Abstract
Humans and animals are colonized by members of the genus Staphylococcus, however only some of these species evolved to cause invasive disease. The genetic basis for conversion of commensal staphylococci into pathogens is not known. We hypothesized that Staphylococcus aureus genes for coagulation and agglutination in vertebrate blood (coa, vwb and clfA) may support pathogenic conversion. Expression of coa and vwb in Staphylococcus epidermidis or Staphylococcus simulans supported a coagulase-positive phenotype but not the ability to cause disease in a mouse model of bloodstream infection. However, the simultaneous expression of coa, vwb and clfA in coagulase-negative staphylococci enabled bacterial agglutination in plasma and enhanced survival of S. simulans in human whole blood. Agglutination of S. simulans in the bloodstream of infected mice upon expression of coa, vwb and clfA provided also a mean for dissemination and replication in distal organs. Thus, the acquisition of genes for bacterial agglutination with fibrin appear sufficient for the conversion of commensal staphylococci into invasive pathogens.
Keywords: coagulation, clumping, agglutination, staphylococcus, abscess, virulence
1. Introduction
Staphylococcus aureus colonizes the nasopharynx, skin and gastrointestinal tract of humans and is also an invasive pathogen, frequently causing skin and soft tissue infection, bacteremia or abscess lesions in any organ tissue [1–3]. The genus Staphylococcus is comprised of more than 40 species, which share 16S ribosomal RNA sequence, low genomic DNA G+C content, cell wall composition (pentaglycine crossbridges, lysostaphin sensitivity), cytochrome and menaquinone profiles as well as susceptibility to erythromycin, bacitracin and furazolidone [4]. Humans and their domesticated animals are colonized by different species of the genus Staphylococcus: S. aureus, S. auricularis, S. capitis, S. epidermidis, S. haemolyticus, S. hominis, S. saprophyticus, S. simulans and S. warneri [5]. Of these, however, only S. aureus evolved to consistently cause invasive disease in healthy immuno-competent individuals [5].
Clinical diagnosis of S. aureus infection and initiation of appropriate antibiotic therapy requires laboratory identification of bacteria from superficial lesions, drainage of deep-seated abscesses or blood cultures [6]. As clinical samples may be contaminated with commensal Staphylococcus species, two laboratory tests, coagulation and clumping, exploit key microbiological traits associated with S. aureus to identify the pathogen [7, 8].
The coagulation test examines the ability of microbes inoculated into plasma of producing clots [9]. S. aureus isolates generate positive test results, owing to the expression of coa and vwb, whose products are secreted into the extracellular medium [10]. Coagulase (Coa) and von-Willebrand factor binding protein (vWbp) each associate with prothrombin (PT), also designated clotting factor II (FII), of the host coagulation cascade and generate enzymatically active complexes: Coa·PT and vWbp·PT [11]. Unlike thrombin, i.e. proteolytically activated FIIa, Coa·PT and vWbp·PT complexes cleave fibrinopeptides A and B off fibrinogen without cutting other thrombin substrates (FV, FVIII, FXI, FXIII, protein C, antithrombin and plasmin)[12, 13]. In addition, the vWbp·PT complex interacts and activates human FXIII in a non-catalytic manner [13].
The clumping test examines the agglutinating attributes of bacteria immersed in plasma. S. aureus isolates test positive in this assay owing to the secretion of coagulases (Coa and vWbp)[14] and to the assembly of clumping factor A (ClfA) in the bacterial envelope [15]. The joint action of coagulases in generating fibrin cables and of ClfA in promoting S. aureus association with fibrin protects bacteria from phagocytosis [14, 16]. Unlike S. aureus, coagulase-negative staphylococcal isolates, for example S. epidermidis or S. simulans, score negative in both coagulase and clumping tests [4].
Several Staphylococcus species produce coagulases, however these microbes (S. delphini, S. intermedius and S. pseudintermedius) adapted to causing invasive disease in other hosts: mink, fox, pigeon, cats or dogs [17, 18]. Genome sequence analysis of pathogenic and non-pathogenic Staphylococcus species suggested that horizontal gene transfer may be responsible for the evolution of pathogenic staphylococci [19]. However, it is not clear what genes may be sufficient for the conversion of commensal staphylococci into an invasive pathogen. This question is addressed here and we show that transfer of the S. aureus genes for coagulation and agglutination (coa, vwb and clfA) is sufficient to convert the coagulase-negative species S. simulans into a pathogen that coagulates vertebrate blood, agglutinates in human and mouse plasma and disseminates from the vasculature of infected mice to replicate in distal organs.
2. Materials and methods
2.1. Bacterial strains and growth conditions
Wild-type isolate S. aureus Newman and its isogenic Δcoa/vwb/clfA variant were described previously [14, 20]. S. epidermidis ATCC 12228 was obtained from American Type Culture Collection (ATCC.org). S. simulans MK148 (ATCC 27848) was a gift from Prof. Friedrich Gotz. All staphylococcal strains were grown in Brain Heart Infusion (BHI) broth at 30°C. Strains harboring plasmids pOS1 and its derivatives were grown in BHI supplemented with 5 μg chloramphenicol/ml. Escherichia coli strain DC10B was cultivated in Luria broth with 100 μg ampicillin/ml at 30°C.
2.2. Cloning procedures and plasmids
The shuttle vector pOS1 (also referred to as vector) was used for all cloning procedures [21]. Plasmid pcoa-vwb expressing the coa and vwb genes under their respective promoters was described previously [10]. The clfA gene with its native promoter was cloned by amplification with the polymerase chain reaction (PCR) using genomic DNA from S. aureus Newman as template and the primer pair 5’-CGGGGATCCAAGCTTTTTCAAGCTAGGATTACATTAGGTA-3’ and 5’-GCGGAATTCGAATCATATGATTAATTTAATATCA-3’. The ends of the PCR product were cut with BamHI and EcoRI restriction enzymes and ligated into pOS1 cut with the same enzymes, thereby generating pclfA. The clfA PCR product was also cut and ligated into the BamHI and SmaI restriction sites of pcoa-vwb to generate pcoa-vwb-clfA. All cloning steps were performed in E. coli DC10B [22]. Plasmid clones were verified by DNA sequencing prior to electroporation into S. epidermidis ATCC 12228 and S. simulans MK148.
2.3. Culture fractionation and immunoblot analysis
Overnight cultures were diluted 1:100 into BHI and grown for 6 hours. Samples were normalized to the same absorbance at 600 nm of 4 units (A600 4) and one milliliter of culture was centrifuged at 8,000 ×g for 5 min. Supernatant containing coagulases was transferred to another tube. Bacterial cells containing cell wall bound ClfA were suspended in 1 ml 50 mM Tris-HCl (pH 7.5), 150 mM NaCl and incubated with 10 μg lysostaphin/ml at 37°C for 30 min. Ice-cold trichloroacetic acid (150 μl TCA) was added to each sample, mixed and incubated for 30 min on ice; TCA precipitated proteins were sedimented by centrifugation (10,000 ×g for 15 min), washed with cold acetone, air-dried and solubilized in 100 μl SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.01 % bromophenol blue). Protein samples were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 30 min with 10 ml TBS-T (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% Tween 20) containing 5% milk and 50 μl human IgG (Sigma) prior to the addition of affinity-purified rabbit antibodies against Coa (α-Coa), vWbp (α-vWbp) or ClfA (α-ClfA) for 1 hour at room temperature. PVDF membranes were washed three times for 10 min with TBS-T and incubated with secondary anti-rabbit HRP-linked IgG for 1 hour at room temperature, washed, and developed using enhanced chemiluminescence substrates.
2.4. Coagulation and clumping assays
Overnight cultures were diluted 1:100 into 10 ml BHI, grown for 6 hours and normalized to A600 4. For the coagulation assay, 10 μl bacterial suspensions were mixed with 90 μl anticoagulated mouse plasma (sodium citrate) or rabbit plasma (EDTA) and incubated at room temperature for 24 hours prior to tilting the tubes. Rabbit plasma anti-coagulated with EDTA was obtained from BBLTM (Coagulase Plasma). Mouse plasma was obtained by drawing blood into 10 mM sodium citrate via cardiac puncture. Mouse blood was incubated at room temperature for 10 min, centrifuged at 2,000 ×g for 10 min and plasma was retrieved. For the clumping assay, bacteria from 10 ml staphylococcal culture were centrifuged, diluted in 1 ml PBS to A600 4 and incubated with 10 μl of 3 mg human fibrinogen (Sigma)/ml PBS. Clumping of staphylococci was observed by briefly inverting the tubes.
2.5. Agglutination assay
The agglutination assay was performed as described previously [14]. Briefly, 10 ml bacterial culture was centrifuged, washed and suspended in PBS and normalized to A600 4. Staphylococci were incubated with SYTO 9 (1:500) (Invitrogen) for 15 min. The cells were washed twice with PBS and suspended in 1 ml PBS. Bacterial suspensions were mixed 1:1 (vol/vol) with human or mouse citrate-plasma or rabbit EDTA-plasma on glass slides and incubated for 30 min. Samples were viewed and images were captured on an IX81 live cell total internal reflection fluorescence microscope using a 20× objective (Olympus). At least 10 images were captured for each sample. Areas of agglutination in each image were measured and quantified using ImageJ software. Statistical significance was determined by ordinary one-way analysis of variance (ANOVA) using Prism (GraphPad Software, Inc.).
2.6. Whole blood killing assay
Overnight cultures of S. simulans MK148 strains were diluted 1:100 into BHI and grown for 3 hours. Bacterial replication ex vivo was measured as described previously [16]. Briefly 0.475 ml of human blood anticoagulated with 5 μg desirudin/ml was incubated with 25 μl bacterial suspension (1.25×107 CFU). Following incubation at 37°C for 0 or 60 min, 0.5 ml of PBS containing 0.5% saponin, 100 U streptokinase K, 50 μg trypsin, 1 μg DNAse, 5 μg RNAse was added to each sample for 10 min at 37 °C, prior to plating on solid medium for enumeration of CFU. Statistical analysis was performed with the two-way ANOVA. Human volunteers were enrolled under a protocol that was reviewed and approved by the University of Chicago's Institutional Review Board.
2.7. Murine infection model
Overnight cultures of S. simulans MK148 strains were diluted 1:100 into BHI and grown for 3 hours. Bacteria were centrifuged, washed and suspended in PBS and normalized to A600 4. Colony forming units (CFU) of the inoculum were determined by serial dilutions of samples and plating on BHI agar plates. For infection, BALB/c mice (6 weeks old, female; Charles River Laboratories) were anesthetized via intraperitoneal injection with 65 mg/kg ketamine and 6 mg/kg xylazine. Mice in groups of 8–10 were infected by injection of a 100 μl bacterial suspension (5×107 CFU) into the periorbital venous plexus. Mice received chloramphenicol in the drinking water (1 mg/ml) for the duration of the experiment to prevent plasmid loss in the infecting bacteria. On day 5 after infection, mice were euthanized by CO2 inhalation and cervical dislocation. Bacterial loads in kidneys were determined by plating serial dilutions on BHI agar containing chloramphenicol using tissues homogenized in PBS with 0.1% Triton X-100; CFU were enumerated after incubation of plates at 30°C [23]. Kidneys were also fixed in 10% formalin for 24 h at room temperature, embedded in paraffin, thin-sectioned, and stained with hematoxylin and eosin. Stained tissues were observed by light microscopy to enumerate abscess lesions. All statistical analyses were performed using the one-way ANOVA on ranks (Kruskal-Wallis test) with Dunn's post-hoc test. P values of < 0.05 were deemed significant. All mouse experiments were performed and conducted in accordance with the institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC) at the University of Chicago.
3. Results
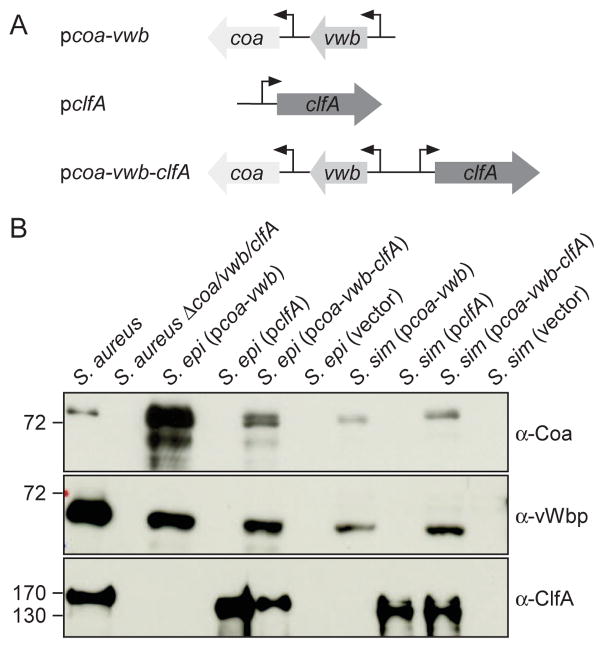
3.1. Expression of agglutination factors in coagulase-negative staphylococci
E. coli/Staphylococcus shuttle vector pOS1 was used to generate expression plasmids for clfA (pclfA), coa-vwb (pcoa-vwb) as well as coa-vwb-clfA (pcoa-vwb-clfA)(Fig. 1A). Plasmids were electroporated into S. simulans MK148 (ATCC27848) and S. epidermidis ATCC12228, both coagulase-negative, non-pathogenic isolates from the human skin [24, 25]. Transformants of both bacterial species stably maintained their plasmids in the presence of chloramphenicol selection. To quantify the production of agglutination factors, staphylococci were grown to early stationary phase, cultures were normalized to the same absorbance at 600 nm, and centrifuged to separate the culture medium from the bacterial sediment. Staphylococci were suspended in buffer and peptidoglycan was digested with lysostaphin to generate a bacterial lysate. Proteins in culture medium and lysate samples were precipitated with trichloroacetic acid and analyzed by immunoblotting with rabbit antibodies specific for Coa, vWbp or ClfA (Fig. 1B). As expected, Coa and vWbp were detected in the culture medium of wild-type S. aureus strain Newman, whereas ClfA was detected in the bacterial lysate (Fig. 1B). As control, Coa, vWbp and ClfA were not detected in the culture medium or lysate of the isogenic Δcoa/vwb/clfA mutant strain (Fig. 1B). Similarly, S. simulans and S. epidermidis carrying pOS1 only (vector) did not produce Coa, vWbp or ClfA (Fig. 1B). ClfA was, however, detected in the lysates of strains harboring pclfA but not in strains carrying pcoa-vwb (Fig. 1B). Reciprocal results were obtained when probing culture media with antibodies specific for Coa and vWbp (Fig. 1B). Finally, S. simulans or S. epidermidis carrying pcoa-vwb-clfA produced all three agglutination factors, Coa, vWbp and ClfA (Fig. 1B). Of note, the abundance of agglutination factors varied between S. aureus, S. simulans and S. epidermidis. In Fig. 1B, one tenth of S. aureus sample size was loaded on SDS-PAGE for a comparative analysis of Coa and vWbp production in coagulase-negative staphylococci. For the assessment of ClfA production, the S. aureus sample size loaded on a gel was concentrated 5-times as compared to S. simulans and S. epidermidis samples. Thus, the strains produced different levels of agglutination factors. Reduced production of some of these factors may at least in part be attributable to the saeS L18P allele of S. aureus Newman, which results in constitutive activation of the SaeS sensory kinase and high level expression of coa and vwb [26, 27].
Fig. 1.
Production of Coa, vWbp and ClfA by S. epidermidis and S. simulans. (A) Diagram illustrating plasmid inserts carrying the coa, vwb or clfA genes from S. aureus Newman. (B) Culture supernatants were examined by immunoblotting with antibodies specific for Coa (α-Coa) and vWbp (α-vWbp) while whole culture lysates were used to examine ClfA production using antibodies specific for ClfA (α-ClfA). Cells cultures were normalized A600 4 and S. aureus samples were diluted 10 times for Coa and vWbp or concentrated 5 times for ClfA analyses as compared to S. epidermidis and S. simulans extracts. Numbers to the left of blots indicate the position of molecular markers in kDa.
3.2. Conversion of coagulase-negative staphylococci
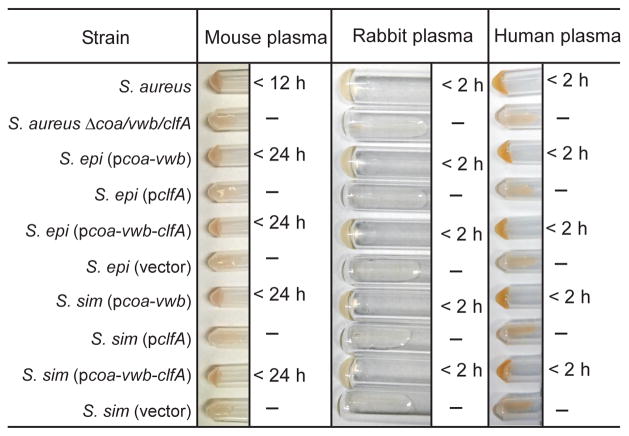
Inoculation of S. aureus into rabbit plasma promotes clotting [7, 8], a diagnostic feature that is critically dependent of the expression of coa and vwb [10]. To determine whether plasmid-borne expression of coa and vwb converted the assay phenotype of coagulase-negative staphylococci, bacteria were inoculated into mouse or rabbit chelated-plasma and incubated at 25°C. As controls, S. aureus Newman inoculation promoted coagulation of human and rabbit plasma in less than 2 hours, and in less than 12 hours in mouse plasma; coagulation was not observed when plasma was inoculated with the Δcoa/vwb/clfA mutant (Fig. 2). Unlike S. aureus, neither S. simulans (vector) nor S. epidermidis (vector) was capable of clotting citrate-plasma. However, inoculation of S. simulans or S. epidermidis carrying pcoa-vwb or pcoa-vwb-clfA into plasma caused clotting in a manner similar to that of S. aureus (Fig. 2). Coagulase-negative staphylococci (pclfA) expressing clfA failed to clot vertebrate plasma (Fig. 2), in agreement with the concept that ClfA alone does not function as a coagulase [14]. Thus, expression of coa and vwb converts S. simulans and S. epidermidis into coagulase-positive staphylococci.
Fig. 2.
Expression of coa and vwb promotes clotting by S. epidermidis and S. simulans. Anti-coagulated mouse, rabbit or human plasma was incubated with bacteria for up to 24 hours at 25°C. Tubes were tilted to assess coagulation. Data are representative of three independent experiments.
3.3. Conversion of clumping-negative staphylococci
Suspension of S. aureus in fibrinogen solutions promotes clumping of the bacteria, which spontaneously sediment to the tube bottom [28](Fig. 3). Earlier work had demonstrated S. aureus clumping to be dependent on the expression of clfA [15]. In agreement with earlier results, the S. aureus Δcoa/vwb/clfA mutant did not display a clumping phenotype (Fig. 3). Further, suspension of S. simulans or S. epidermidis in fibrinogen solution did not result in bacterial sedimentation to the bottom of reaction tubes (Fig. 3). In contrast, coagulase-negative staphylococci harboring pclfA or pcoa-vwb-clfA sedimented following bacterial suspension in fibrinogen solution, similar to wild-type S. aureus (Fig. 3). S. simulans and S. epidermidis expressing coagulases without clfA (pcoa-vwb) also did not display a clumping phenotype (Fig. 3).
Fig. 3.
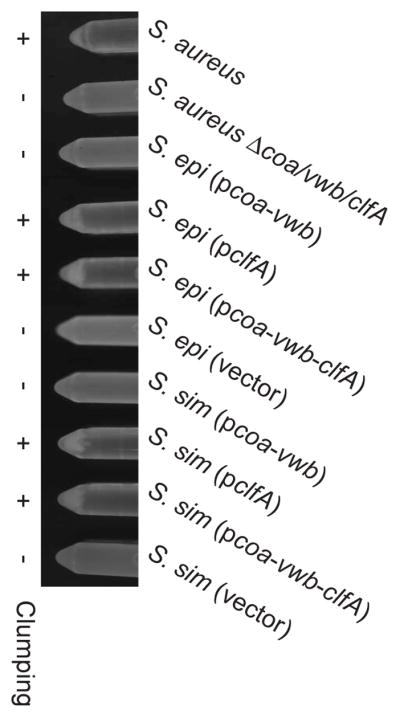
Expression of clfA promotes clumping by S. epidermidis and S. simulans. Sedimentation of bacteria was monitored immediately after mixing cultures with soluble fibrinogen and inverting the tubes. Data are representative of three independent experiments.
3.4. Conversion of agglutination-negative staphylococci
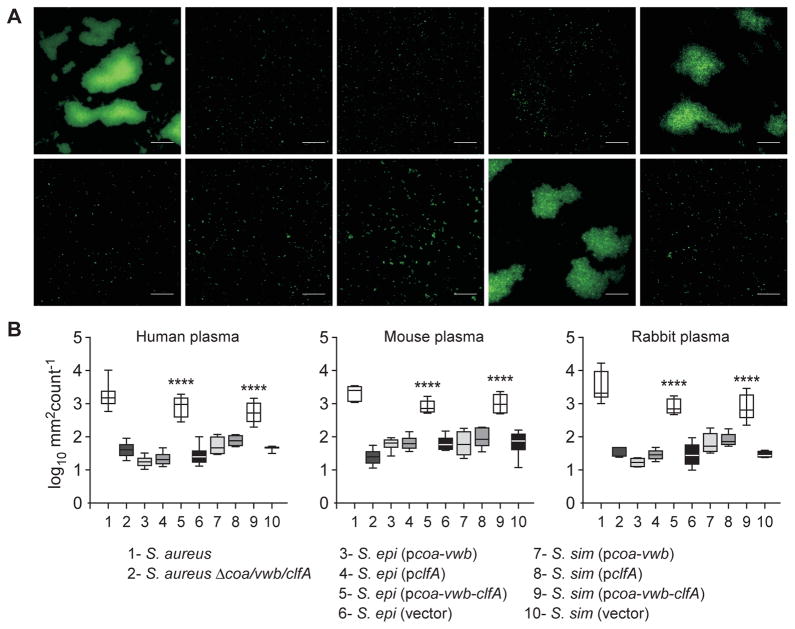
Another diagnostic tool, the slide agglutination test, monitors agglutination of bacteria immersed in calcium-chelated plasma (reviewed by [7]). This rapid test is used for positive identification of S. aureus and differential diagnosis with non-pathogenic staphylococci such as S. simulans and S. epidermidis. Bacterial agglutination requires two host factors, fibrinogen and prothrombin [29], as well as staphylococcal coagulases and clumping factor A [14]. As controls, Syto-9 stained wild-type, but not Δcoa/vwb/clfA mutant S. aureus, generated aggregates of fluorescent bacteria in human plasma. S. epidermidis and S. simulans did not agglutinate in human plasma. However, coagulase-negative staphylococci expressing coa, vwb and clfA (pcoa-vwb-clfA) agglutinated in human plasma, whereas strains expressing only coa-vwb (pcoavwb) or clfA (pclfA) did not (Fig. 4A). Similar results were obtained when staphylococcal agglutination was measured in mouse or in rabbit plasma (Fig. 4B).
Fig. 4.
Staphylococcal agglutination in citrate-treated plasma. (A) Agglutination in human plasma of Syto-9 stained bacteria. Scale bar, 50 μm. (B) Box and whisker plot representation of agglutination areas with anti-coagulated human, mouse and rabbit plasma. Statistical significance was determined using ANOVA (****, P < 0.0001).
3.5. Expression of coa-vwb-clfA promotes virulence
Virulence associated with expression of coagulation and agglutination factors was measured using a whole blood killing assay and a sub-lethal mouse model of infection. For these experiments, only the S. simulans variants were used. All plasmid-bearing variants of S. simulans replicated similarly. However, the introduction of plasmid pcoa-vwb-clfA significantly reduced replication of S. epidermidis as compared to plasmids pcoa-vwb or pclfA (data not shown).
Earlier work demonstrated that S. aureus evades opsonophagocytosis and replicates in human blood via mechanisms involving coa-vwb induced fibrin formation and clfA-mediated assembly of fibrin shields [16]. This work established that S. aureus exploits the host factors prothrombin and fibrinogen to induce the formation of fibrin agglutinates and shield bacteria from phagocytes [16]. Enumeration of S. aureus in blood requires release of bacteria from fibrin cables, which is achieved by treating samples with streptokinase, an activator of plasminogen [30]. When incubated in fresh human blood, approximately 99% of the inoculum of S. simulans carrying vector alone (1.25×107 CFU) was killed within 60 min (Fig. 5). Expression of coa-vwb-clfA, but not of coa-vwb or clfA alone, significantly improved the survival of S. simulans in whole blood (Fig. 5).
Fig. 5.
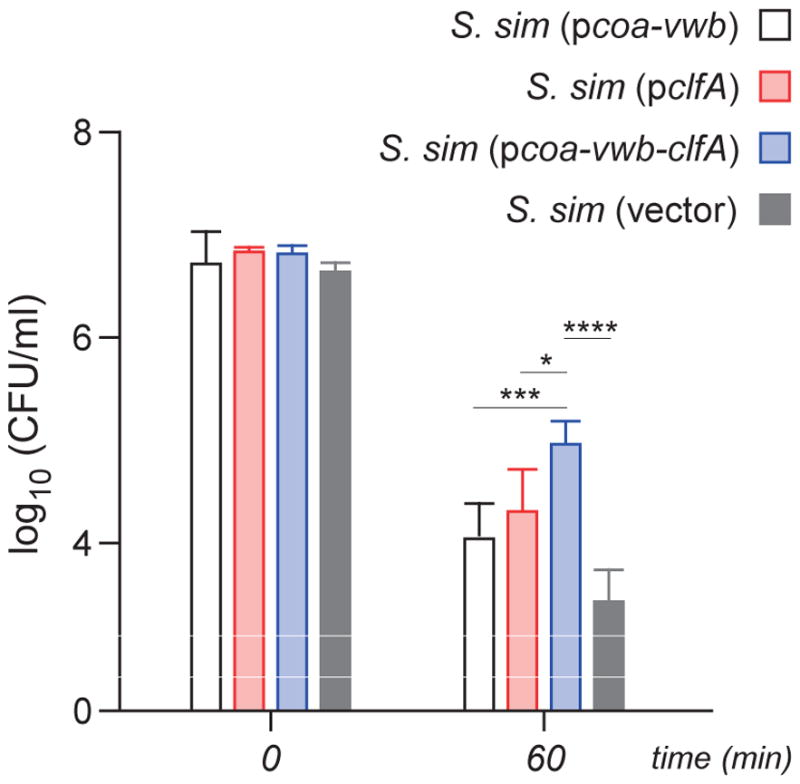
Staphylococcal survival in human blood. Mid-log phase bacteria (1.25 × 107 CFU) were inoculated into freshly drawn human blood anti-coagulated with desirudin. At 0 min and 60 min, saponin- and streptokinase-containing buffer was added to lyse host cells and release agglutinated bacteria. Bacteria were counted by plating serial dilutions on solid medium and reported as CFU/ml. Data are from three independent experiments; error bars indicate standard deviation. Statistical significance was performed by two-way ANOVA analysis, (****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05).
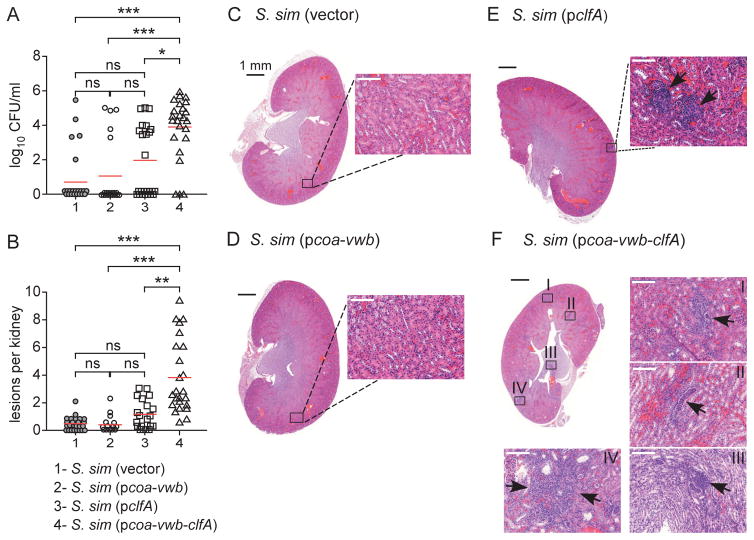
Next, S. simulans variants were inoculated intravenously in mice. Earlier work has shown that inoculation of 1×107 CFU S. aureus Newman into immune-competent mice leads to disseminated abscess formation in many different organ tissues within 4–5 days [23]. Abscess lesions caused by S. aureus are highly structured [31]. The pathogenesis of these lesions requires expression of coagulases, i.e. coa and vwb, but not of clfA [10]. On the other hand, expression of all three genes, coa-vwb-clfA, contributes to the pathogenesis of staphylococcal bacteremia [14]. Intravenous inoculation of 5×107 CFU S. simulans strains into BALB/c mice did not cause lethal disease. Five days following infection, animals were euthanized, necropsied and kidneys removed for the enumeration of bacterial load in tissue homogenates (left kidney of necropsied animals) or subjected to histopathological analysis (right kidney). The data showed that mice infected with S. simulans (vector) or S. simulans (pcoa-vwb) harbored very modest bacterial loads (Fig. 6A). Most kidneys were sterile (Fig. 6A) and H&E staining of kidney sections revealed healthy tissues (Fig. 6CD) without lesions (Fig. 6B). Infection of BALB/c mice with S. simulans (pclfA) resulted in a modest enhancement of virulence whereby bacterial counts increased in some animals (Fig. 6A) and occasional lesions could be observed in the kidney sections (Fig. 6BE). ClfA-induced lesions presented as immune cell infiltrates without detectable staphylococcal community at the center of these lesions (Fig. 6E). Inoculation of BALB/c mice with S. simulans (pcoa-vwb-clfA) resulted in a dramatic increase in bacterial load in renal tissue (Fig. 6A). Histopathology analysis revealed numerous lesions in kidneys with large infiltrates of immune cells, predominantly granulocytes (Fig. 6BF). These lesions appeared heterogeneous in shape and staining but were found throughout the organ as if small hemorrhages enabled access of bacteria from the vasculature and into organ tissue. Taken together, these data reveal that expression of the three agglutination factors coa, vwb and clfA in coagulase-negative staphylococci promotes bacterial escape from the bloodstream and replication in infected host tissues.
Fig. 6.
Pathogenic conversion of S. simulans with S. aureus coa, vwb and clfA. Mice were injected in the peri-orbital venous plexus with 5×107 of S. simulans carrying either vector, pcoa-vwb, pclfA or pcoa-vwb-clfA, and euthanized 5 days following challenge. (A) Following necropsy, renal tissues were assessed for bacterial load. (B) Lesions observed in panels C through F were enumerated using 20–25 slides per infected group. The data in panels A and B represent the cumulative result of three independent experiments and were analyzed using the one-way ANOVA on ranks (Kruskal-Wallis test) with Dunn's post-hoc test (***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant). (C-F) Microscopy images of hematoxylin-eosin-stained, thin-sectioned renal tissue. Whole kidney sections are shown and areas boxed in black are shown as a 20× enlarged images. Images and insets in panels C and D did not reveal pathological features in renal tissues. In panels E and F, arrows point to replicating bacteria and immune cell infiltrates.
4. Discussion
Members of the genus Staphylococcus evolved to colonize the epidermis, mucous membranes and gastrointestinal tracts of vertebrates [4]. Coagulase-negative staphylococci colonize a wide range of host species [32]. In contrast, pathogenic Staphylococcus species acquiring genetic traits for invasive disease target a narrow range of hosts [17, 19, 33]. Laboratory studies with S. aureus, the most prominent human pathogen of this genus, characterized coagulase and agglutination activities as hallmarks for the diagnosis of clinical S. aureus isolates [7, 8]. Subsequent identification of the genetic determinants for coagulation and agglutination enabled experiments that assessed the contributions of these factors towards infectious disease pathogenesis in animal models by infecting animals with defined genetic mutants [10, 14]. This research revealed that genes encoding coagulases (coa and vwb) as well as clumping factor A (clfA) contribute to the establishment of S. aureus bacteremia [10, 34] and that mutants lacking all three determinants are essentially avirulent [14]. These experiments represent a classic genetic approach and ask whether a gene is required for disease. Here, we ask whether these genes are sufficient to cause disease by expressing coa, vwb and clfA in a heterologous and related bacterial host. We find that the simultaneous expression of genes for staphylococcal coagulation and agglutination is sufficient to convert coagulase-negative S. simulans into an invasive pathogen.
S. aureus agglutination with fibrin cables requires two host factors, prothrombin and fibrinogen (reviewed by [7]). More recent publications use the clumping test to describe the same reaction as agglutination [9]. However, Hawiger and colleagues showed that S. aureus binds to purified fibrinogen, specifically the C-terminal 17 residues of the γ-chain, which also functions as a pocket in the D domain of fibrin enabling polymerization of monomers into fibrin cables [35, 36]. This fibrinogen binding assay was subsequently used to identify clfA [15]. Several other S. aureus surface proteins also bind to fibrinogen, however ClfA is a key factor for staphylococcal agglutination with coagulase-derived fibrin cables [14]. Expression of clfA in the non-pathogenic commensal Lactococcus lactis leads to functional assembly of clumping factor A with fibrinogen binding activity in the bacterial envelope [37]. Injection of 1×109 CFU L. lactis expressing clfA into the tail vein of BALB/c mice kills the experimental animals within 24 hours, whereas animals challenged with L. lactis expressing clfAY338A, a variant that cannot bind fibrinogen [38], are not [39]. The pathophysiology of the lethal outcome in this model is not known, however we presume that the association of large numbers of Lactococci expressing clfA with fibrinogen generates thrombi blocking blood perfusion to vital organs. In the rat endocarditis model, L. lactis expressing clfA associate with and colonize the pre-damaged heart valve tissues for 48 hours but are subsequently eradicated by the host immune system [40]. As is shown here, expression of clfA in S. simulans is not sufficient to increase the virulence attributes of the heterologous host towards the establishment of abscess lesions. Similar results were obtained with the heterologous expression of coa and vwb. Although coagulase-negative staphylococci expressing coa and vwb produce abundant coagulase activity, this is not sufficient for the establishment of infectious lesions in mice. Co-expression of clfA and coa-vwb, which reconstitutes agglutination with fibrin in S. simulans, enhanced the virulence potential of this heterologous host. S. aureus abscess lesions present distinct morphological features at day 5 post infection [23]. Histology of S. aureus infected organs reveals that bacteria replicate at the center of abscess lesions and are protected by a fibrin deposit from concentric layers of immune cells which in turn are engulfed within eosinophilic deposits comprised of fibrin [10]. Infection with S. simulans (pcoa-vwb-clfA) resulted in the formation of small lesions with immune infiltrates not quite as mature as S. aureus abscesses yet numerous small lesions could be seen throughout the organ reminiscent of small hemorrhages or petechiae as is observed when capillaries leak blood into the skin. In S. aureus bloodstream infection, the agglutination factors Coa, vWbp and ClfA promote endothelial adherence by inducing microthrombi with staphylococci embedded with fibrin shields [41]. This process has been proposed as one of the mechanisms responsible for the dissemination of S. aureus and subsequent invasion into organ tissues [7]. Data presented here support a model whereby Coa, vWbp, and ClfA are sufficient to promote S. simulans exit from the bloodstream by mechanically occluding blood flow in the microvasculature through the formation of bacterial agglutinates.
Stutzmann Meier and colleagues examined the virulence contributions of coa and clfA expressed in Streptococcus gordonii, a commensal of the human oropharynx [42]. Expression of clfA in S. gordonii enabled these bacteria (105 CFU inoculum) to adhere to rat endocardial tissue 12 hours post challenge [42]. However, adherence was not affected by co-expression of coa and clfA in S. gordonii [42]. It is not clear why co-expression of coa and clfA in streptococci does not generate increased virulence attributes in the rat endocarditis model. Nonetheless, we discuss several possibilities. First, S. aureus expresses two coagulases, coa and vwb, and cooperative effects between activators of hemostasis and clfA may require co-expression of all three genes, as is described here. In particular, adhesion of S. aureus to the wall of blood vessels under flow has been shown to be specifically mediated by vWbp [41]. Second, SD-repeat sequences of S. aureus ClfA are glycosylated and mutants lacking this post-translational modification display defects in agglutination with fibrin and in the murine abscess model [43]. Surface protein genes encoding SD repeats and fibrinogen binding activity are known to be expressed in coagulase-negative staphylococci [44]. The translational product of ClfA is likely glycosylated in S. epidermidis and S. simulans, as the mobility of ClfA on SDS-PAGE is similar to that of ClfA from S. aureus [43](Fig. 1). In agreement with this hypothesis, sequenced genomes of S. epidermidis and S. simulans isolates harbor homologues of S. aureus glycosyl-transferase genes (sdgA and sdgB) targeting SD repeats [43]. Third, Stutzmann Meier and colleagues measured adherence after a single 12 hour time interval, however it is not clear whether recombinant S. gordonii replicate on rat endocardial tissue to cause disease and whether such processes are impacted by the co-expression of clfA and coa [42].
In conclusion, expression of S. aureus coa, vwb and clfA is sufficient to confer onto coagulase-negative staphylococci the ability of agglutination with fibrin. Expression of coa-vwb-clfA increased the virulence of S. simulans, enabling the pathogenesis of invasive infections. We therefore propose that pathogenic conversion of commensal Staphylococcus species involves the acquisition of genes for agglutination with fibrin. In addition to S. aureus, similar conversions occurred in S. delphini, S. intermedius, S. pseudintermedius and S. lugdnunensis [45, 46]. These microbes share the attributes of coagulating vertebrate plasma and of binding to fibrinogen/fibrin on the bacterial surface. The product of these reactions, a fibrin shield covering the bacterial surface, provides protection against host phagocytes [16, 34].
Acknowledgments
The authors thank Professor Friedrich Gotz for the kind gift of S. simulans MK148 and members of our laboratory for discussion. This work was supported by the National Institute of Allergy and Infectious Diseases (award AI110937) and the Deutsche Forschungsgemeinschaft (award YU 181/1-1).
Footnotes
Conflict of interest
HKK, DM and OS declare a conflict of interest as inventors of patent applications related to the development of S. aureus vaccines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Kluytmans J, van Belkum A, Verburgh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Belkum A. Hidden Staphylococcus aureus carriage: overrated or underappreciated? MBio. 2016;7:e00079–16. doi: 10.1128/mBio.00079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götz F, Bannerman T, Schleifer K-H. The Genera Staphylococcus and Macrococcus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. Springer; New York: 2006. pp. 5–75. [Google Scholar]
- 5.Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5:183–95. doi: 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomer L, Schneewind O, Missiakas D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu Rev Pathol. 2016;11:343–64. doi: 10.1146/annurev-pathol-012615-044351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeb L. The influence of certain bacteria on the coagulation of blood. J Med Res. 1903;10:407. [PMC free article] [PubMed] [Google Scholar]
- 9.Cadness-Graves B, Williams REO, Harper GJ, Miles AA. Slide-test for coagulase-positive staphylococci. Lancet. 1943;244:736. [Google Scholar]
- 10.Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, Anderson PJ, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–9. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 12.McAdow M, Missiakas DM, Schneewind O. Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. J Innate Immun. 2012;4:141–8. doi: 10.1159/000333447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. J Biol Chem. 2013;288:28283–92. doi: 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdow M, Kim HK, DeDenta AC, Hendrickx APA, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDevitt D, Francois P, Vaudaux P, Foster TJ. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–48. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomer L, Emolo C, Thammavongsa V, Kim HK, McAdow ME, Yu W, et al. Antibodies against a secreted product of Staphylococcus aureus trigger phagocytic killing. J Exp Med. 2016;213:293–301. doi: 10.1084/jem.20150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guardabassi L, Schmidt KR, Petersen TS, Espinosa-Gongora C, Moodley A, Agersø Y, et al. Mustelidae are natural hosts of Staphylococcus delphini group A. Vet Microbiol. 2012;159:351–3. doi: 10.1016/j.vetmic.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. 2012;23:253–66. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Lefébure T, Bitar PP, Stanhope MJ. Comparative genomic analysis of the genus Staphylococcus including Staphylococcus aureus and its newly described sister species Staphylococcus simiae. BMC Genomics. 2012;13:38. doi: 10.1186/1471-2164-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes. J Bacteriol. 2007;190:300–10. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneewind O, Model P, Fischetti VA. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–81. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 22.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. MBio. 2012;3:e00277–11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloos WE, Schleifer KH. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975;1:82–8. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228) Mol Microbiol. 2003;49:1577–93. doi: 10.1046/j.1365-2958.2003.03671.x. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer D, Lâm TT, Geiger T, Mainiero M, Engelmann S, Hussain M, et al. A point mutation in the sensor histidine kinase SaeS of Staphylococcus aureus strain Newman alters the response to biocide exposure. J Bacteriol. 2009;191:7306–14. doi: 10.1128/JB.00630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinhuber A, Goerke C, Bayer M, Doring G, Wolz C. Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on expression of virulence factors. J Bacteriol. 2003;185:6278–86. doi: 10.1128/JB.185.21.6278-6286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawiger J, Timmons S, Strong DD, Cottrell BA, Riley M, Doolittle RF. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982;21:1407–13. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- 29.Birch-Hirschfeld L. Über die Agglutination von Staphylokokken durch Bestandteile des Säugetierblutplasmas. Klinische Woschenschrift. 1934;13:331. [Google Scholar]
- 30.Verhamme IM, Panizzi PR, Bock PE. Pathogen activators of plasminogen. J Thromb Haemost. 2015;(Suppl 1):S106–14. doi: 10.1111/jth.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng AG, DeDent AC, Schneewind O, Missiakas DM. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19:225–32. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderhaeghen W, Piepers S, Leroy F, Van Coillie E, Haesebrouck F, De Vliegher S. Identification, typing, ecology and epidemiology of coagulase negative staphylococci associated with ruminants. Vet J. 2015;203:44–51. doi: 10.1016/j.tvjl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 33.McCarty AJ, Lindsay JA. Staphylococcus aureus innate immune evasion is lineage-specific: a bioinformatics study. Infect Genet Evol. 2013;19:7–14. doi: 10.1016/j.meegid.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Palmqvist N, Patti JM, Tarkowski A, Josefsson E. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect. 2004;6:188–95. doi: 10.1016/j.micinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Hawiger J, Hammond DK, Timmons S. Human fibrinogen possesses binding sites for staphylococci on Aalpha and Bbeta polypeptide chains. Nature. 1975;258:643–5. doi: 10.1038/258643a0. [DOI] [PubMed] [Google Scholar]
- 36.Strong DD, Laudano AP, Hawiger J, Doolittle RF. Isolation, characterization and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982;21:1414–20. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- 37.Que YA, Francois P, Haefliger JA, Entenza JM, Vaudaux P, Moreillon P. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect Immun. 2001;69:6296–302. doi: 10.1128/IAI.69.10.6296-6302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loughman A, Fitzgerald JR, Brennan MP, JH, Downer R, Cox D, et al. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol. 2005;57:804–18. doi: 10.1111/j.1365-2958.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 39.Scully IL, Timofeyeva Y, Keeney D, Matsuka YV, Severina E, McNeil LK, et al. Demonstration of the preclinical correlate of protection for Staphylococcus aureus clumping factor A in a murine model of infection. Vaccine. 2015;33:5452–7. doi: 10.1016/j.vaccine.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 40.Que YA, Haefliger JA, Piroth L, François P, Widmer E, Entenza JM, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005;201:1627–35. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claes J, Vanassche T, Peetermans M, Liesenborghs L, Vandenbriele C, Vanhoorelbeke K, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–76. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stutzmann Meier P, Entenza JM, Vaudaux P, Francioli P, Glauser MP, Moreillon P. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect Immun. 2001;69:657–64. doi: 10.1128/IAI.69.2.657-664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomer L, Becker S, Emolo C, Quach A, Kim HK, Rauch S, et al. N-acetylglucosaminylation of serine-aspartate repeat proteins promotes Staphylococcus aureus bloodstream infection. J Biol Chem. 2014;289:3478–86. doi: 10.1074/jbc.M113.532655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCrea KW, Hartford O, Davis S, Eidhin DN, Lina G, Speziale P, et al. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology. 2000;146:1535–46. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 45.Geoghegan JA, Ganesh VK, Smeds E, Liang X, Höök M, Foster TJ. Molecular characterization of the interaction of staphylococcal microbial surface components recognizing adhesive matrix molecules (MSCRAMM) ClfA and Fbl with fibrinogen. J Biol Chem. 2010;285:6208–16. doi: 10.1074/jbc.M109.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson M, Bjerketorp J, Wiebensjö A, Ljungh A, Frykberg L, Guss B. A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol Lett. 2004;234:155–61. doi: 10.1016/j.femsle.2004.03.024. [DOI] [PubMed] [Google Scholar]