Abstract
Objective
To determine the birth prevalence of congenital heart defects (CHDs) across the spectrum of common defects in very/extremely premature infants and to compare mortality rates between premature infants with and without CHDs.
Study design
The Kids' Inpatient Databases (2003-2012) were used to estimate the birth prevalence of CHDs (excluding patent ductus arteriosus) in very/extremely premature infants born between 25 and 32 weeks gestational age (GA). Birth prevalence was compared with term infants for a subset of “severe” defects expected to be near universally diagnosed in the neonatal period. Weighted multivariable logistic regression was used to calculate adjusted odds ratios of mortality comparing very and extremely premature infants with versus without CHDs.
Results
We identified 249,011 very/extremely premature infants, including 28,806 with CHDs. The overall birth prevalence of CHDs was 116 per 1000 very/extremely premature births. Severe CHDs had significantly higher birth prevalence in very/extremely premature infants when compared with term infants (7.4 per 1000 very/premature births versus 1.5 per 1000 term births, p<0.001). Very/extremely premature infants with severe CHDs had an overall 26.3% in-hospital mortality and a 7.5-fold increased adjusted odds of death compared with those without CHDs. Mortality varied widely by defect in very/extremely premature infants, ranging from 12% for interrupted aortic arch to 67% for truncus arteriosus.
Conclusion
Given the increased birth prevalence of severe CHDs in very/extremely premature infants, and significantly higher mortality, there is justification for intensive interventions aimed at decreasing the likelihood of premature delivery for patients where CHD is diagnosed in utero.
Keywords: congenital heart defects, very premature, extremely premature, mortality
Congenital heart defects (CHDs) are the most common congenital anomaly, occurring in approximately 6 to 10 infants per 1000 live births1–5. While advances in surgery have improved outcomes for term and near-term infants with CHDs, for those born very prematurely (28-32 weeks gestational age (GA)) or extremely prematurely (<28 weeks GA), neonatal heart surgery is often infeasible or associated with excess risk6. Therefore, consideration of cardiac intervention is typically delayed to allow these infants to grow and develop. During this time, they are exposed to physiologic derangements inherent to both their prematurity and CHD7,8. These derangements may cause death or major morbidities that preclude the possibility of intervention7,8.
Despite recognition that premature infants with CHDs are a very vulnerable population, little is known about the epidemiology or outcomes of very/extremely premature infants with CHDs. Prior epidemiologic studies have largely focused on term infants. Similarly, prior multi-center analyses evaluating the association between GA and mortality in infants with CHDs have all focused on either infants born at term (37 to 40 weeks completed GA) or late preterm (34 to 37 weeks GA)9–11. Studies evaluating very/extremely premature infants with CHDs are generally small (n< 300) and encompass a limited variety of defects12–16.
To better understand the epidemiology and outcomes of very/extremely premature infants with CHDs, we used a large, nationally representative administrative database to determine the birth prevalence of CHDs across the spectrum of common defects in very/extremely premature infants, and to compare mortality between premature infants with and without CHDs.
Methods
We performed a retrospective cohort study using the 2003, 2006, 2009, and 2012 Healthcare Cost and Utilization Project Kids’ Inpatient Database (KID). The KID is the largest publicly-available all-payer pediatric inpatient database and includes approximately seven million estimated hospitalizations in community, academic, and private hospitals in 44 states. It is a 20% stratified sample of discharges for patients younger than 21 years across the country collected by the Agency for Healthcare Research and Quality. Stratification, cluster, and weighting variables account for complex survey design and allow for calculation of national estimates.
All birth hospitalizations from the ‘03, ‘06, ‘09, and ‘12 KIDs were included in the analysis. ICD-9-CM diagnosis codes were used to define GA at birth by week from 25 to 32 weeks GA (Table I; available at www.jpeds.com). Neonates born at < 25 weeks GA were excluded because of the high mortality rates related to being born at such extremes of prematurity17,18. Term birth was defined as any birth hospitalization that did not have a prematurity or post-maturity related ICD-9-CM diagnosis. CHDs were classified using ICD-9 CM codes for the various defects. Hypoplastic left heart syndrome (HLHS) included ICD-9-CM codes for mitral atresia and HLHS. A subset of severe CHDs were defined consistent with previous studies and included atrioventricular canal defects, truncus arteriosus, total anomalous pulmonary venous return (TAPVR), tetralogy of Fallot, pulmonary atresia, tricuspid atresia, HLHS, Ebstein’s anomaly, aortic stenosis, transposition of the great arteries (TGA), coarctation, double outlet right ventricle (DORV) and interrupted aortic arch (IAA)4,19. These are all CHDs that would be expected to be nearly universally diagnosed during a prolonged neonatal hospital stay, which is expected for very and extremely premature neonates. Patent ductus arteriosus (PDA) was not included when calculating CHD birth prevalence. For epidemiological analyses, defects were not mutually exclusive and infants with multiple defects could be counted in several diagnostic categories. However when determining the overall prevalence of CHD, each hospitalization was only counted once.
Table 1.
Online: Gestational age ICD-9-CM codes
| Classification | ICD-9-CM | ICD-9-CM Short description |
|---|---|---|
| 25 to 26 weeks | 765.23 | 25-26 completed weeks of gestation |
| 27 to 28 weeks | 765.24 | 27-28 completed weeks of gestation |
| 29 to 30 weeks | 765.25 | 29-30 completed weeks of gestation |
| 31 to 32 weeks | 765.26 | 31-32 completed weeks of gestation |
For outcomes assessments, the cohort was limited to hospitalizations in children’s hospitals or pediatric units in the ’03, ’06 and ’09 KIDs for infants less than one month of age (i.e. not just birth hospitalizations). The 2012 KID was not used because this database does not have information on pediatric hospital or unit type. The outcomes cohort was limited to children’s hospitals and pediatric units to ensure outcomes were representative of infants receiving advanced care and to prevent erroneously capturing a positive outcome (i.e. survival) for infants who were born in community hospitals and immediately transferred following diagnosis of their CHD to tertiary care centers. Hospitalizations containing a diagnosis code for non-cardiac fatal anomalies including trisomy 13, trisomy 18, anencephaly, and renal agenesis were excluded. For the outcomes analysis, it was important to attribute outcomes to the appropriate diagnostic category. Thus, a hierarchy of defects (Table II; available at www.jpeds.com) was created and outcomes for these hospitalizations were attributed to the highest risk defect in the hierarchy. Small for gestational age (SGA), Down’s syndrome, and DiGeorge syndrome were defined by ICD-9-CM codes.
Table 2.
online: Hierarchy of CHD
| 1 | HLHS |
| 2 | Truncus arteriosus |
| 3 | Non-HLHS single ventricle |
| 4 | Interrupted aortic arch |
| 5 | Pulmonary atresia |
| 6 | Total anomalous pulmonary venous return |
| 7 | Double outlet right ventricle |
| 8 | Transposition of the great arteries |
| 9 | Tetralogy of Fallot |
| 10 | Coarctation |
| 11 | Aortic stenosis |
| 12 | AV canal defects |
| 13 | VSDs |
| 14 | Pulmonary stenosis |
| 15 | ASDs |
1 is the highest on the hierarchy
Statistical analyses
Primary outcomes included birth prevalence of CHDs in premature infants, calculated as number of infants with CHD per 1000 births, and in-hospital mortality. Summary statistics were used to describe patient characteristics. Weighted chi square and t-tests were performed where appropriate. Weighted multivariable logistic regression was used to calculate adjusted odds ratios of mortality between very and extremely premature infants with and without CHD. For this analysis, neonates with CHD were assigned a single diagnosis based on our noted hierarchy of CHD diagnoses (Table II). In addition to diagnostic cohort, other model covariates were chosen a priori to represent a set of variables that were considered clinically relevant and included race, sex, GA in weeks, year, and the dichotomous variables: SGA status, and hospital teaching status. For regression analysis, CHDs with a sample size <30 were excluded to avoid misinterpreting the data. Diagnoses of tricuspid atresia and common ventricle were combined into a category called non-HLHS single ventricle. Statistical significance was defined as a p-value less than 0.05. All statistical analysis was performed in SAS 9.3, Cary, NC.
Results
An estimated 249,011 infants were born between 25 and 32 weeks gestational age, of which 28,806 (116 per 1000 birth hospitalizations of very/extremely premature infants) had CHDs. Of those infants with CHDs, 48.6% were female and 5.9% were SGA (Table III).
Table 3.
Characteristics of very and extremely premature infants with CHD (n= 28,806)
| Gestational Age (weeks) | n (%) |
|---|---|
| 25 to 26 | 4874 (16.9) |
| 27 to 28 | 6389 (22.2) |
| 29 to 30 | 7698 (26.7) |
| 31 to 32 | 9846 (34.2) |
| Female | 13982 (48.6) |
| Race | |
| White | 11398 (45.2) |
| Black | 6068 (24.1) |
| Hispanic | 5054 (20.1) |
| Asian & Native American | 1099 (4.3) |
| Other | 1579 (6.3) |
| SGA | 1705 (5.9) |
| Down Syndrome | 339 (1.2) |
| DiGeorge syndrome | 22 (0.1) |
Table IV summarizes the birth prevalence of various CHDs. ASDs were the most commonly diagnosed defect followed by VSDs and pulmonary stenosis. Severe CHDs had an aggregate birth prevalence of 7.4 (95% CI: 6.9, 7.9) per 1000 at-risk births, significantly higher than the birth prevalence in term neonates (1.5/1000 term birth hospitalizations, p<0.001). HLHS was the most common severe CHD followed by tetralogy of Fallot and coarctation of the aorta. The Figure compares birth hospitalization prevalence between very/extremely premature and term infants; 13 out of 14 defects had higher odds of diagnosis in very/extremely premature infants versus term infants but there was wide variability by defect with unadjusted odds ratios (OR) ranging from 6.2 for HLHS to 2.3 for transposition of the great arteries. Total anomalous pulmonary venous return had an OR of 0.3. In aggregate the birth prevalence of severe defects was significantly higher for infants born more prematurely (8.8 at 25 to 26 weeks GA vs 6.7 at 31 to 32 weeks per 1000 birth hospitalizations, p=0.005). There was no significant change in birth prevalence by GA for any single severe defect; however these analyses were underpowered.
Table 4.
Birth Prevalence of CHD per 1000 very/extremely premature births (n=249,011 very/extremely premature births with GA 25 to 32 weeks)
| n | Birth Prevalence (95% CI) | |
|---|---|---|
| All simple defects | 27681 | 111.2 (105.5, 116.8) |
| ASD | 23299 | 93.6 (88.1, 99.1) |
| VSD | 4514 | 18.1 (17.3, 18.9) |
| Pulmonary stenosis | 1771 | 7.11 (6.32, 7.91) |
| All severe defects | 1846 | 7.41 (6.90, 7.93) |
| HLHS | 556 | 2.23 (1.95, 2.51) |
| Coarctation | 286 | 1.15 (0.98, 1.31) |
| TOF | 306 | 1.22 (1.06, 1.39) |
| AV Canal defect | 172 | 0.69 (0.56, 0.82) |
| Interrupted aortic arch | 102 | 0.41 (0.31, 0.51) |
| Aortic stenosis | 94 | 0.38 (0.29, 0.47) |
| Pulmonary atresia | 94 | 0.38 (0.28, 0.47) |
| Double outlet right ventricle | 87 | 0.35 (0.25, 0.44) |
| TGA | 84 | 0.34 (0.25, 0. 42) |
| Tricuspid atresia | 47 | 0.19 (0.12, 0.25) |
| Truncus Arteriosus | 62 | 0.25 (0.17, 0.33) |
| Ebstein anomaly | 41 | 0.16 (0.10, 0.22) |
| Common Ventricle | 35 | 0.14 (0.08, 0.20) |
| TAPVR | 27 | 0.11 (0.06, 0.16) |
| Any defect | 28806 | 115.7 (110.0, 121.4) |
Figure.
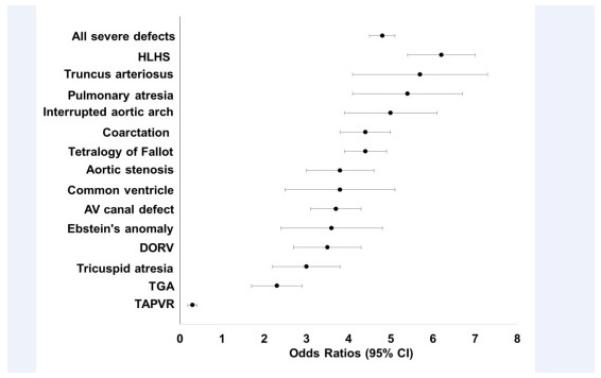
Odds of severe CHD in very/extremely premature infants versus term infants
For the outcomes assessment, we restricted the cohort to a subset of 49,893 hospitalizations for very/extremely premature infants in children’s hospitals or pediatric units of which 7060 (14%) had CHDs. Of these, 87 (1%) were excluded because they were associated with a diagnostic code for a lethal genetic syndrome or non-cardiac anomaly. The CHD cohort was significantly more likely to be born at earlier GAs when compared with very/extremely premature infants without CHDs (18.3% vs. 13.3% at 25-26 weeks, 23.5% vs. 17.4% at 27-28 weeks, 26.1% vs. 24.7% at 29-30 weeks GA, 32.2 vs. 44.6% at 31-32 weeks GA, p<0.001), have a diagnosis of Down’s syndrome (1.4 vs. 0.1%, p<0.001) and be born SGA (5.8% vs. 4.3%, p< 0.001). Otherwise very/extremely premature infants with and without CHDs were similar.
Collectively very/extremely premature infants with severe CHDs had a 26.3% in-hospital mortality rate versus 5.3% for very/extremely premature infants without CHDs (p<0.001) and 10.9% for term neonates with severe CHDs (p<0.001). However, there was a wide distribution by defect. For severe defects mortality rates ranged from 12% for coarctation of the aorta to 67% for truncus arteriosus. Simpler defects, such as ASDs and VSDs, had mortality rates (4% and 5% respectively) that were similar to those seen for very/extremely premature infants without CHD (Table V). In multivariable analysis, very/extremely premature infants with severe CHDs had a 7.5 fold higher adjusted odds of death (95% CI: 5.9, 9.6) than those without CHDs and a 3.4 (95% CI: 2.8, 4.1) fold higher adjusted odds of death than term infants with severe defects after controlling for race, sex, GA, year, SGA status, and hospital teaching status. Of those severe defects with adequate numbers for evaluation, all except interrupted aortic arch had significantly higher odds of dying in the hospital compared with very/extremely premature infants without CHDs (Table V). Specifically, DORV, pulmonary atresia, HLHS, non-HLHS single ventricle and TOF had the highest increase in odds of in-hospital mortality. Numbers for defect-specific mortality comparisons were insufficient for truncus arteriosus, aortic stenosis, D-transposition, Ebstein’s anomaly and total anomalous pulmonary pulmonary venous return (TAPVR). There was no difference in odds of death for very/extremely premature infants with VSDs, and those with ASDs or pulmonary stenosis had significantly lower odds of dying in the hospital compared with infants without CHDs (adjusted OR 0.60, 95% CI: 0.49, 0.73 for ASD and 0.45, 95% CI: 0.22, 0.93 for pulmonary stenosis).
Table 5.
Overall mortality and frequency of CHDs and adjusted odds ratios for mortality compared to very/extremely premature infants without CHD (n=6973)
| Mortality | ||
|---|---|---|
|
| ||
| % died (95% CI) | Adjusted OR (95% CI) | |
| DORV (n=43, 0.6%) | 52.6 (29.8, 75.4) | 17.5 (6.0, 51.0) |
| Pulmonary atresia (n=35, 0.6%) | 30.3 (9.1, 51.5) | 13.2 (4.1, 42.3) |
| Non-HLHS single ventricle (n=48 0.7%) | 29 (13.1, 44.6) | 10.5 (4.7, 23.3) |
| HLHS (n=207, 3.0%) | 31.1 (23.9, 38.2) | 9.8 (6.4, 15.1) |
| Tetralogy of Fallot (n=96, 1.4%) | 26.4 (16.9, 35.9) | 7.1 (4.0, 12.4) |
| AV canal defect (n=42, 0.6%) | 25.5 (10.7, 40.3) | 6.6 (2.2, 19.8) |
| Coarctation (n=139, 2.0%) | 11.6 (4.3, 19.0) | 2.7 (1.2, 6.0) |
| VSD (n=1059, 15.2%) | 5.2 (3.4, 7.0) | 0.8 (0.5, 1.2) |
| Interrupted aortic arch (n=47, 0.7%) | 12.8 (1.0, 24.7) | 2.9 (0.8, 11.0) |
| ASD (n=4834, 69.3%) | 4.3 (3.5, 5.1) | 0.6 (0.5, 0.7) |
| Pulmonary stenosis (n=323, 4.6%) | 2.8 (0.8, 4.8) | 0.5 (0.2, 0.9) |
| Truncus arteriosus+ (n=22, 0.3%) | 67.4 (5.7, 59.4) | - |
| TAPVR+ (n=19, 0.3%) | 35.3 (5.7, 65.0) | - |
| Aortic stenosis+ (n=17, 0.2%) | 19.7 (0.0, 43.5) | - |
| TGA+ (n=24, 0.3%) | 19.0 (0.2, 37.8) | - |
| Ebstein anomaly+ (n=17, 0.2%) | 17.2 (13.0, 80.9) | - |
| Any severe defect (n=758, 10.9%) | 26.3 (22.7, 29.9) | 7.5 (5.9, 9.6) |
| No congenital heart defect | 5.3 (4.9, 5.8) | Reference |
Adjusted for GA, sex, race, SGA, Downs syndrome, hospital teaching status, year
Regression analysis was not performed for defects with sample size < 30
Discussion
In this analysis of very and extremely premature infants born 25 to 32 weeks GA, the overall birth prevalence of CHDs (116/1000 births of similar GA) was significantly higher than the reported birth prevalence of CHDs in term neonates (6-10/1000 births) 1–5. Importantly, the subset of severe CHDs were almost five-fold more likely in very/extremely premature neonates when compared with term neonates, and very/extremely premature neonates with severe CHDs had a more than seven-fold increased odds of mortality when compared with very/extremely premature neonates without CHD.
qThe overall high prevalence of CHDs in very/extremely premature infants is partly driven by a high prevalence of ASDs which are undoubtedly more commonly diagnosed in premature infants due to the increased likelihood of receiving an echocardiogram1–5. However, echocardiography utilization practices should minimally impact the prevalence of severe CHDs as these defects would be expected to be almost universally diagnosed during the neonatal period. In our cohort, the birth prevalence of severe defects in very/extremely premature infants was 7.4/1000 birth hospitalizations compared with 1.5/1000 birth hospitalizations in term infants. Other epidemiological studies of severe CHDs in term infants have reported slightly higher prevalence than our analysis (~2 per 1000 live births)4,19. It is possible that we underestimated the true birth prevalence of severe CHDs in term infants as we restricted the analysis to birth hospitalizations. Nonetheless these relatively smaller differences would not change our overall conclusion that severe CHDs are strongly associated with very/extremely premature birth. This conclusion is consistent with the findings of Laas et al who demonstrated that European children with CHDs (excluding ASDs and PDAs) were twice as likely to be born prematurely (<37 weeks GA) or very/extremely prematurely (<32 weeks GA) than children without CHDs20. Importantly in our analysis the relative prevalence of severe CHDs was even higher at earlier GAs.
We also found wide variation in the odds of having individual defects when comparing premature and term infants. Again there are similarities between our data and the findings of Laas et al who demonstrated that the subset of single ventricle anomalies were most strongly associated with preterm birth while pulmonary venous anomalies were the only defects with higher birth prevalence in term neonates20. Interestingly we also found that pulmonary venous anomalies were more common in term neonates and we had adequate sample size to look at specific subsets of single ventricle anomalies, finding that HLHS was most strongly associated with very/extreme premature birth. It is not clear why these differences exist but we speculate that they reflect differences in each defect’s impact on fetal physiology.
Few studies have examined mortality of very and extremely premature infants across the spectrum of CHDs. As expected, these infants had markedly higher in-hospital mortality rates compared with those without CHDs (adjusted odds ratio of mortality = 7.5). The overall mortality rate of 26.3% that we report is actually lower than the 44% - 55% in-hospital mortality rates reported in two previous database studies focused on very low birth weight neonates (< 1500 grams) with severe CHDs12,21. Some of these differences may reflect that our study included patients with birth weight > 1500 grams and that our outcomes analysis was restricted to a subset of hospitals with more experience caring for children with heart disease (i.e. excluding community hospitals). It should be noted that all of these studies (including ours) report in-hospital mortality rates only and therefore will underestimate infant mortality related to CHDs. Despite this, it is clear that severe CHDs are an important risk factor for mortality with odds of mortality varying from approximately 3-fold higher for the arch anomalies (coarctation and interrupted aortic arch) to greater than 10-fold higher for more complex defects (DORV, single ventricle defects and pulmonary atresia). Unexpectedly we found that having a diagnosed ASD or pulmonary stenosis was associated with improved survival. This finding requires further study. Plausibly one could speculate that ASDs might offer a survival benefit in very/extremely premature infants with pulmonary hypertension. However this hypothesis is contrary to several prior reports suggesting that larger ASDs actually exacerbate underlying chronic lung disease of prematurity22,23. It is unclear why isolated pulmonary stenosis may confer an improvement in survival. An alternate hypothesis is that there is a survival bias in our study as the most critically ill preterm neonates, including those that cannot be successfully resuscitated in the delivery room, may not undergo echocardiography.
When evaluating mortality by GA for severe CHD, greater prematurity was associated with markedly higher mortality (40% at 25-26 weeks GA vs 22% at 31 to 32 weeks GA, p=0.036). These data highlight the substantial differences that small increments of prematurity can have on outcomes in neonates with CHD, and may influence future decisions surrounding surgical interventions, medical management, and palliative care. Additionally, these mortality rates underscore the need for targeted prenatal care in fetuses with known high risk CHDs to delay delivery to the latest possible GA.
Our study has several limitations. The KID is an administrative database of only inpatient stays that relies on provider ICD-9-CM coding and is prone to error. Diagnostic codes may not be accurate which could result in misclassification biases that may affect our birth prevalence and outcomes data. Similarly GA and other important model covariates were based on ICD-9-CM codes. With respect to GA, the method of dating is not known and we cannot verify accuracy. We are not aware of any previous studies that have validated the use of ICD-9-CM codes for GA by week in very and extremely premature infants and it is possible that inaccurate coding may bias our data. Most other model covariates are typically well coded administrative variables and should be accurate (i.e. race, sex, year of birth, hospital teaching status) with the notable exception of SGA status which was less prevalent in our analysis (5.9%) than has been reported in prior studies and could reflect suboptimal coding of this variable by providers. Also important to note, data outside of the hospitalization are not captured by the KID and readmissions/transfer data are not tracked across patients; therefore, for our epidemiologic analysis, we are missing information on neonates who were diagnosed outside of a birth hospitalization or who were undiagnosed until after transfer to a congenital heart center. Similarly, for our outcomes analysis, we may underestimate mortality by “double counting” patients readmitted in the first month of life. These factors likely impact the birth prevalence and outcomes of CHDs in term infants but are less of a concern for the premature cohort because these neonates typically have an extended birth hospitalization. Term neonates are also likely less accurately classified because our definition of “term” relied upon a diagnosis of exclusion (i.e. no documented ICD9 prematurity code). Additionally, mortality rates represent in-hospital mortality only and do not account for mortality in infants sent home with palliative care, infants who died outside of the hospital after discharge, or who died before being transferred to a more advanced pediatric facility for care. We also were unable to ascertain the cause of death including those that may have had an operative mortality. Another limitation is the lack of maternal risk factor data in the KID which prevented inclusion of these variables as covariates in the regression model to calculate the adjusted odds of death. Thus, we could not account for possible confounding variables such as smoking or diabetes status that are both associated with increased probability of death and CHD.
Providers should target interventions aimed at decreasing the likelihood of premature delivery for patients where a CHD is diagnosed in utero as our data highlight that outcomes are substantially improved if delivery can be delayed even by only a few weeks. Future directions include evaluating prematurity-related comorbidities in very and extremely premature infants with CHD and evaluating outcomes after surgical interventions.
Acknowledgments
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (1TL1TR001116 [to P.C.] and UL1TR001117 [to J.L., C.H., K.H.]). K.H. receives funding from the Gilead Cardiovascular Scholars Research Program and is a consultant for Kowa Pharmaceuticals.
Abbreviations
- ASD
atrial septal defect
- CHD
congenital heart defects
- DORV
double outlet right ventricle
- GA
gestational age
- HLHS
hypoplastic left heart syndrome
- IAA
interrupted aortic arch
- KID
Kids’ Inpatient Database
- PDA
patent ductus arteriosus
- SGA
small for gestational age
- TAPVR
Total anomalous pulmonary venous return
- TGA
transposition of the great arteries
- VSD
ventricular septal defect
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807–13. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PKA, Melbye M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J. 2009;157:467–73. doi: 10.1016/j.ahj.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Khoshnood B, Lelong N, Houyel L, Thieulin A-C, Jouannic J-M, Magnier S, et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population-based study. Heart. 2012;98:1667–73. doi: 10.1136/heartjnl-2012-302543. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardio. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Curzon CL, Milford-Beland S, Li JS, O’Brien SM, Jacobs JP, Jacobs ML, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008;135(3):546–51. doi: 10.1016/j.jtcvs.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 7.Ades A, Johnson BA, Berger S. Management of low birth weight infants with congenital heart disease. Clin Perinatol. 2005;32(4):999–1015. x–xi. doi: 10.1016/j.clp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod DM, Chock VY, Reddy VM. Management of the Preterm Infant with Congenital Heart Disease. Clin Perinato. 2016;43(1):157–71. doi: 10.1016/j.clp.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Birth before 39 weeks’ gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126(2):277–84. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 10.Costello JM, Pasquali SK, Jacobs JP, He X, Hill KD, Cooper DS, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129(24):2511–7. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cnota JF, Gupta R, Michelfelder EC, Ittenbach RF. Congenital heart disease infant death rates decrease as gestational age advances from 34 to 40 weeks. J Pediatr. 2011;159(5):761–5. doi: 10.1016/j.jpeds.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AW, Smith PB, Corey KM, Hill KD, Zimmerman KO, Clark RH, et al. Clinical outcomes in very low birth weight infants with major congenital heart defects. Early Hum Dev. 2014;90(12):791–5. doi: 10.1016/j.earlhumdev.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dees E, Lin H, Cotton RB, Graham TP, Dodd DA. Outcome of preterm infants with congenital heart disease. J Pediatr. 2000;137(5):653–9. doi: 10.1067/mpd.2000.108568. [DOI] [PubMed] [Google Scholar]
- 14.Polito A, Piga S, Cogo PE, Corchia C, Carnielli V, Da Frè M, et al. Increased morbidity and mortality in very preterm/VLBW infants with congenital heart disease. Intensive Care Med. 2013;39:1104–12. doi: 10.1007/s00134-013-2887-y. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HH, Almodovar MC, Laussen PC, Wypij D, Polito A, Brown DW, et al. Outcomes and risk factors for mortality in premature neonates with critical congenital heart disease. Pediatr Cardiol. 32:1139–46. doi: 10.1007/s00246-011-0036-3. 201. [DOI] [PubMed] [Google Scholar]
- 16.Lynema S, Fifer CG, Laventhal NT. Perinatal Decision Making for Preterm Infants with Congenital Heart Disease: Determinable Risk Factors for Mortality. Pediatr Cardiol. 2016;37:938–45. doi: 10.1007/s00246-016-1374-y. [DOI] [PubMed] [Google Scholar]
- 17.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 2013;132:62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 19.Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–9. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 20.Laas E, Lelong N, Thieulin A-C, Houyel L, Bonnet D, Ancel P-Y, et al. Preterm birth and congenital heart defects: a population-based study. Pediatrics. 2012;130:e829–37. doi: 10.1542/peds.2011-3279. [DOI] [PubMed] [Google Scholar]
- 21.Archer JM, Yeager SB, Kenny MJ, Soll RF, Horbar JD. Distribution of and mortality from serious congenital heart disease in very low birth weight infants. Pediatrics. 2011;127:293–9. doi: 10.1542/peds.2010-0418. [DOI] [PubMed] [Google Scholar]
- 22.Thomas VC, Vincent R, Raviele A, Diehl H, Qian H, Kim D. Transcatheter closure of secundum atrial septal defect in infants less than 12 months of age improves symptoms of chronic lung disease. Congenit Heart Dis. 2012;7:204–11. doi: 10.1111/j.1747-0803.2010.00442.x. [DOI] [PubMed] [Google Scholar]
- 23.Wood AM, Holzer RJ, Texter KM, Hill SL, Gest AL, Welty SE, et al. Transcatheter elimination of left-to-right shunts in infants with bronchopulmonary dysplasia is feasible and safe. Congenit Heart Dis. 2011;6:330–7. doi: 10.1111/j.1747-0803.2011.00540.x. [DOI] [PubMed] [Google Scholar]


