Abstract
Objective
To determine whether brain volume is reduced at one year and whether these volumes are associated with neurodevelopment in biventricular congenital heart disease (CHD) repaired in infancy.
Study design
Infants with biventricular CHD (n = 48) underwent brain magnetic resonance imaging (MRI) and neurodevelopmental testing with the Bayley Scales of Infant Development-II (BSID-II) and the MacArthur-Bates Communicative Development Inventories (CDI) at one year. A multi-template based probabilistic segmentation algorithm was applied to volumetric MRI data. We compared volumes with those of 13 healthy control infants of comparable ages. In the CHD group, we measured Spearman correlations between neurodevelopmental outcomes and the residuals from linear regression of the volumes on corrected chronological age at MRI and sex.
Results
Compared with controls, CHD infant had reductions of 54 mL in total brain (P = 0.009), 40 mL in cerebral white matter (P < 0.001), and 1.2 mL in brainstem (P = 0.003) volumes. Within the CHD group, brain volumes were not correlated with BSID-II scores but did correlate positively with CDI language development.
Conclusion
Infants with biventricular CHD show total brain volume reductions at one year of age, driven by differences in cerebral white matter. White matter volume correlates with language development, but not broader developmental indices. These findings suggest that abnormalities in white matter development detected months after corrective heart surgery may contribute to language impairment.
Trial registration
ClinicalTrials.gov: NCT00006183
Keywords: brain, MRI, neurodevelopment, language, infant
Congenital heart disease (CHD) is among the most common birth defects. Moderate or severe forms of CHD affect 0.5-1% of all live births.1, 2 Children with CHD have an elevated risk of neurodevelopmental impairment, which typically affects attention, executive function, social cognition, and/or language.3-6 Language in particular was the most common isolated delay in one longitudinal study of children with CHD.7 In biventricular forms of CHD, such as transposition of the great arteries (TGA) or tetralogy of Fallot (TOF), over half of children require special services such as special education or early intervention.4, 8 Thus, neurodevelopmental impairments significantly influence quality of life and daily functioning.9-12
Brain magnetic resonance imaging (MRI) has demonstrated both overt brain injury and subtle quantitative differences in the brain structures of children and adolescents with CHD. Cerebral white matter abnormalities are the most frequently reported neuroimaging abnormality in this population, though diffusely scattered punctate hemorrhages are also common.13, 14 Subtle differences in cerebral white matter microstructure and cortical thickness are measurable in adolescents with TGA even without overt brain injury by qualitative MRI.15, 16 Abnormalities in brain development are thought to precede surgery with reduced cortical folding, smaller cerebral volumes, and abnormal metabolism apparent in newborns and infants prior to surgery and even in utero.17-19 Recent studies in adolescents indicate a relationship between brain MRI findings and neurodevelopmental functioning.20, 21
In the present study, we sought to determine whether brain volumes are reduced following corrective surgery for biventricular CHD, and to determine the relationship between quantitative brain volumes and neurodevelopment in infancy. The study sample is unique in that the corrective heart surgery was performed in infancy, and the MRI was performed approximately one year following surgery, allowing for a period of recovery and growth after surgical correction. In addition, we specifically evaluated language, given that it is commonly affected by CHD and may be more sensitive to subtle cognitive differences not detectable with instruments that assess global development. We hypothesized that both cerebral white and gray matter volumes would be smaller in CHD than control infants, and that cerebral volumes would be more closely associated with language scores than with broader neurodevelopmental measures.
Methods
Cardiac subjects for these analyses were participants in a clinical trial (ClinicalTrials.gov: NCT00006183) comparing two hematocrit strategies during cardiopulmonary bypass surgery in infants. Detailed trial methods were previously published.22 Briefly, the trial sample consisted of infants who underwent biventricular repair at less than 9 months of age with a diagnosis of one of the following: (1) TGA, (2) TOF with or without pulmonary atresia or truncus arteriosus, or (3) ventricular septal defect (VSD) or complete common atrioventricular canal defect. Exclusion criteria were birth weight less than 2.3 kg, recognizable phenotypic syndrome of congenital anomalies detected during routine clinical care, extracardiac anomalies of greater than minor severity which could impede recovery of myocardial or brain function in the perioperative period, previous cardiac surgery, or associated cardiovascular anomalies necessitating aortic arch reconstruction or additional open surgical procedures before the planned developmental follow-up. Genetic testing was not performed for all subjects, but no CHD children were identified as having chromosomal or genetic problems at one year by treating clinicians.
A comparison sample of 13 healthy control infants was selected from the National Institutes of Health MRI study of normal brain development, including all infants with neuroimaging performed on the same MRI scanner as the CHD infants, and who had acceptable imaging quality and were of comparable age. These control infants had no known risk factors for brain disorders such as intra-uterine exposure to toxins, history of closed head injury with loss of consciousness, language disorder or Axis 1 psychiatric disorder, first degree relative with a lifetime history of an Axis 1 psychiatric disorder, or abnormality on neurological examination.23 This study was approved by the Boston Children's Hospital Institutional Review Board and adhered to both institutional guidelines and the Declaration of Helsinki.
Image Acquisition and Analysis
At approximately 12 months of age, subjects underwent brain MRI with sequences including a standard clinical axial fast spin echo T2-weighted sequence for skull stripping and coronal three-dimensional Spoiled Gradient Recalled for volumetric analyses. Detailed MRI acquisition parameters for both the CHD trial subjects and the healthy control infants have been previously published.14, 23
For volumetric analyses, we applied a whole brain probabilistic segmentation algorithm Local MAP PSTAPLE.24 This innovative algorithm, which was not available at the time primary trial results were published, computes probabilistic segmentations of a target brain simultaneously from multiple templates. Each of the template images is registered to the target image. Label and intensity information from each template is used to compute label probabilities of the target image. An Expectation Maximization algorithm is used both to estimate the segmentation of the target image and to measure the ability of each template to predict locally the correct segmentation of the target image. The algorithm converges on a local optimum, labeling both cortical and subcortical structures based on information stored in the template library.
This segmentation algorithm yields 134 brain regions. To reduce the problem of multiple comparisons, we selected 22 discrete regions thought likely to be affected by CHD, and from these 22 discrete regions created six aggregate regions for group comparisons and correlation analyses. The 22 discrete regions are listed in Table I (available at www.jpeds.com). The six aggregate regions were total brain, cerebral white matter, cerebral gray matter, subcortical gray matter, cerebellum, and brainstem.
Table I. (online only). Volumes of discrete brain regions according to group (N = 61).
| Discrete brain region (mL)* | Cardiac (N = 48) | Control (N = 13) | P value† |
|---|---|---|---|
| Cerebrum | |||
| Cerebral white matter | |||
| Right cerebral white matter | 116.9 ± 14.0 | 137.6 ± 16.0 | <0.001 |
| Left cerebral white matter | 114.4 ± 14.4 | 133.4 ± 16.4 | <0.001 |
| Cerebral gray matter | |||
| Right frontal | 53.5 ± 6.0 | 55.9 ± 7.0 | 0.11 |
| Left frontal | 53.3 ± 6.4 | 54.7 ± 7.5 | 0.38 |
| Right perisylvian | 11.7 ± 1.3 | 11.3 ± 1.3 | 0.24 |
| Left perisylvian | 11.7 ± 1.3 | 11.5 ± 1.2 | 0.57 |
| Right parietal | 49.6 ± 5.2 | 50.4 ± 4.7 | 0.49 |
| Left parietal | 49.2 ± 5.1 | 51.4 ± 5.9 | 0.12 |
| Subcortical gray matter | |||
| Right thalamus | 6.1 ± 0.5 | 5.7 ± 0.5 | 0.02 |
| Left thalamus | 6.2 ± 0.6 | 5.8 ± 0.4 | 0.007 |
| Right caudate | 2.9 ± 0.4 | 2.8 ± 0.4 | 0.40 |
| Left caudate | 2.7 ± 0.4 | 2.6 ± 0.3 | 0.31 |
| Right putamen | 3.8 ± 0.4 | 3.5 ± 0.4 | 0.01 |
| Left putamen | 3.9 ± 0.4 | 3.6 ± 0.4 | 0.05 |
| Right pallidum | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.18 |
| Left pallidum | 1.1 ± 0.1 | 1.0 ± 0.2 | 0.23 |
| Right hippocampus | 2.8 ± 0.4 | 2.7 ± 0.3 | 0.12 |
| Left hippocampus | 2.7 ± 0.3 | 2.7 ± 0.3 | 0.79 |
| Cerebellum | |||
| Cerebellar gray matter | 83.3 ± 9.3 | 80.3 ± 9.2 | 0.15 |
| Cerebellar white matter | 14.5 ± 1.8 | 16.2 ± 2.8 | <0.001 |
| Vermis | 8.0 ± 0.9 | 8.3 ± 1.0 | 0.18 |
| Brainstem | 11.2 ± 1.5 | 12.5 ± 2.0 | 0.003 |
Data are expressed as mean ± standard deviation.
P values were determined by linear regression adjusting for corrected chronological age at MRI and sex.
Demographic and Medical Variables
For correlation analyses we evaluated demographic and medical factors thought to be associated with brain volumes in the CHD subjects. Demographic characteristics included sex and race. Birth characteristics were weight, gestational age, and Apgar score at 5 minutes. Preoperative variables were catheterizations, balloon atrial septostomy, age at operation, and endotracheal intubation before surgery. Operative variables were hematocrit treatment group, crossclamp time, total support time, total bypass time, low-flow bypass time, duration of circulatory arrest, hematocrit at onset of low-flow, lowest tympanic temperature, lowest pCO2, pH at lowest pCO2, highest pCO2, and lowest pO2. Of note, the pH-stat blood gas strategy was used during core cooling on cardiopulmonary bypass. Postoperative variables were lactate 60 minutes after bypass, lowest PaCO2, lowest PaO2, PRISM-III25 score at 12 and 24 hours postoperative, hours intubated, postoperative intensive care unit length of stay, postoperative hospital length of stay, hypertension, and hypothermia.
Neurologic and Neurodevelopmental Outcomes
A standardized neurological exam was performed on all CHD subjects and was an entry criterion for control subjects. Neurodevelopmental evaluation performed at one year included the Bayley Scales of Infant Development-II (BSID-II)26 for all subjects; the MacArthur-Bates Communicative Development Inventories (CDI)27 was completed for CHD subjects only. We evaluated the Words and Gestures form of the CDI Part I: Early Words including the following scores: Phrases, Vocabulary Words Understood, and Vocabulary Words Produced. We also evaluated Part II: Actions and Gestures with the Total Gestures summary score. Scores were converted into age- and sex-specific percentiles for analyses. We excluded other sections of the CDI due to a narrow range of responses limiting the utility of statistical analyses. The scores included are designed to be measured in children ages 8-18 months and describe the extent to which a child has begun to respond to familiar words, phrases, and routines, understand and produce words, and employ communicative and symbolic actions or gestures.
Statistical analyses
Wilcoxon rank-sum, Kruskal-Wallis, or Fisher exact tests were used to compare groups with respect to demographic and birth history variables. We used linear regression adjusting for corrected chronological age at MRI and sex to compare brain volumes between groups. To calculate average percent change in brain volumes between groups, we compared fitted volumes for cardiac subjects with those estimated for control subjects at the same corrected chronological age and sex. T-tests or analysis of variance were used to compare follow-up data (BSID-II scores and CDI, head circumference, and weight percentiles) between groups. We used one-sample t-tests to compare the CHD group mean for each CDI percentile and the CHD and control group means for head circumference with the expected population mean of 50th percentile.
For the CHD subjects, Spearman correlation analysis was used to evaluate the relationship between the medical variables and residuals from linear regression of the brain volumes on corrected chronological age, sex, and diagnosis. We adjusted for diagnosis in these correlation analyses given the inherent association between diagnosis and medical variables. In addition, Spearman correlation analysis was used to evaluate the association between the CDI percentiles and residuals from linear regression of the brain volumes on corrected chronological age and sex. The significance level was set at 0.05 for all correlation analyses. With a sample size of 48 CHD subjects for most correlations and 38 for the correlations with CDI percentiles, we had 80% power to detect a correlation of ≥0.40 or ≥0.44, respectively, at a 2-sided 0.05 level.
Results
Of 126 subjects enrolled in the trial, 106 returned for the one-year clinical evaluation. Medical characteristics of the CHD group have been previously published.14 The two hematocrit groups did not differ in BSID-II or CDI scores, weight, or head circumference.22 Forty-eight subjects (19 TGA, 20 TOF, and 9 VSD) underwent brain MRI. The majority without brain MRI were parental refusals mostly due to the need for general anesthesia (N=55), with two cancelled due to medical concerns and one for lack of MRI availability. The median duration between surgery and MRI was 373 days (interquartile range 339-408; range 194-605). CHD subjects who underwent MRI did not differ in head circumference percentiles or BSID-II scores from those who did not. However, CHD subjects who underwent MRI had both significantly lower phrases and total gestures CDI percentiles (mean ± standard deviation, phrases: 43 ± 28 vs 59 ± 29, P = 0.02; total gestures: 32 ± 27 vs 52 ± 29, P = 0.002).
Comparing the 48 CHD subjects with MRI to control subjects, demographic characteristics and birth history did not differ (Table II). At one year, compared with the control subjects, the CHD subjects had significantly lower weight and smaller head circumference percentiles, though head circumference was similar to age norms in the CHD group, and the control group had above average head circumference. Within the CHD group, the head circumference increased from the 30th percentile preoperatively to the 48th percentile at one year (paired t-test P < 0.001).
Table II. Patient characteristics according to group (N = 61).
| Characteristics* | Cardiac (N = 48) | Control (N = 13) | P value† |
|---|---|---|---|
| Medical history and demographic information | |||
| Birth weight, kg | 3.4 ± 0.5 | 3.3 ± 0.4 | 0.68 |
| Gestational age, wk | 39.2 ± 1.3 | 39.4 ± 0.8 | 0.54 |
| Apgar score at 5 min‡ | 8.5 ± 1.2 | 9.0 ± 0.4 | 0.10 |
| Male, n (%) | 28 (58) | 7 (54) | 1.0 |
| Nonwhite, n (%) | 10 (21) | 6 (46) | 0.08 |
| MRI at one year of age | |||
| Corrected chronological age at MRI, median (range), mo | 13.4 (11.3-23.5) | 14.8 (11.9-18.9) | 0.77 |
| Total brain volume, mL | 870.9 ± 85.5 | 924.8 ± 89.1 | 0.009 |
| Cerebral white matter volume, mL | 231.2 ± 28.2 | 271.0 ± 32.3 | <0.001 |
| Cerebral gray matter volume, mL | 500.3 ± 50.0 | 516.3 ± 48.8 | 0.20 |
| Subcortical gray matter volume, mL | 41.6 ± 3.5 | 40.9 ± 3.3 | 0.46 |
| Cerebellum volume, mL | 105.9 ± 11.5 | 104.9 ± 12.2 | 0.65 |
| Brainstem volume, mL | 11.2 ± 1.5 | 12.5 ± 2.0 | 0.003 |
| Follow-up data at one year of age | |||
| Bayley Scales of Infant Development II | |||
| Psychomotor Development Index | 85.8 ± 16.3 | 96.2 ± 9.8 | 0.03 |
| Mental Development Index§ | 94.7 ± 11.3 | 101.8 ± 11.7 | 0.049 |
| MacArthur-Bates Communicative Development Inventory (CDI) percentiles¶ | |||
| Phrases | 43 ± 28 | - | 0.13 |
| Vocabulary words understood | 34 ± 30 | - | 0.003 |
| Vocabulary words produced | 42 ± 24 | - | 0.052 |
| Total gestures | 32 ± 27 | - | <0.001 |
| Head circumference percentile | 48 ± 29 | 74 ± 19** | 0.004 |
| Weight percentile | 39 ± 33 | 66 ± 24 | 0.009 |
MRI, magnetic resonance imaging.
Data are expressed as mean ± standard deviation unless otherwise specified.
P values are for differences between groups. For binary variables, P values were determined by Fisher's exact tests. For brain volumes, P values were determined by linear regression adjusting for corrected chronological age at MRI and sex. For follow-up data, P values were determined by t-tests. For all other continuous variables, P values were determined by Wilcoxon rank-sum tests. For CDI percentiles, P values were determined by one-sample t-tests comparing the cardiac group mean with the expected population mean of 50th percentile.
N = 44 cardiac and 11 control patients.
N = 47 cardiac and 13 control patients.
N = 38 cardiac patients. MacArthur-Bates Communicative Development Inventory was not assessed in control patients.
P<0.001 comparing the control group mean with the expected population mean of 50th percentile.
Neurologic and Neurodevelopmental Outcomes
Neurological exam at one year was abnormal in 28 CHD subjects (58%), with 26 having abnormal motor function (54%). Per enrollment criteria, all control subjects had normal neurological examination findings. BSID-II psychomotor developmental index (PDI) and mental developmental index (MDI) were significantly lower in the CHD group compared with controls (Table II). Neurologic and BSID-II data by CHD subgroup have been previously published.14 No subjects in the trial had post-operative clinical seizures detected.22 CDI was completed for 38 CHD subjects; scores were significantly below age and sex norms for understanding of vocabulary and development of gestures (Table II). Parents of many CHD children reported delays, defined as being below the 10th percentile, in development of phrases (18%) and gestures (16%) and understanding of vocabulary (34%). Only understanding of vocabulary differed significantly from the expected 10% (one-sample exact binomial test P < 0.001).
Qualitative MRI Outcomes
Detailed qualitative MRI findings have been published for the CHD group; subtle hemorrhagic injury was the most common neuroimaging abnormality, which correlated with BSID-II PDI score.14 In general, brain mineralization/hemosiderin was not associated with language scores, except that the 16 subjects (42%) who had brain mineralization/hemosiderin had lower CDI phrases percentiles than the 22 subjects who did not (32 ± 27 vs 51 ± 27, P = 0.0495). Hemosiderin did not correlate with head circumference percentile.
Quantitative MRI Group Comparison
Among the six aggregate regions, total brain, cerebral white matter, and brainstem volumes were significantly smaller in the CHD subjects than control subjects after adjusting for corrected chronological age at MRI and sex (Table II). Compared with healthy controls, the CHD group had a reduction of 54 mL (6%) in total brain (P = 0.009), 40 mL (15%) in cerebral white matter (P < 0.001), and 1.2 mL (10%) in brainstem (P = 0.003) volumes, but there were no significant differences in cerebral gray matter, subcortical gray matter, and cerebellum volumes. Even after further adjustment for total brain volume, the reductions in cerebral white matter (P < 0.001) and brainstem (P = 0.04) persisted. There were no appreciable differences among the three cardiac diagnostic groups in the aggregate regions (Table III; available at www.jpeds.com). In the 22 discrete regions, CHD subjects had smaller right and left cerebral white matter and cerebellar white matter volumes than controls (Table I).
Table III. (online only). Cardiac patient characteristics according to diagnostic group (N = 48).
| Characteristic* | TGA (N = 19) | TOF (N = 20) | VSD (N = 9) | P value† |
|---|---|---|---|---|
| Medical history and demographic information | ||||
| Male, n (%) | 12 (63) | 10 (50) | 6 (67) | 0.64 |
| Age at operation, median (range), d | 6 (2-13) | 63 (2-198) | 156 (39-264) | <0.001 |
| Lowest operative pCO2 | 32.6 ± 5.1 | 32.5 ± 5.0 | 33.0 ± 5.9 | 0.98 |
| Postoperative ICU length of stay, d | 4.8 ± 4.0 | 4.0 ± 2.0 | 2.3 ± 0.7 | 0.01 |
| MRI at one year of age | ||||
| Corrected chronological age at MRI, median (range), mo | 13.0 (11.3-16.4) | 13.5 (11.7-23.5) | 14.6 (12.2-21.4) | 0.08 |
| Total brain volume, mL | 881.9 ± 86.4 | 852.6 ± 91.6 | 888.5 ± 68.7 | 0.16 |
| Cerebral white matter volume, mL | 235.9 ± 28.3 | 224.3 ± 28.7 | 237.0 ± 26.8 | 0.11 |
| Cerebral gray matter volume, mL | 506.6 ± 52.3 | 492.8 ± 53.8 | 503.8 ± 37.7 | 0.23 |
| Subcortical gray matter volume, mL | 41.9 ± 3.9 | 40.7 ± 2.9 | 42.9 ± 3.8 | 0.25 |
| Cerebellum volume, mL | 105.7 ± 10.7 | 102.7 ± 12.0 | 113.3 ± 9.2 | 0.16 |
| Brainstem volume, mL | 11.3 ± 1.3 | 10.7 ± 1.4 | 12.2 ± 1.7 | 0.04 |
| Follow-up data at one year of age | ||||
| Overall abnormal neurologic examination result, n (%) | 7 (37) | 14 (70) | 7 (78) | 0.06 |
| Abnormal motor function, n (%) | 6 (32) | 13 (65) | 7 (78) | 0.03 |
| Bayley Scales of Infant Development II | ||||
| Psychomotor Development Index | 84.8 ± 14.9 | 86.5 ± 19.7 | 86.3 ± 11.9 | 0.95 |
| Mental Development Index‡ | 96.6 ± 10.2 | 93.9 ± 13.3 | 92.3 ± 8.5 | 0.61 |
| MacArthur-Bates Communicative Development Inventory | ||||
| percentiles§ | ||||
| Phrases | 46 ± 21 | 43 ± 33 | 33 ± 34 | 0.61 |
| Vocabulary words understood | 41 ± 26 | 33 ± 34 | 18 ± 28 | 0.27 |
| Vocabulary words produced | 47 ± 25 | 39 ± 27 | 36 ± 14 | 0.56 |
| Total gestures | 39 ± 28 | 28 ± 28 | 23 ± 17 | 0.36 |
| Head circumference percentile | 55 ± 29 | 42 ± 28 | 45 ± 31 | 0.34 |
| Weight percentile | 48 ± 33 | 31 ± 31 | 40 ± 36 | 0.26 |
MRI, magnetic resonance imaging; TGA, D-transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventral septal defect.
Data are expressed as mean ± standard deviation unless otherwise specified.
P values are for differences between diagnostic groups. For binary variables, the P value was determined by Fisher's exact test. For follow-up data, P values were determined by analysis of variance. For brain volumes, P values were determined by linear regression adjusting for corrected chronological age at MRI and sex. For all other continuous variables, P values were determined by Kruskal-Wallis tests.
N = 19 TGA, 20 TOF, and 8 VSD patients.
N = 17 TGA, 15 TOF, and 6 VSD patients.
Brain Volumes and Medical Factors
Total brain volume correlated with lowest operative pCO2 (r = 0.33, P = 0.02) (Table IV; available at www.jpeds.com). Cerebral gray matter volume correlated directly with lowest operative pCO2 (r = 0.31, P = 0.03) and inversely with postoperative intensive care unit length of stay (r = −0.29, P = 0.047). The lowest pCO2 typically occurred shortly after induction of anesthesia prior to cooling (N = 16, 33%) or after rewarming to 35 degrees Celsius (N = 31, 65%).
Table IV. (online only). Correlations of brain volumes with medical variables and neurologic/neurodevelopmental outcomes for cardiac patients (N = 48).
| Variable | Total brain | Cerebral white | Cerebral gray | Subcortical gray | Cerebellum | Brainstem |
|---|---|---|---|---|---|---|
| Medical variable* | ||||||
| Preoperative characteristics | ||||||
| Birth weight | 0.20 | 0.12 | 0.24 | 0.18 | 0.16 | 0.05 |
| Gestational age | 0.06 | −0.14 | 0.13 | 0.05 | 0.21 | −0.09 |
| Apgar score at 5 min | −0.10 | −0.14 | 0.00 | −0.11 | −0.13 | −0.11 |
| Catheterizations | 0.08 | −0.13 | 0.11 | −0.02 | 0.21 | −0.01 |
| Balloon atrial septostomy | 0.06 | 0.01 | 0.05 | 0.13 | 0.11 | 0.16 |
| Age at operation | 0.10 | 0.13 | 0.11 | −0.01 | 0.02 | −0.07 |
| Intubated before surgery | −0.12 | −0.09 | −0.11 | −0.06 | −0.16 | −0.12 |
| Operative characteristics | ||||||
| Hematocrit treatment group (25%) | 0.05 | 0.10 | 0.01 | 0.18 | 0.12 | 0.15 |
| Crossclamp time | 0.04 | 0.06 | −0.03 | 0.17 | 0.15 | 0.16 |
| Total support time | −0.02 | 0.00 | −0.05 | 0.07 | 0.09 | 0.13 |
| Total bypass time | 0.00 | 0.00 | −0.03 | 0.07 | 0.11 | 0.07 |
| Low-flow bypass time | 0.21 | 0.21 | 0.17 | 0.22 | 0.17 | 0.11 |
| Duration of circulatory arrest | −0.09 | −0.04 | −0.11 | −0.03 | −0.06 | 0.17 |
| Hematocrit at onset of low-flow | 0.02 | 0.13 | −0.03 | 0.16 | 0.15 | 0.08 |
| Lowest tympanic temperature | 0.13 | 0.08 | 0.13 | 0.07 | 0.02 | −0.03 |
| Lowest pCO2 | 0.33§ | 0.24 | 0.31§ | 0.17 | 0.28 | 0.11 |
| pH at lowest pCO2 | −0.23 | −0.17 | −0.23 | −0.11 | −0.15 | −0.02 |
| Highest pCO2 | 0.01 | 0.00 | 0.00 | 0.17 | 0.21 | 0.11 |
| Lowest pO2 | 0.11 | 0.02 | 0.09 | 0.06 | 0.13 | −0.10 |
| Postoperative characteristics | ||||||
| Lactate 60 min after bypass | −0.13 | −0.25 | −0.08 | −0.13 | −0.16 | −0.07 |
| Lowest PaCO2 | 0.14 | 0.16 | 0.11 | 0.20 | 0.20 | 0.05 |
| Lowest PaO2 | −0.10 | −0.23 | −0.01 | −0.19 | −0.21 | −0.23 |
| PRISM 12 hr postoperative | −0.13 | −0.13 | −0.14 | −0.12 | −0.16 | −0.09 |
| PRISM 24 hr postoperative | 0.05 | 0.05 | 0.07 | −0.05 | −0.14 | −0.01 |
| Hours intubated | −0.16 | −0.11 | −0.16 | −0.07 | −0.07 | −0.07 |
| Postoperative ICU length of stay | −0.27 | −0.21 | −0.29§ | −0.07 | 0.01 | −0.02 |
| Postoperative hospital length of stay | −0.10 | −0.08 | −0.13 | 0.03 | −0.06 | −0.02 |
| Hypertension | 0.22 | 0.23 | 0.25 | 0.10 | 0.08 | −0.04 |
| Hypothermia | 0.13 | 0.15 | 0.19 | −0.04 | −0.07 | −0.19 |
| Neurologic/neurodevelopmenta l outcomes† | ||||||
| Overall abnormal neurologic examination result | 0.05 | 0.13 | 0.01 | −0.02 | 0.03 | 0.02 |
| Abnormal motor function | 0.06 | 0.10 | 0.04 | −0.04 | 0.08 | 0.05 |
| Bayley Scales of Infant Development-II | ||||||
| Psychomotor Development Index (PDI) | −0.23 | −0.22 | −0.21 | −0.08 | −0.14 | −0.03 |
| Mental Development Index (MDI)‡ | 0.01 | −0.02 | 0.04 | 0.04 | −0.01 | −0.03 |
| MacArthur-Bates Communicative Development Inventories (CDI)‡ | ||||||
| Phrases | 0.19 | 0.32§ | 0.14 | 0.25 | 0.24 | 0.43¶ |
| Vocabulary words understood | 0.29 | 0.43¶ | 0.23 | 0.29 | 0.22 | 0.38§ |
| Vocabulary words produced | 0.03 | 0.11 | 0.05 | 0.10 | 0.00 | 0.10 |
| Total gestures | 0.39§ | 0.44¶ | 0.36§ | 0.45¶ | 0.29 | 0.39§ |
Values are Spearman correlations between the medical variables and residuals from linear regression of the brain volumes on corrected chronological age at MRI, sex, and diagnosis.
Values are adjusted Spearman correlations between the neurologic/neurodevelopmental outcomes and residuals from linear regression of the brain volumes on corrected chronological age at MRI and sex.
N = 47 for MDI and N = 38 for CDI percentiles.
P < 0.05,
P < 0.01.
Brain Volumes and Neurologic/Neurodevelopmental Outcomes
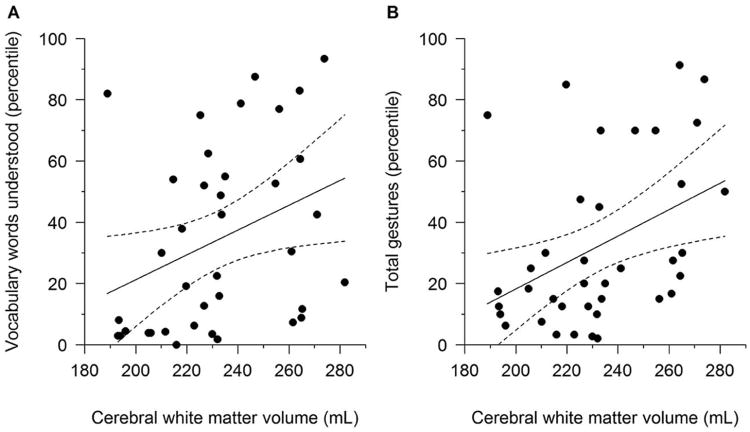
Brain volumes were not correlated with BSID-II scores or abnormal neurological exam in the CHD group, but they were associated with language development by CDI (Tables IV and V). Cerebral white matter and brainstem volumes correlated significantly with development of phrases, understanding of vocabulary, and development of gestures (Figure). Total brain, cerebral gray matter, and subcortical gray matter volumes also correlated with development of gestures.
Table V. Correlations between MacArthur-Bates Communicative Development Inventory percentiles and brain volumes for cardiac patients (N = 38).
| MacArthur-Bates Communicative Development Inventory Percentiles | Brain Volume | Spearman Correlation Coefficient | P value* |
|---|---|---|---|
| Phrases | Cerebral white matter | 0.32 | 0.049 |
| Brainstem | 0.43 | 0.008 | |
| Vocabulary words understood | Cerebral white matter | 0.43 | 0.008 |
| Brainstem | 0.38 | 0.02 | |
| Total gestures | Total brain | 0.39 | 0.02 |
| Cerebral white matter | 0.44 | 0.006 | |
| Cerebral gray matter | 0.36 | 0.03 | |
| Subcortical gray matter | 0.45 | 0.005 | |
| Brainstem | 0.39 | 0.02 |
P values were determined by Spearman correlation analysis between the MacArthur-Bates Communicative Development Inventory percentiles and residuals from linear regression of the brain volumes on corrected chronological age at MRI and sex. Only correlations with P < 0.05 are presented.
Figure 1.
Regression lines and 95% confidence intervals showing the associations between MacArthur- Bates Communicative Development Inventories (CDI) percentiles and cerebral white matter volume. Cerebral white matter volume was adjusted for corrected chronological age and sex by adding the mean value of cerebral white matter volume for the 38 cardiac patients to the residuals from linear regression of the volume on corrected chronological age and sex. CDI percentiles increase as cerebral white matter volume increases (adjusted Spearman correlation r = 0.43, P = 0.008, Panel A for vocabulary words understood; r = 0.44, P = 0.006, Panel B for total gestures).
Discussion
We found that biventricular CHD subjects had smaller total brain, cerebral white matter, and brainstem volumes than healthy controls at one year, and that these brain volumes correlated with receptive language and gestures, but not with broader neurodevelopmental measures. Interestingly, cortical and subcortical gray matter and cerebellar volumes in CHD subjects were not significantly different compared with control subjects. In addition, we found that although there was significant recovery of head circumference to the normal range following cardiac surgery, this recovery did not include normalization of all brain volumes. Interpreted within the context of existing literature, our results provide insight into trajectories of brain growth and development following surgery for biventricular CHD.
We found that the smaller total brain volume in infants with CHD was largely driven by reduced cerebral white matter volume. This finding at one year of age complements p r i o r investigations that showed reduced total brain volume in fetuses, neonates, and adolescents with CHD.17, 21, 28 Fetuses with hypoplastic left heart syndrome had smaller total brain volume than controls, most prominent in cerebral white matter and cortex.18 Pre-operative neonates with predominantly biventricular CHD, scanned at less than two weeks of age, had global reductions in brain volume without pronounced regional differences.28 Despite years of further brain growth and development, adolescents with CHD have reduced total brain volume, most prominent in cerebral white matter.16, 21 Together with qualitative studies indicating that white matter injury is the most common neuroimaging finding in CHD,13, 29 our data suggest that early developmental disturbances and brain injury lead to persistent reductions in total and cerebral white matter volume long after surgical repair. This specific reduction in white matter may relate to the timing of cerebral insult in biventricular CHD. The late fetal period through early infancy is a time of rapid white matter development and myelination; thus, an insult during this developmental stage might have long-lasting effects on white matter growth and maturation.30, 31
Our results contrast with those of Watanabe et al who found smaller gray but not white matter volume in infants with CHD compared to healthy infants at a similar average age.32 Although cerebral gray matter volume was smaller than controls in our CHD group, this difference did not reach statistical significance in our study. Their study methods differed from ours. First, they obtained MRI scans over a wide age range, from 2 to 23 months. This variation in age at MRI acquisition may have obscured a varying pattern of brain growth and/or may introduce inaccuracy in volumetric measures owing to marked changes in gray and white matter signal intensity over this age range. Second, their study included infants with various forms of single ventricle and biventricular CHD, whereas our cohort was confined to a biventricular group. Differences in underlying cardiac disease may influence rates of brain injury and affect subsequent brain growth.13, 33 Neither study can accurately classify rates of brain injury in early infancy, as brain MRI months after surgical repair of CHD may be insensitive for detecting earlier brain injury.34 Finally, the lack of significantly reduced gray matter volume in our cohort may be due to sample size or other factors related to characteristics of our study population.
Few studies have specifically assessed brainstem and cerebellar volume. However our findings are consistent with those of Ortinau et al who demonstrated smaller brainstem but normalization of cerebellum volume at 3 months of age in a mixed cohort of CHD.35 The cerebellum shows tremendous volumetric gains relative to other regions of brain tissue over the first year.31 Thus, robust cerebellar growth in the first postnatal year may compensate for any early volume reductions, and brainstem volume differences reflect a persistent difference in descending tracts from the cerebral white matter.
Interestingly, we found significant correlations between cerebral white matter and total brain volumes with measures of language at one year. These correlations are similar to those found in adolescents with TGA in whom regional white matter microstructure in the cerebrum and brainstem correlated with specific cognitive deficits but rarely with global measures of intelligence.20 Similarly, in a mixed cohort of adolescents who underwent cardiopulmonary bypass in infancy for CHD, cerebral white matter correlated with verbal comprehension.21 Although there are few studies evaluating the relationship between quantitative MRI and neurodevelopment in CHD, our findings align with those of extremely preterm girls in whom language correlated with white matter volume.36 Our findings support the notion that insults to the rapidly developing white matter in young infants may have at least a short-term impact on language development.30
Brain volumes did not correlate with global indices of cognitive and motor development as measured with the BSID-II. Several studies in other populations have suggested that PDI and MDI measured at <18-24 months of age have poor predictive value. Thus, PDI and MDI may not be sensitive to detecting significant but subtle differences in cognitive functions (such as language) at one year of age.37, 38
We found few correlations of brain volumes with medical variables, although length of stay inversely correlated with cerebral gray matter volume. Prior data indicate that children with TGA who had longer hospital stay had worse cognitive function.39 In addition, lowest operative pCO2 was directly correlated with total brain and cerebral gray matter volumes. Typically lower pCO2 causes reduced cerebral blood flow and potentially increased risk of cerebral injury, hence a lower brain volume is concordant with this pathophysiology.
Overall, our findings fill a gap in the literature by describing brain volumes at one year of age following corrective cardiac surgery, a timeframe that has not often been studied.. In prior studies of this population, imaging was typically obtained shortly before and/or shortly after surgery, or much later in adolescence. In our study, there was some time for brain growth between surgery and imaging, but not such an extended period where other factors, such as environment, additional surgery, or medical complications, may play a larger role in recovery of brain growth and development. In addition, our study clarifies the relationship between structural brain volumes and neurodevelopment. Our study reports detailed infant language data and suggests that these early measures may be particularly sensitive to the effects of CHD on brain development and injury. This finding suggests that specific evaluation of language development is an important component of early neurodevelopmental follow-up in these children. Future studies should investigate whether early brain volumes predict later differences in language performance.
Our study has some limitations. First, our study was a secondary analysis from a trial designed to examine other outcomes. Second, because the initial trial did not recruit a control group, we used a dataset from a control group that was acquired on the same scanner at the same age as our CHD group. However, the slightly different sequence parameters could potentially introduce bias, and so we visually compared the segmented MRI data between the two groups to ensure that the segmentation algorithm was similarly effective for both groups. Another limitation to this control group is that they did not undergo the same detailed language evaluation as our CHD group. Third, our control sample had a larger head circumference than normative data. This above-average head size in control subjects may in part account for group differences in total brain volume. However, it would not account for the relatively greater reduction in white matter than other brain regions in the CHD subjects. Further, our finding of reduced total brain volume is consistent with data from other cohorts both younger and older than our subjects.17, 21, 28 Fourth, given the era during which our neurodevelopmental data were collected, the BSID-II was used, and the third edition has been used in more recent cohorts. The significant findings with the CDI in contrast to the lack of correlations with BSID-II may reflect a lower sensitivity of the BSID-II in this regard. It remains to be shown if the newer Bayley-III is more sensitive for detection of language differences in this population given its separate language composite score. Fifth, formal genetic evaluation and testing was not performed at the time of the trial, although subjects were excluded from the trial if phenotypic characteristics suggested a syndromic diagnosis. Sixth, MRI was not routinely obtained in the neonatal and perioperative period. Although we can infer that brain growth likely improved following surgery given the recovery of head circumference to the normal range, we cannot directly describe temporal patterns of brain growth within this cohort. Finally, in order to reduce the problem of multiple comparisons, we restricted our correlation analyses to aggregate brain regions and therefore did not explore the relationships between smaller discrete brain regions and neurodevelopment.
Despite these limitations, our findings have important implications for the trajectory of brain growth and neurodevelopment in children with biventricular forms of CHD when placed in the context of prior literature. Following reparative cardiac surgery, head growth normalized yet cerebral white matter and brainstem volumes remained small. Our findings suggest that persistent reduction in brain volumes in biventricular CHD months after surgery may be driven by early injury to and/or altered development of white matter, and that these observed structural differences are associated with language development. Language should be closely monitored at early neurodevelopmental follow-up visits, and future studies will be needed to determine the long-term consequences for language development.
Acknowledgments
Funded by the Pediatric Heart Network (PHN), supported by the National Heart, Lung, and Blood Institute/National Institutes of Health (NIH; U10HL068270), the NIH/National Institute of Neurological Disorders and Stroke (K12 NS079414, HL063411, RR02172), the Farb Family Fund, and the Boston Children's Hospital Intellectual And Developmental Disabilities Research Center (P30 HD18655). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- CHD
congenital heart disease
- MRI
magnetic resonance imaging
- TGA
transposition of the great arteries
- TOF
tetralogy of Fallot
- VSD
ventricular septal defect
- BSID-II
Bayley Scales of Infant Development-II
- CDI
MacArthur-Bates Communicative Development Inventories
Footnotes
The authors declare no conflicts of interest.
Portions of the study were presented as a poster at the meeting of the Pediatric Academic Societies, April 30-May 3, 2016, Baltimore, MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:e32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 3.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–7. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, et al. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol. 2013;55:1143–9. doi: 10.1111/dmcn.12242. [DOI] [PubMed] [Google Scholar]
- 6.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–67. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 7.Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, et al. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation. 2015;132:755–61. doi: 10.1161/CIRCULATIONAHA.114.014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, et al. Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiol Young. 2015;25:338–47. doi: 10.1017/S1047951114000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira FM, Coelho RM, Proenca C, Silva AM, Vieira D, Vaz C, et al. Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatr Cardiol. 2011;32:1132–8. doi: 10.1007/s00246-011-0039-0. [DOI] [PubMed] [Google Scholar]
- 10.Neal AE, Stopp C, Wypij D, Bellinger DC, Dunbar-Masterson C, DeMaso DR, et al. Predictors of health-related quality of life in adolescents with tetralogy of Fallot. J Pediatr. 2015;166:132–8. doi: 10.1016/j.jpeds.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussatto K, Tweddell JS. Quality of life following surgery for congenital cardiac malformations in neonates and infants. Cardiol Young. 2005;15:174–8. doi: 10.1017/s1047951105001253. [DOI] [PubMed] [Google Scholar]
- 12.Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, et al. Health-related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. 2014;164:781–8 e1. doi: 10.1016/j.jpeds.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–9. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 14.Soul JS, Robertson RL, Wypij D, Bellinger DC, Visconti KJ, du Plessis AJ, et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–81. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson CG, Asaro LA, Wypij D, Robertson RL, Jr, Newburger JW, Rivkin MJ. Altered gray matter in adolescents with d-transposition of the great arteries. J Pediatr. 2016;169:36–43 e1. doi: 10.1016/j.jpeds.2015.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivkin MJ, Watson CG, Scoppettuolo LA, Wypij D, Vajapeyam S, Bellinger DC, et al. Adolescents with d-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J Thorac Cardiovasc Surg. 2013;146:543–9 e1. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2013;23:2932–43. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 20.Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–44. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–76. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Jonas RA, Soul J, Kussman BD, Bellinger DC, Laussen PC, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347–54. 54 e1–4. doi: 10.1016/j.jtcvs.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Evans AC Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 24.Akhondi-Asl A, Warfield SK. Simultaneous truth and performance level estimation through fusion of probabilistic segmentations. IEEE Transactions on Medical Imaging. 2013;32:1840–52. doi: 10.1109/TMI.2013.2266258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Bayley N. Bayley scales of infant development. 2nd. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 27.Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, Bates E. MacArthur-Bates Communicative Development Inventories User's Guide and Technical Manual. 2nd. Baltimore, MD: Paul H. Brooke's Publishing Company; 2007. [Google Scholar]
- 28.von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr. 2015;167:1259–63 e1. doi: 10.1016/j.jpeds.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–16. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppi P, Warfield SK, Kikinis R, Barnes PD, Zientara GP, Jolesz F, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–35. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 31.Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–82. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Matsui M, Matsuzawa J, Tanaka C, Noguchi K, Yoshimura N, et al. Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2009;137:146–53. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Inder TE, Huppi P, Warfield SK, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–14. [PubMed] [Google Scholar]
- 35.Ortinau C, Inder T, Lambeth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr Cardiol. 2012;33:1138–46. doi: 10.1007/s00246-012-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164:1012–8. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–41. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 38.Harris SR, Megens AM, Backman CL, Hayes VE. Stability of the Bayley II Scales of Infant Development in a sample of low-risk and high-risk infants. Dev Med Child Neurol. 2005;47:820–3. doi: 10.1017/S0012162205001738. [DOI] [PubMed] [Google Scholar]
- 39.Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KCK, Rappaport LA, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143:67–73. doi: 10.1016/S0022-3476(03)00183-5. [DOI] [PubMed] [Google Scholar]