Abstract
Objective
To assess associations between breastfeeding and iron status at 9 months in two samples of Chinese infants.
Study design
Associations between feeding at 9 months (breastfed [BF] as sole milk source, mixed-fed [MF], or formula-fed [FF]) and iron deficiency anemia (IDA), iron deficiency (ID), and iron sufficiency were determined in infants from Zhejiang and Hebei provinces (ns = 142 and 813). ID was defined as body iron < 0 mg/kg, IDA as ID + hemoglobin < 110 g/L. Multiple logistic regression assessed associations between feeding pattern and iron status.
Results
Breastfeeding was associated with iron status (P-values < .001). In Zhejiang, 27.5% of BF infants had IDA compared with 0% of FF infants. The odds of ID/IDA were increased in BF and MF infants compared with FF: BF vs. FF odds ratio (OR): 28.8, 95% CI: 3.7–226.4; MF vs. FF OR: 11.0, 95% CI: 1.2–103.2. In Hebei, 44.0% of BF infants had IDA compared with 2.8% of FF infants. With covariable adjustment, odds of IDA were increased in BF and MF groups: BF vs. FF OR: 78.8, 95% CI: 27.2–228.1; MF vs. FF OR: 21.0, 95% CI: 7.3–60.9.
Conclusions
In both cohorts, the odds of ID/IDA at 9 months were increased in BF and MF infants, and ID/IDA was common. Although the benefits of breastfeeding are indisputable, these findings add to the evidence that breastfeeding in later infancy identifies infants at risk for ID/IDA in many settings. Protocols for detecting and preventing ID/IDA in BF infants are needed.
Trial registration
ClinicalTrials.gov: NCT00642863 and NCT00613717
Keywords: feeding, human, breastfeeding, iron deficiency
Iron deficiency is among the most common single nutrient deficiencies in the world, affecting women and young children disproportionately.1 ID is the most common cause of anemia. Both ID and iron deficiency anemia (IDA) in early life are associated with poorer cognitive, motor, and social- emotional development as well as neurophysiologic alterations.2
The risk for developing ID in infancy increases with factors such as male sex, rapid growth, economic stress and other family disadvantages.3 Evidence is accumulating that breastfeeding into the second half-year of life in otherwise healthy infants may be another risk factor. In the U.S., infants breastfed for 6 months or more were reported to be at higher risk of ID than infants who were not.4 Other studies worldwide5–16 reported associations between poorer iron status and breastfeeding into the second half-year of postnatal life and beyond. A study in Bangladesh,17 however, did not find an association between breastfeeding and anemia at 6 months. Studies restricted to breastfed infants alone do not address the question but show the wide range in prevalence of ID or IDA in breastfed infants in different settings.18–21
It is important to ascertain associations between ID and breastfeeding over 6 months in different contexts. The World Health Organization (WHO) recommends continuation of breastfeeding for up to 2 years of age or beyond, in addition to nutritionally adequate and safe complementary foods. The 6- to 24-month age period coincides with the maximal risk of ID, because iron requirements during the second half-year of life are higher than at any other time of life.22 However, there is debate about the iron needs of breastfed infants4,23,24 and the risks of giving iron to iron-sufficient infants.23,25 The purpose of this secondary analysis was to determine associations between breastfeeding at 9 months and iron status in two cohorts of healthy Chinese infants.
Methods
This secondary analysis of an observational study involved Chinese infants with data on iron status and feeding at 9 months, obtained in the course of two studies on neurodevelopmental effects of ID in early life (ClinicalTrials.gov: NCT00642863 and NCT00613717). For the cohort in Zhejiang province, in southeastern China, 142 of 204 infants with feeding data at 9 months had classifiable iron status; they constitute about 12% of 1196 infants screened for study participation at birth. For the cohort in Hebei province, in northeastern China, 813 of 927 infants with feeding data at 9 months had classifiable iron status; they constitute about 54% of 1512 infants considered at birth. Signed informed consent was obtained from parents. Studies were approved by the Institutional Review Boards of the University of Michigan and Zhejiang University School of Medicine (Zhejiang cohort) or Peking University First Hospital (Hebei cohort). A program of multimicronutrient fortification that was being rolled out in some regions of China at the time26 did not involve the areas where our participants lived.
In Zhejiang, mother-neonate pairs in Fuyang County were enrolled between December 2008 and November 2011. Pregnant women with normal, uncomplicated pregnancies were invited to participate after random screening at routine prenatal visits at 36–37 weeks gestation. After birth, inclusion criteria were confirmed as previously reported.27 Infants with cord hemoglobin [Hb] 2 SD below the mean for term infants (< 130 g/L)28 were provided iron supplements on ethical grounds (~1 mg/kg per day iron as liquid iron proteinsuccynilate from 6 weeks to 9 months). Infants were randomly assigned to receive the same supplement or placebo from 6 weeks to 9 months if they had marginally low cord Hb (130 – 140 g/L) or cord serum ferritin < 60 μg/L (<5th percentile in our previous study in the same province29). Other infants did not receive iron.
For the Hebei cohort, infants in Sanhe County were invited to participate in a randomized controlled trial of iron supplementation30 if their mothers had participated in a Peking University First Hospital randomized controlled trial of iron supplementation in pregnancy.31 Enrollment in the infancy study occurred between December 2009 and June 2012. Inclusion/exclusion criteria for mothers have been previously published.30,31 Infants with very low cord serum ferritin (< 35 μg/L) were provided iron supplements (~1 mg/kg per day iron as oral iron proteinsuccynilate from 6 weeks to 9 months) and not randomized. They were included in the current analysis if they had data on feeding. Other infants were randomly assigned to same iron supplement or placebo.30 Background characteristics of the Zhejiang and Hebei cohorts are shown in Table I.
Table 1.
Sample characteristics and comparison of cohortsa
| Zhejiang | Hebei | P valueb | |
|---|---|---|---|
| N | 142 | 813 | |
| Sex, % male (n) | 52.8 (75) | 52.5 (427) | .95 |
| Age at testing, days | 280.3 (278.6 – 282.0) | 282.4 (281.4 – 283.3) | .08 |
| Growth | |||
| Birth weight, g | 3388.4 (3321.4 – 3455.4) | 3382.7 (3357.4 – 3408.0) | .87 |
| Gestational age, weeks | 39.5 (39.4 – 39.7) | 39.7 (39.7 – 39.8) | .02 |
| Weight gain to 9 months, g | 6103.5 (5922.5 – 6284.5) | 6296.2 (6220.3 – 6372.1) | .06 |
| Weight-for-age z-scorec | 0.77 (0.60 – 0.94) | 0.92 (0.85 – 0.99) | .10 |
| Length-for-age z-scorec | 0.66 (0.49 – 0.82) | 0.33 (0.26 – 0.40) | .001 |
| Weight-for-length z-scorec | 0.65 (0.48 – 0.83) | 1.05 (0.98 – 1.13) | .000 |
| Maternal anemia at late gestation/delivery, % (n/total n)d | 30.0 (42/140) | 24.1 (97/403) | .17 |
| Fetal-neonatal ID, % (n/total n)e | 32.6 (46/141) | 44.5 (359/807) | .01 |
| Iron supplemented in infancy, % (n) | 28.2 (40) | 53.9 (438) | .000 |
| Iron status at 9 monthsf | |||
| IDA, % (n) | 12.0 (17) | 31.2 (254) | .000 |
| ID, % (n) | 2.8 (4) | 20.7 (168) | .000 |
| Iron Sufficient, % (n) | 85.2 (121) | 48.1 (391) | .000 |
| C-reactive protein at 9 months, mg/L | NA | 0.94 (0.61 – 1.27) | NA |
| Feeding at 9 months | |||
| Breastfed, % (n) | 35.9 (51) | 50.6 (411) | .001 |
| Mixed-fed, % (n) | 19.0 (27) | 32.0 (260) | .002 |
| Formula-fed, % (n) | 45.1 (64) | 17.5 (142) | .000 |
| Parental education, ≥ high school, % (n/total n) | 58.2 (82/141) | 27.7 (221/797) | .000 |
| Net family income < 50,000 yuan/year, % (n/total n)g | 41.0 (57/139) | 83.7 (667/797) | .000 |
Values are mean (95% CI) for continuous variables, % (n) for categorical variables.
P values for comparisons between Zhejiang and Hebei samples based on χ2 and t-test.
z-scores based on WHO growth curves.32
Prevalence of anemia in the Hebei sample for women who were not randomly assigned to receive prenatal iron supplementation. Including women who received iron supplementation in pregnancy, the prevalence of maternal anemia in Hebei was 17.9% (143/801).
Fetal-neonatal ID was defined as cord blood zinc protoporphyrin/heme > 118 μmol/mol heme or serum ferritin < 75 ng/ml.30
Anemic infants who did not meet criteria for ID were excluded, because their iron status could not be categorized (see Methods for details and definitions).
The definition of low income (<50,000 yuan per year) was based on the Fuyang County Housing Assistance Policy for Low Income Families, 2012 (Zhejiang) and the Sanhe Public Housing Benefits Guidelines, Sanhe People’s Government, 2013 (Hebei).
In each cohort, project personnel obtained information on feeding from mothers at a 9-month developmental assessment. We grouped infants based on feeding at 9 months. The breastfed (BF) group consisted of infants receiving breast milk as the sole source of milk. The mixed-fed group (MF) included those receiving some human milk and some formula. The formula-fed (FF) group consisted of those receiving formula as the sole source of milk. Formula in both settings was typically commercially prepared and iron-fortified. Because data on consumption of juice and solids were not complete, we could not determine exclusive breastfeeding as defined by WHO.37 However, complementary feeding of nutrient-containing solids and liquids besides breast milk typically starts at or around 4–6 months of age in China.38
Iron status at 9 months was determined from venous blood samples in Zhejiang and capillary samples in Hebei. The measures of iron status included Hb, serum ferritin, serum transferrin receptor (sTfR), and zinc protoporphyrin/heme (ZPP/H). Serum C-reactive protein was also analyzed in Hebei. Laboratories maintained standard quality control procedures; methods have been previously published for both cohorts.27,30 BI was calculated using serum ferritin and sTfR according to the formula in Cook et al33: Body iron (mg/kg) = − [log10(sTfR*1000/ferritin) − 2.8229]/0.1207. This formula used a sTfR assay described in Flowers et al34 The Beckman Coulter sTfR concentrations in the Hebei project required conversion for use in the formula. To do so, we built on published data for Flowers, Ramco, and Beckman Coulter sTfRs. As reported in Pfeiffer et al,35 the Ramco assay was similar to Flowers et al. Ramco and Beckman Coulter assays were part of a World Health Organization study that used a standard reference reagent for sTfR.36 The Ramco assay yielded sTfR concentrations 4.3 times higher than Beckman Coulter, so the Flowers sTfR equivalent was calculated by the following formula: Flowers sTfR = 4.3 x Beckman Coulter sTfR. The assay used in Zhejiang did not require conversion.
BI ≥ 0 mg/kg indicates iron surplus in stores; BI < 0 mg/kg indicates iron deficit in tissues.39 ID at 9 months was defined as BI < 0 mg/kg.39 IDA was defined as ID and anemia (Hb < 110 g/L per WHO guidelines1) and iron sufficiency (IS) as BI ≥ 0 mg/kg and no anemia. As previously reported,30,31 we considered fetal-neonatal ID as cord serum ferritin < 75 μg/L or ZPP/H > 118 μmol/mol. The serum ferritin cutoff has been used in studies of neurodevelopmental effects;40–42 the ZPP/H cutoff is the US 90th percentile.43
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics Version 22 (IBM Corp. Released 2013). We used t-tests for continuous measures and χ2 for categorical data to describe and compare cohorts. Associations between feeding pattern and iron status were analyzed separately for each cohort using χ2 and logistic regression. A single unified analysis was not appropriate due to differences in study design, prevalence of ID, and background characteristics. Separate analyses could also make results more generalizable if findings were similar in the different contexts.
We conducted univariate logistic regression to determine associations at 9 months between the predictor (feeding group) and the dependent variable (iron status group) in each cohort. Anemic infants (Hb < 110 g/L)1 who did not meet criteria for ID were excluded, because their iron status could not be categorized: 62 in Zhejiang and 114 in Hebei, leaving final ns of 142 and 813, respectively. Multiple logistic regression modeling was used to adjust for clinically relevant, potentially important covariables (sex, age at testing, birth weight, gestational age, parental education, weight gain from birth to 9 months, iron supplementation in infancy, fetal-neonatal ID, and inflammation as measured by C-reactive protein [available for Hebei but not Zhejiang]). Covariates were included and then removed if they were not at least marginally significant (P < .10) until the most parsimonious model remained.
Results
Sample characteristics are shown in Table I. In both cohorts, there were slightly more males than females, infants were born at term with mean birth weights > 3 kg, and continued to grow well. The cohorts differed in most other background characteristics (Table I). Infants in the Hebei cohort had lower length-for-age z-scores but higher weight-for-length z-scores at 9 months, meaning that, on average, they were shorter and heavier than infants in the Zhejiang cohort. A greater percentage of families in the Hebei cohort than the Zhejiang cohort were low income and parents were less educated. A higher proportion of Hebei cohort infants had ID or IDA at 9 months.
Zhejiang Cohort
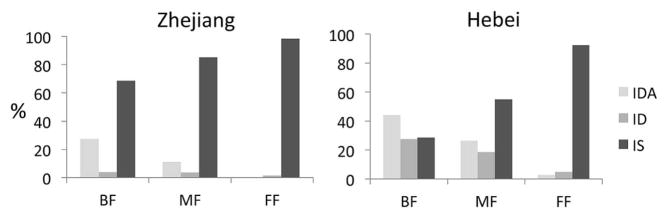
At 9 months, feeding pattern was strongly associated with iron status (overall P-value < .001; Figure). Because no FF infants had IDA, we combined ID and IDA in further analyses. BF and MF groups differed significantly in ID/IDA from the FF group (P values=.001 and .04), but BF and MF groups did not (P = .12). Nearly one-third of BF infants (31.4%) had ID/IDA. The odds of ID/IDA were increased in both BF and MF groups, compared with the FF group (Table II). No other variables were significant in the model.
Figure.
Percentage of 9-month-old infants with IDA, ID, and iron sufficiency by feeding pattern in Zhejiang (n = 142) and Hebei (n = 813). The overall difference in iron status by feeding groups was significant in each cohort: Zhejiang, χ2 = 26.5, P < .001; Hebei, χ2 = 202.5, P < .001.
Table 2.
Logistic regression model for predicting odds of ID/IDA (Zhejiang) and IDA or ID (Hebei)
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |
| ID/IDA vs. IS (Zhejiang) | ||||
| BF | 28.8 (3.7 – 226.4) | < .001 | - | - |
| MF | 11.0 (1.2 – 103.2) | .04 | - | - |
| FF | Reference | - | - | |
| IDA vs. IS (Hebei) | ||||
| BF | 50.7 (18.2 – 140.7) | < .001 | 78.8 (27.2 – 228.1) | < .001 |
| MF | 15.8 (5.6 – 44.5) | < .001 | 21.0 (7.3 – 60.9) | < .001 |
| FF | Reference | - | Reference | - |
| Male sex | - | - | 1.7 (1.1 – 2.5) | .01 |
| Fetal-neonatal ID vs. IS | - | - | 2.4 (1.6 – 3.5) | < .001 |
| Birth weight1 | - | - | 0.91 (0.86 – 0.96) | .001 |
| Weight gain to 9 months1 | - | - | 1.05 (1.03 – 1.07) | < .001 |
| Age at testing, months | - | - | 0.6 (0.3 – 0.9) | .01 |
| ID vs. IS (Hebei) | ||||
| BF | 18.1 (8.1 – 40.3) | < .001 | 21.9 (9.7 – 49.7) | < .001 |
| MF | 6.3 (2.7 – 14.4) | < .001 | 6.7 (2.9 – 15.4) | < .001 |
| FF | Reference | - | Reference | - |
| Male sex | - | - | 1.8 (1.2 – 2.7) | .005 |
| Log C-reactive protein at 9 months, mg/L | - | - | 0.8 (0.7 – 0.9) | .001 |
Per 100 g.
Hebei Cohort
Feeding pattern was also strongly associated with iron status at 9 months in Hebei (overall P-value <.001; Figure). Forty-four percent of BF infants had IDA and 27.5% had ID. BF, MF, and FF groups differed significantly from one another in iron status (P <.001). The odds of IDA were significantly increased in both the BF and MF groups compared with the FF group (Table II). Male sex, fetal-neonatal ID, lower birth weight, higher weight gain from birth to 9 months, and age at testing remained as significant covariables. For ID, the odds were also increased in both the BF and MF groups compared with the FF group (Table II): Male sex and higher C-reactive protein were significant covariables.
Discussion
A high percentage of breastfed infants had IDA at 9 months in two separate Chinese cohorts. The relation between breastfeeding as the sole source of milk and ID/IDA at 9 months was similar in both Hebei and Zhejiang cohorts despite differences in background characteristics. These findings in two distinct regions of China mirror those in other studies,4–16 indicating that breastfeeding beyond 6 months of age is associated with poor iron status in a wide range of settings.
We identified 13 studies that examined associations between breastfeeding for 6 months or more and infant iron status.4–16 In all but four,5,7,11,16 iron status was assessed only by Hb or Hb in combination with serum ferritin. Our panel of iron status measures was more comprehensive, particularly in its inclusion of sTfR, which allowed BI to be calculated. The US Centers for Disease Control included BI < 0 mg/kg as a way to define ID in recent National Health and Nutrition Examination Surveys,39 as BI captures both iron storage and function in a single index and does not depend on specific cutoffs for individual iron status measures. Nonetheless, BI may be a conservative indicator of ID in infancy, because other iron measures, such as ZPP/H or reticulocyte Hb, may indicate iron insufficiency before serum ferritin or sTfR are altered enough to make the BI calculation < 0 mg/kg. We could not consider these options, however, because ZPP/H data were missing for about a third of the Hebei cohort, and the hospitals were not set up to measure reticulocyte Hb.
The infants in our study were relatively heavy and growing rapidly, as evidenced by birth weight, weight gain, and z-scores. Their rapid growth could explain the limited ability of breastfeeding to protect against IDA. Although the benefits of breastfeeding are indisputable, rapid growth of infants in many settings may now exceed the capacity of breast milk to meet iron needs. However, the ability to consider the importance of growth in most previous studies is limited. Four studies did not report birth weight or growth rate9–11,16 and four others reported birth weight only.4,7,8,13 Nonetheless, it is likely that birth weights and growth rates are higher now than in most of human history.44 That infants in Hebei were shorter and heavier at 9 months than infants in Zhejiang raises the question of other micronutrient deficiencies or other factors.
The observational nature of our study means that it cannot establish a causal relation between breastfeeding and ID/IDA. It is not ethical to randomize women to breastfeeding or for different durations. Therefore, making a causal connection must rely on the robustness of the relation in the face of different contexts, populations, study designs, and definitions of breastfeeding and iron status. Careful statistical control of potential confounding factors is also critical. Our study adds to the literature in these respects. Biological plausibility is another consideration. A new study by Cai et al provides an explanation for the low iron content of breast milk and the limited capacity of breast milk to meet infant iron needs.45 The study found that human mammary epithelial cells do not have the only known membrane iron transporter and thus apparently have no way to secrete iron into breast milk.
The clinical challenge is to how best to prevent ID/IDA in breastfed infants globally. In our cohorts, some infants received supplemental iron as drops from 6 weeks to 9 months. As reported previously for the Hebei study,30 there was limited hematologic response to iron supplementation, likely due to insufficient supplement intake and rapid growth. Further studies with carefully supervised early iron supplementation are warranted. Home fortification is another promising approach for breastfed infants. Adding to the growing body of literature, a recent study of China’s home fortification program showed its effectiveness in reducing anemia in 6- to 23-month-old children.26 An unanswered question is whether starting at 6 months is too late to protect the developing brain from ID effects. In an editorial accompanying the Cai et al paper, Friel challenges the rationale for exclusive breastfeeding for 6 months and argues cogently for providing external sources of iron earlier.46
Our study has several further limitations. The analysis was cross-sectional. Feeding information was based on maternal report collected when infants were 9 months old. The duration of exclusive breastfeeding, defined by WHO, was not known. Incomplete data on complementary foods means that we cannot determine if groups within each cohort differed in other facets of feeding or the quality of the diet. We also cannot compare diet across cohorts. The use of venous blood in Zhejiang and capillary blood in Hebei may affect iron status differences across cohorts. ZPP/H was missing for part of the Zhejiang cohort, and we were able to adjust for C-reactive protein in the Hebei cohort only.
Despite limitations in the available studies, including ours, the accumulating evidence has global clinical implications. Specifically, breastfed infants in many settings worldwide warrant additional and earlier monitoring to prevent them from developing ID or IDA and to detect and treat ID/IDA before it becomes chronic and severe. Although more research is needed to determine the optimal timing, vehicle, and amount of iron supplementation, clinicians need updated protocols to guide their care of the breastfed infant.
Acknowledgments
Supported by the China National Sciences Foundation (30671773) and the National Institutes of Health (HD039386 and R01 HD052069), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Iron supplements and placebo were prepared by Lee’s Pharmaceutical Holdings Limited (Hong Kong) as a donation for the Zhejiang study and provided at cost for the Hebei study. B.L. was an unpaid speaker at 2 seminars supported by Lee’s Pharmaceutical Holdings Limited (April 2010 and May 2011), which provided hotel accommodations and/or airfare. The other authors declare no conflicts of interest.
We thank Liqin Chen (Zhejiang University Children’s Hospital) and Yaping Jiang (Peking University First Hospital) for their work on the laboratory analyses. We also thank Zhixiang Zhang, MD (Peking University First Hospital), and Twila Tardif, PhD (University of Michigan), for their intellectual contributions to the study.
Abbreviations
- Hb
hemoglobin
- IDA
iron deficiency anemia
- sTfR
serum transferrin receptor
- WHO
World Health Organization
- ZPP/H
zinc protoporphyrin/heme
Footnotes
Portions of the study were presented at the meeting of the Pediatric Academic Societyes, Vancouver, BC, Canada, May 3–6, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva, Switzerland: WHO press; 2008. [Google Scholar]
- 2.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients. 2014;6:4093–4114. doi: 10.3390/nu6104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and risk for iron deficiency in U.S. infants. Breastfeeding Med. 2007;2:63–73. doi: 10.1089/bfm.2007.0002. [DOI] [PubMed] [Google Scholar]
- 5.Calvo EB, Galindo AC, Aspres NB. Iron status in exclusively breast-fed infants. Pediatrics. 1992;90:375–379. [PubMed] [Google Scholar]
- 6.Maguire JL, Salehi L, Birken CS, Carsley S, Mamdani M, Thorpe K, Lebovic, Khoratovich, Parkin P. Association between total duration of breastfeeding and iron deficiency. Pediatrics. 2013;131:1530–1537. doi: 10.1542/peds.2012-2465. [DOI] [PubMed] [Google Scholar]
- 7.Pizarro F, Yip R, Dallman PR, Olivares M, Hertrampf E, Walter T. Iron status with different infant feeding regimens: relevance to screening and prevention of iron deficiency. J Pediatr. 1991;118:687–692. doi: 10.1016/s0022-3476(05)80027-7. [DOI] [PubMed] [Google Scholar]
- 8.Luo R, Shi Y, Zhou H, Yue A, Zhang L, Sylvia S, Medina A, Rozelle S. Anemia and feeding practices among infants in rural Shaanxi province in China. Nutrients. 2014;6:5975–5991. doi: 10.3390/nu6125975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S, Tan H, Peng A, Jiang H, Wu J, Guo S, Qian X. Disparity of anemia prevalence and associated factors among rural to urban migrant and the local children under two years old: a population based cross-sectional study in Pinghu, China. BMC Public Health. 2014;14:601. doi: 10.1186/1471-2458-14-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hipgrave DB, Fu X, Zhou H, Jin Y, Wang X, Chang S, Scherpbier RW, Guo S. Poor complementary feeding practices and high anaemia prevalence among infants and young children in rural central and western China. Eur J Clin Nutr. 2014;68:916–924. doi: 10.1038/ejcn.2014.98. [DOI] [PubMed] [Google Scholar]
- 11.Elalfy MS, Hamdy AM, Maksoud SS, Megeed RI. Pattern of milk feeding and family size as risk factors for iron deficiency anemia among poor Egyptian infants 6 to 24 months old. Nutr Res. 2012;32:93–99. doi: 10.1016/j.nutres.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Meinzen-Derr JK, Guerrero ML, Altaye M, Ortega-Gallegos H, Ruiz-Palacios GM, Morrow AL. Risk of infant anemia is associated with exclusive breast-feeding and maternal anemia in a Mexican cohort. J Nutr. 2006;136:452–458. doi: 10.1093/jn/136.2.452. [DOI] [PubMed] [Google Scholar]
- 13.Sultan AN, Zuberi RW. Late weaning: the most significant risk factor in the development of iron deficiency anaemia at 1–2 years of age. J Ayub Med Coll Abbottabad. 2003;15:3–7. [PubMed] [Google Scholar]
- 14.Hopkins D, Emmett P, Steer C, Rogers I, Noble S, Emond A. Infant feeding in the second 6 months of life related to iron status: an observational study. Arch Dis Child. 2007;92:850–854. doi: 10.1136/adc.2006.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altucher K, Rasmussen KM, Barden EM, Habicht JP. Predictors of improvement in hemoglobin concentration among toddlers enrolled in the Massachusetts WIC Program. J Am Diet Assoc. 2005;105:709–715. doi: 10.1016/j.jada.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Innis SM, Nelson CM, Wadsworth LD, MacLaren IA, Lwanga D. Incidence of iron- deficiency anaemia and depleted iron stores among nine-month-old infants in Vancouver, Canada. Can J Public Health. 1997;88:80–84. doi: 10.1007/BF03403865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakur YA, Choudhury N, Hyder SM, Zlotkin SH. Unexpectedly high early prevalence of anaemia in 6-month-old breast-fed infants in rural Bangladesh. Public Health Nutr. 2010;13:4–11. doi: 10.1017/S1368980009005886. [DOI] [PubMed] [Google Scholar]
- 18.Domellof M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lonnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr. 2001;138:679–687. doi: 10.1067/mpd.2001.112895. [DOI] [PubMed] [Google Scholar]
- 19.Pisacane A, de Vizia B, Valiante A, Vaccaro F, Russo M, Grillo G, Giustardi A. Iron status in breast-fed infants. J Pediatr. 1995;127:429–431. doi: 10.1016/s0022-3476(95)70076-5. [DOI] [PubMed] [Google Scholar]
- 20.Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams R. A double-masked, randomized control trial of iron supplementation in early infancy in healthy full-term breast- fed infants. J Pediatr. 2003;143:582–586. doi: 10.1067/S0022-3476(03)00301-9. [DOI] [PubMed] [Google Scholar]
- 21.Olaya GA, Lawson M, Fewtrell M. Iron status at age 6 months in Colombian infants exclusively breast-fed for 4–5 versus 6 months. JPGN. 2016 doi: 10.1097/MPG.0000000000001301. First published ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Rawat R, Saha KK, Kennedy A, Rohner F, Ruel M, Menon P. Anaemia in infancy in rural Bangladesh: contribution of iron deficiency, infections and poor feeding practices. Br J Nutr. 2013;111:172–181. doi: 10.1017/S0007114513001852. [DOI] [PubMed] [Google Scholar]
- 23.Furman LM. Exclusively breastfed infants: Iron recommendations are premature. Pediatrics. 2011;126:e1098–e1099. doi: 10.1542/peds.2011-0201B. [DOI] [PubMed] [Google Scholar]
- 24.AAP Section on Breastfeeding. Schanler RJ, Executive Committee, Feldman-Winter L, Landers S, Noble L, Szucs KA, Viehmann L. Concerns with early universal iron supplementation of breastfeeding infants. Pediatrics. 2011;127:e1097. doi: 10.1542/peds.2011-0201A. [DOI] [PubMed] [Google Scholar]
- 25.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–1276. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo J, Sun J, Fang Z, Chang S, Zhao L, Fu P, Wang J, Huang J, Wang L, Begin F, Hipgrave DB, Ma G. Effect of home-based complementary food fortification on prevalence of anemia among infants and young children aged 6 to 23 months in poor rural regions of China. Food Nutr Bull. 2015;36:405–414. doi: 10.1177/0379572115616001. [DOI] [PubMed] [Google Scholar]
- 27.Armony-Sivan R, Zhu B, Clark KM, Richards B, Ji C, Kaciroti N, Shao J, Lozoff B. Iron deficiency (ID) at both birth and 9 months predicts right frontal EEG asymmetry in infancy. Dev Psychobiol. 2016;58:462–470. doi: 10.1002/dev.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenz L, Peter A, Poets CF, Franz AR. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology. 2013;104:194–202. doi: 10.1159/000353161. [DOI] [PubMed] [Google Scholar]
- 29.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao ZY, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–2009. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozoff B, Jiang Y, Li X, Zhou M, Richards B, Xu G, Clark KM, Liang F, Kaciroti N, Zhao G, Santos DCC, Zhang Z, Tardif T, Li M. Low-dose iron supplementation in infancy modestly increases infant iron status at 9 months without decreasing growth or increasing illness in a randomized clinical trial in rural China. J Nutr. 2016;146:612–621. doi: 10.3945/jn.115.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, Kaciroti N, Georgieff MK, Zhang Z, Tardif T, Li M, Lozoff B. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145:1916–1923. doi: 10.3945/jn.114.208678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO Anthro (version 3.2.2, January 2011) and macros. Geneva: World Health Organization; 2011. Child growth standards. [Internet] Available from: http://www.who.int/childgrowth/software/en/ [Google Scholar]
- 33.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 34.Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med. 1989;114:368–377. [PubMed] [Google Scholar]
- 35.Pfeiffer CM, Cook JD, Mei Z, Cogswell ME, Looker AC, Lacher DA. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin Chim Acta. 2007;382:112–116. doi: 10.1016/j.cca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe SJ, Heath A, Sharp G, Cook J, Ellis R, Worwood M. A WHO reference reagent for the Serum Transferrin Receptor (sTfR): international collaborative study to evaluate a recombinant soluble transferrin receptor preparation. Clin Chem Lab Med. 2010;48:815–820. doi: 10.1515/CCLM.2010.167. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Infant and young child nutrition. Global strategy on infant and young child feeding. 2002;A55/15:4–16. [Google Scholar]
- 38.Zhang YQ, Li H, Xia XL. Complementary feeding practice in nine cities of China. Chinese J Child Health. 2008;3:009. [Google Scholar]
- 39.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89:1334–1342. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 40.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 41.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156:377–381. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol. 2004;24:757–762. doi: 10.1038/sj.jp.7211178. [DOI] [PubMed] [Google Scholar]
- 43.McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer B, Kling PJ. Impact of multiple risk factors on newborn iron status. J Pediatr Hematol Oncol. 2013;35:473–477. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozoff B. Do breast-fed babies benefit from iron before 6 months? J Pediatr. 2003;143:554–556. doi: 10.1067/S0022-3476(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 45.Cai C, Eck P, Friel JK. Gene expression profiles suggest iron transport pathway in the lactating human epithelial cell. JPGN. 2016 doi: 10.1097/MPG.0000000000001303. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Friel JK. There is no iron in human milk. JPGN. 2016 doi: 10.1097/MPG.0000000000001364. in press. [DOI] [PubMed] [Google Scholar]