloroacetate exposure and withdrawal. Drug Metab Dispos. 2006;34:36–42. doi: 10.1124/dmd.105.003996. [DOI] [PubMed] [Google Scholar]
- Habela CW, Ernest NJ, Swindall AF, Sontheimer H. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J Neurophysiol. 2009;101:750–757. doi: 10.1152/jn.90840.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452:3–7. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC WG. Some drinking-water disinfectants and contaminants, including arsenic. Monographs on chloramine, chloral and chloral hydrate, dichloroacetic acid, trichloroacetic acid and 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. IARC Monogr Eval Carcinog Risks Hum. 2004;84:269–477. [PMC free article] [PubMed] [Google Scholar]
- IARC WG. Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. IARC Monogr Eval Carcinog Risks Hum. 2014;106:1–512. [PMC free article] [PubMed] [Google Scholar]
- Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271:861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- Jahn SC, Rowland-Faux L, Stacpoole PW, James MO. Chloride concentrations in human hepatic cytosol and mitochondria are a function of age. Biochem Biophys Res Commun. 2015;459:463–468. doi: 10.1016/j.bbrc.2015.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn SC, Solayman MH, Lorenzo RJ, Langaee T, Stacpoole PW, James MO. GSTZ1 expression and chloride concentrations modulate sensitivity of cancer cells to dichloroacetate. Biochim Biophys Acta. 2016;1860:1202–1210. doi: 10.1016/j.bbagen.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO, Cornett R, Yan Z, Henderson GN, Stacpoole PW. Glutathione-dependent conversion to glyoxylate, a major pathway of dichloroacetate biotransformation in hepatic cytosol from humans and rats, is reduced in dichloroacetate-treated rats. Drug Metab Dispos. 1997;25:1223–1227. [PubMed] [Google Scholar]
- James MO, Stacpoole PW. Pharmacogenetic considerations with dichloroacetate dosing. Pharmacogenomics. 2016 doi: 10.2217/pgs-2015-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO, Yan Z, Cornett R, Jayanti VM, Henderson GN, Davydova N, Katovich MJ, Pollock B, Stacpoole PW. Pharmacokinetics and metabolism of [14C]dichloroacetate in male Sprague-Dawley rats. Identification of glycine conjugates, including hippurate, as urinary metabolites of dichloroacetate. Drug Metab Dispos. 1998;26:1134–1143. [PubMed] [Google Scholar]
- Johnstone TC, Kulak N, Pridgen EM, Farokhzad OC, Langer R, Lippard SJ. Nanoparticle encapsulation of mitaplatin and the effect thereof on in vivo properties. ACS Nano. 2013;7:5675–5683. doi: 10.1021/nn401905g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankotia S, Stacpoole PW. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta. 2014;1846:617–629. doi: 10.1016/j.bbcan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Karakas-Celik S, Aras N, Ates C. Glutathione S-transferase Z1 (GSTZ1) gene polymorphism in gastric cancer: a preliminary study in a Turkish population. Lab Med. 2014;45:37–42. doi: 10.1309/lmbprab33kk5ejba. [DOI] [PubMed] [Google Scholar]
- Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, De Vivo DC. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- Knoechel TR, Tucker AD, Robinson CM, Phillips C, Taylor W, Bungay PJ, Kasten SA, Roche TE, Brown DG. Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry. 2006;45:402–415. doi: 10.1021/bi051402s. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005;7:1–6. doi: 10.1593/neo.04373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langaee TY, Zhong G, Li W, Hamadeh I, Solayman MH, McDonough CW, Stacpoole PW, James MO. The influence of human GSTZ1 gene haplotype variations on GSTZ1 expression. Pharmacogenet Genomics. 2015;25:239–245. doi: 10.1097/FPC.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantum HB, Baggs RB, Krenitsky DM, Board PG, Anders MW. Immunohistochemical localization and activity of glutathione transferase zeta (GSTZ1-1) in rat tissues. Drug Metab Dispos. 2002a;30:616–625. doi: 10.1124/dmd.30.6.616. [DOI] [PubMed] [Google Scholar]
- Lantum HB, Board PG, Anders MW. Kinetics of the biotransformation of maleylacetone and chlorofluoroacetic acid by polymorphic variants of human glutathione transferase zeta (hGSTZ1-1). Chem Res Toxicol. 2002b;15:957–963. doi: 10.1021/tx010095y. [DOI] [PubMed] [Google Scholar]
- Lantum HB, Cornejo J, Pierce RH, Anders MW. Perturbation of maleylacetoacetic acid metabolism in rats with dichloroacetic Acid-induced glutathione transferase zeta deficiency. Toxicol Sci. 2003;74:192–202. doi: 10.1093/toxsci/kfg104. [DOI] [PubMed] [Google Scholar]
- Li T, Schultz I, Keys DA, Campbell JL, Fisher JW. Quantitative evaluation of dichloroacetic acid kinetics in human--a physiologically based pharmacokinetic modeling investigation. Toxicology. 2008;245:35–48. doi: 10.1016/j.tox.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Li W, Gu Y, James MO, Hines RN, Simpson P, Langaee T, Stacpoole PW. Prenatal and postnatal expression of glutathione transferase zeta 1 in human liver and the roles of haplotype and subject age in determining activity with dichloroacetate. Drug Metab Dispos. 2012;40:232–239. doi: 10.1124/dmd.111.041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, James MO, McKenzie SC, Calcutt NA, Liu C, Stacpoole PW. Mitochondrion as a novel site of dichloroacetate biotransformation by glutathione transferase zeta 1. J Pharmacol Exp Ther. 2011;336:87–94. doi: 10.1124/jpet.110.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CE, Matthaei KI, Blackburn AC, Davis RP, Dahlstrom JE, Koina ME, Anders MW, Board PG. Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases. Am J Pathol. 2004;165:679–693. doi: 10.1016/S0002-9440(10)63332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg. 2013;58:219–230. doi: 10.1016/j.jvs.2013.02.240. [DOI] [PubMed] [Google Scholar]
- Lipscomb JC, Mahle DA, Brashear WT, Barton HA. Dichloroacetic acid: metabolism in cytosol. Drug Metab Dispos. 1995;23:1202–1205. [PubMed] [Google Scholar]
- Loftus RM, Finlay DK. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J Biol Chem. 2016;291:1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorini M, Ciman M. Hypoglycaemic action of diisopropyl-ammonium salts in experimental diabetes. Biochem Pharmacol. 1962;11:823–827. doi: 10.1016/0006-2952(62)90177-6. [DOI] [PubMed] [Google Scholar]
- Lu TH, Su CC, Tang FC, Chen CH, Yen CC, Fang KM, Lee k I, Hung DZ, Chen YW. Chloroacetic acid triggers apoptosis in neuronal cells via a reactive oxygen species-induced endoplasmic reticulum stress signaling pathway. Chem Biol Interact. 2015;225:1–12. doi: 10.1016/j.cbi.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Maurus R, Begum A, Williams LK, Fredriksen JR, Zhang R, Withers SG, Brayer GD. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity. Biochemistry. 2008;47:3332–3344. doi: 10.1021/bi701652t. [DOI] [PubMed] [Google Scholar]
- McCommis KS, Finck BN. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J. 2015;466:443–454. doi: 10.1042/BJ20141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Calvert LD, Singh SJ, Hageman GJ, Schols AM, Steiner MC. Dichloroacetate modulates the oxidative stress and inflammatory response to exercise in COPD. Chest. 2009;136:744–751. doi: 10.1378/chest.08-2890. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martinez-Palma L, Bolatto C, Trias E, Gandelman M, Radi R, Barbeito L, Cassina P. Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis. PLoS One. 2012;7:e34776. doi: 10.1371/journal.pone.0034776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi H, Ikeuchi H, Yamashita S, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Increased levels of serum sulfite in patients with acute pneumonia. Shock. 2004;21:99–102. doi: 10.1097/01.shk.0000105501.75189.85. [DOI] [PubMed] [Google Scholar]
- Moore GW, Swift LL, Rabinowitz D, Crofford OB, Oates JA, Stacpoole PW. Reduction of serum cholesterol in two patients with homozygous familial hypercholesterolemia by dichloroacetate. Atherosclerosis. 1979;33:285–293. doi: 10.1016/0021-9150(79)90180-1. [DOI] [PubMed] [Google Scholar]
- National Toxicology P. NTP report on the toxicology studies of dichloroacetic acid (CAS No. 79-43-6) in genetically modified (FVB Tg.AC hemizygous) mice (dermal and drinking water studies) and carcinogenicity studies of dichloroacetic acid in genetically modified [B6.129-Trp53(tm1Brd) (N5) haploinsufficient] mice (drinking water studies). Natl Toxicol Program Genet Modif Model Rep. 2007:1–168. [PMC free article] [PubMed] [Google Scholar]
- NCT01083524 A Phase I, Open-Label, Two Centre Study to Evaluate Dichloroacetate (DCA) in Advanced Pulmonary Arterial Hypertension. 2010 https://clinicaltrials.gov/ct2/show/NCT01083524?term=NCT01083524&rank=1.
- NCT02616484 Phase 3 Trial of Dichloroacetate in Pyruvate Dehydrogenase Complex Deficiency. 2016 https://clinicaltrials.gov/ct2/show/NCT02616484?term=dichloroacetate&rank=1.
- Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, Alam R. The role of low-level lactate production in airway inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2012;302:L300–307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamiuc L, Schlagowski A, Ngo ST, Vernay A, Dirrig-Grosch S, Henriques A, Boutillier AL, Zoll J, Echaniz-Laguna A, Loeffler JP, Rene F. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol Med. 2015;7:526–546. doi: 10.15252/emmm.201404433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- Pathak RK, Marrache S, Harn DA, Dhar S. Mito-DCA: a mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS Chem Biol. 2014;9:1178–1187. doi: 10.1021/cb400944y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf D, Lambert M, Laframboise R, Mitchell G, Lettre F, Tanguay RM. Type 1 hereditary tyrosinemia. Evidence for molecular heterogeneity and identification of a causal mutation in a French Canadian patient. J Clin Invest. 1992;90:1185–1192. doi: 10.1172/JCI115979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polekhina G, Board PG, Blackburn AC, Parker MW. Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity. Biochemistry. 2001;40:1567–1576. doi: 10.1021/bi002249z. [DOI] [PubMed] [Google Scholar]
- Pritchard JB, French JE, Davis BJ, Haseman JK. The role of transgenic mouse models in carcinogen identification. Environ Health Perspect. 2003;111:444–454. doi: 10.1289/ehp.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang DB, Li T, Chen YH, Li Y. Identification of differentially methylated genes in the malignant transformation of ovarian endometriosis. J Ovarian Res. 2014;7:73. doi: 10.1186/1757-2215-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat I, Khalili M, Nafissi S, Omidvari S, Saadat M. Susceptibility to breast cancer and three polymorphisms of GSTZ1. DNA Cell Biol. 2012a;31:337–341. doi: 10.1089/dna.2011.1334. [DOI] [PubMed] [Google Scholar]
- Saadat M, Khalili M, Nasiri M, Rajaei M, Omidvari S, Saadat I. Clinical response to chemotherapy in locally advanced breast cancer was not associated with several polymorphisms in detoxification enzymes and DNA repair genes. Biochem Biophys Res Commun. 2012b;419:117–119. doi: 10.1016/j.bbrc.2012.01.143. [DOI] [PubMed] [Google Scholar]
- Saghir SA, Schultz IR. Low-dose pharmacokinetics and oral bioavailability of dichloroacetate in naive and GST-zeta-depleted rats. Environ Health Perspect. 2002;110:757–763. doi: 10.1289/ehp.02110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Ghosh M, Dutta SK. A potent tumoricidal co-drug ‘Bet-CA’--an ester derivative of betulinic acid and dichloroacetate selectively and synergistically kills cancer cells. Sci Rep. 2015;5:7762. doi: 10.1038/srep07762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2016;138:809–817. doi: 10.1002/ijc.29564. [DOI] [PubMed] [Google Scholar]
- Schoormans D, Li J, Darabi H, Brandberg Y, Sprangers MA, Eriksson M, Zwinderman KH, Hall P. The genetic basis of quality of life in healthy Swedish women: a candidate gene approach. PLoS One. 2015;10:e0118292. doi: 10.1371/journal.pone.0118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz IR, Merdink JL, Gonzalez-Leon A, Bull RJ. Dichloroacetate toxicokinetics and disruption of tyrosine catabolism in B6C3F1 mice: dose-response relationships and age as a modifying factor. Toxicology. 2002;173:229–247. doi: 10.1016/s0300-483x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- Sharma V, Schwille PO. Sulfite inhibits oxalate production from glycolate and glyoxylate in vitro and from dichloroacetate infused i.v. into male rats. Biochem Med Metab Biol. 1993;49:265–269. doi: 10.1006/bmmb.1993.1028. [DOI] [PubMed] [Google Scholar]
- Shiozaki A, Otsuji E, Marunaka Y. Intracellular chloride regulates the G(1)/S cell cycle progression in gastric cancer cells. World J Gastrointest Oncol. 2011;3:119–122. doi: 10.4251/wjgo.v3.i8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroads AL, Coats BS, McDonough CW, Langaee T, Stacpoole PW. Haplotype variations in glutathione transferase zeta 1 influence the kinetics and dynamics of chronic dichloroacetate in children. J Clin Pharmacol. 2015;55:50–55. doi: 10.1002/jcph.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroads AL, Guo X, Dixit V, Liu HP, James MO, Stacpoole PW. Age-dependent kinetics and metabolism of dichloroacetate: possible relevance to toxicity. J Pharmacol Exp Ther. 2008;324:1163–1171. doi: 10.1124/jpet.107.134593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroads AL, Langaee T, Coats BS, Kurtz TL, Bullock JR, Weithorn D, Gong Y, Wagner DA, Ostrov DA, Johnson JA, Stacpoole PW. Human Polymorphisms in the Glutathione Transferase Zeta 1/Maleylacetoacetate Isomerase Gene Influence the Toxicokinetics of Dichloroacetate. J Clin Pharmacol. 2012;52:837–849. doi: 10.1177/0091270011405664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi F, Howes BD, Smulevich G, Ciaccio C, Coletta M, Santucci R. Anion concentration modulates the conformation and stability of the molten globule of cytochrome c. Journal of Biological Inorganic Chemistry. 2003;8:663–670. doi: 10.1007/s00775-003-0462-7. [DOI] [PubMed] [Google Scholar]
- Smith RA, Curran JE, Weinstein SR, Griffiths LR. Investigation of glutathione S-transferase zeta and the development of sporadic breast cancer. Breast Cancer Res. 2001;3:409–411. doi: 10.1186/bcr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ, Jr., Sontheimer H. Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers. J Neurosci. 1999;19:5942–5954. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW. Review of the pharmacologic and therapeutic effects of diisopropylammonium dichloroacetate (DIPA). J Clin Pharmacol J New Drugs. 1969;9:282–291. [PubMed] [Google Scholar]
- Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW. Lactic acidosis and other mitochondrial disorders. Metabolism. 1997;46:306–321. doi: 10.1016/s0026-0495(97)90259-6. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW. The Congenital Lactic Acidoses. In NORD Guide to Rare Disorders. Lippincott, Williams and Wilkins; Philadelphia: 2003. pp. 462–464. [Google Scholar]
- Stacpoole PW. The Dichloroacetate Dilemma: Environmental Hazard versus Therapeutic Goldmine-Both or Neither? Environ Health Perspect. 2011;119:155–158. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell. 2012;11:371–377. doi: 10.1111/j.1474-9726.2012.00805.x. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Barnes CL, Hurbanis MD, Cannon SL, Kerr DS. Treatment of congenital lactic acidosis with dichloroacetate. Arch Dis Child. 1997;77:535–541. doi: 10.1136/adc.77.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Bridge DM, Alvarez IM, Goldberg RB, Harwood HJ., Jr. In vivo regulation of human mononuclear leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase. Decreased enzyme catalytic efficiency in familial hypercholesterolemia. J Clin Invest. 1987;80:1401–1408. doi: 10.1172/JCI113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Felts JM. Diisopropylammonium dichloroacetate (DIPA) and sodium dichloracetate (DCA): effect on glucose and fat metabolism in normal and diabetic tissue. Metabolism. 1970;19:71–78. doi: 10.1016/0026-0495(70)90119-8. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Felts JM. Diisopropylammonium dichloroaccetate: regulation of metabolic intermediates in muscle of alloxan diabetic rats. Metabolism. 1971;20:830–834. doi: 10.1016/0026-0495(71)90044-8. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, Shuster JJ. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223–1228. doi: 10.1542/peds.2007-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Harman EM, Curry SH, Baumgartner TG, Misbin RI. Treatment of lactic acidosis with dichloroacetate. N Engl J Med. 1983a;309:390–396. doi: 10.1056/NEJM198308183090702. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Harwood HJ, Jr., Varnado CE. Dietary carbohydrate decreases 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in rat liver. Biochem Biophys Res Commun. 1983b;113:888–894. doi: 10.1016/0006-291x(83)91082-3. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Harwood HJ, Jr., Varnado CE. Regulation of rat liver hydroxymethylglutaryl coenzyme A reductase by a new class of noncompetitive inhibitors. Effects of dichloroacetate and related carboxylic acids on enzyme activity. J Clin Invest. 1983c;72:1575–1585. doi: 10.1172/JCI111116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. 1998a;30:499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, James MO. Clinical pharmacology and toxicology of dichloroacetate. Environ Health Perspect. 1998b;106(Suppl 4):989–994. doi: 10.1289/ehp.98106s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O'Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Lorenz AC, Thomas RG, Harman EM. Dichloroacetate in the treatment of lactic acidosis. Ann Intern Med. 1988;108:58–63. doi: 10.7326/0003-4819-108-1-58. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Moore GW, Kornhauser DM. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N Engl J Med. 1978;298:526–530. doi: 10.1056/NEJM197803092981002. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, Duncan CA, Harman EM, Henderson GN, Jenkinson S, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. N Engl J Med. 1992;327:1564–1569. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- Starr JM, Fox H, Harris SE, Deary IJ, Whalley LJ. GSTz1 genotype and cognitive ability. Psychiatr Genet. 2008;18:211–212. doi: 10.1097/YPG.0b013e328304dea8. [DOI] [PubMed] [Google Scholar]
- Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the “Warburg Effect” in a human cancer patient: complete response in non-Hodgkin's lymphoma after disease progression with rituximab-CHOP. J Bioenerg Biomembr. 2013;45:307–315. doi: 10.1007/s10863-012-9496-2. [DOI] [PubMed] [Google Scholar]
- Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2:44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Valet JP, Lescault A, Duband JL, Laberge C, Lettre F, Plante M. Different molecular basis for fumarylacetoacetate hydrolase deficiency in the two clinical forms of hereditary tyrosinemia (type I). Am J Hum Genet. 1990;47:308–316. [PMC free article] [PubMed] [Google Scholar]
- Tennant RW, Spalding J, French JE. Evaluation of transgenic mouse bioassays for identifying carcinogens and noncarcinogens. Mutat Res. 1996;365:119–127. doi: 10.1016/s0165-1110(96)90016-0. [DOI] [PubMed] [Google Scholar]
- Theodoratos A, Tu WJ, Cappello J, Blackburn AC, Matthaei K, Board PG. Phenylalanine-induced leucopenia in genetic and dichloroacetic acid generated deficiency of glutathione transferase Zeta. Biochem Pharmacol. 2009;77:1358–1363. doi: 10.1016/j.bcp.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Tong Z, Board PG, Anders MW. Glutathione transferase zeta-catalyzed biotransformation of dichloroacetic acid and other alpha-haloacids. Chem Res Toxicol. 1998a;11:1332–1338. doi: 10.1021/tx980144f. [DOI] [PubMed] [Google Scholar]
- Tong Z, Board PG, Anders MW. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem J. 1998b;331(Pt 2):371–374. doi: 10.1042/bj3310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapella C, Voltan R, Melloni E, Tisato V, Celeghini C, Bianco S, Fantinati A, Salvadori S, Guerrini R, Secchiero P, Zauli G. Design, Synthesis, and Biological Characterization of Novel Mitochondria Targeted Dichloroacetate-Loaded Compounds with Antileukemic Activity. J Med Chem. 2016;59:147–156. doi: 10.1021/acs.jmedchem.5b01165. [DOI] [PubMed] [Google Scholar]
- Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphism- and species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem Res Toxicol. 2000;13:231–236. doi: 10.1021/tx990175q. [DOI] [PubMed] [Google Scholar]
- Valbuena GN, Rizzardini M, Cimini S, Siskos AP, Bendotti C, Cantoni L, Keun HC. Metabolomic Analysis Reveals Increased Aerobic Glycolysis and Amino Acid Deficit in a Cellular Model of Amyotrophic Lateral Sclerosis. Mol Neurobiol. 2016;53:2222–2240. doi: 10.1007/s12035-015-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Akker CHR, Van Goudoever JB. Recent advances in our understanding of protein and amino acid metabolism in the human fetus. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13:75–80. doi: 10.1097/MCO.0b013e328333aa4f. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S, Randle PJ. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication). Biochem J. 1973;134:651–653. doi: 10.1042/bj1340651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson EM, Dickerson JW. The effect of growth and function on the chemical composition of soft tissues. Biochem J. 1960;77:30–43. doi: 10.1042/bj0770030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev O, Barker K, Sikka A, Almeida GS, Hallsworth A, Smith LM, Jamin Y, Ruddle R, Koers A, Webber HT, Raynaud FI, Popov S, Jones C, Petrie K, Robinson SP, Keun HC, Chesler L. p53 Loss in MYC-Driven Neuroblastoma Leads to Metabolic Adaptations Supporting Radioresistance. Cancer Res. 2016;76:3025–3035. doi: 10.1158/0008-5472.CAN-15-1939. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Hu X, Zhang W, Tam KY. Unexpected Discovery of Dichloroacetate Derived Adenosine Triphosphate Competitors Targeting Pyruvate Dehydrogenase Kinase To Inhibit Cancer Proliferation. J Med Chem. 2016;59:3562–3568. doi: 10.1021/acs.jmedchem.5b01828. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu Q, Sun HW, Rao J, Kannan K. Perchlorate and iodide in whole blood samples from infants, children, and adults in Nanchang, China. Environ Sci Technol. 2010;44:6947–6953. doi: 10.1021/es101354g. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang SL, Hu X, Tam KY. Targeting Tumor Metabolism for Cancer Treatment: Is Pyruvate Dehydrogenase Kinases (PDKs) a Viable Anticancer Target? Int J Biol Sci. 2015;11:1390–1400. doi: 10.7150/ijbs.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Li W, Gu Y, Langaee T, Stacpoole PW, James MO. Chloride and other anions inhibit dichloroacetate-induced inactivation of human liver GSTZ1 in a haplotype-dependent manner. Chem Biol Interact. 2014a;215C:33–39. doi: 10.1016/j.cbi.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Smeltz M, Jahn SC, Langaee T, Stacpoole P, James MO. Age-related changes in expression and activity of human mitochondrial glutathione transferase zeta-1. Drug Metabolism Reviews. 2014b:1. doi: 10.1124/dmd.118.081810. [DOI] [PMC free article] [PubMed] [Google Scholar]