Abstract
Background
Extended-release naltrexone (XR-NTX), is an effective treatment for opioid use disorder but is rarely initiated in US prisons or with criminal justice populations. Mobile treatment for chronic diseases have been implemented in a variety of settings. Mobile treatment may provide an opportunity to expand outreach to parolees to surmount barriers to traditional clinic treatment.
Methods
Male and female prisoners (240) with pre-incarceration histories of opioid use disorder who are within one month of release from prison will be enrolled in this randomized clinical trial. Participants are randomized to one of two study arms: 1) [XR-NTX-OTx] One injection of long-acting naltrexone in prison, followed by 6 monthly injections post-release at a community opioid treatment program; or 2) [XR-NTX+ MMTx] One injection of long-acting naltrexone in prison followed by 6 monthly injections post-release at the patient's place of residence utilizing mobile medical treatment. The primary outcomes are: treatment adherence; opioid use; criminal activity; re-arrest; reincarceration; and HIV risk-behaviors.
Results
We describe the background and rationale for the study, its aims, hypotheses, and study design.
Conclusions
The use of long-acting injectable naltrexone may be a promising form of treatment for pre-release prisoners. Finally, as many individuals in the criminal justice system drop out of treatment, this study will assess whether treatment at their place of residence will improve adherence and positively affect treatment outcomes.
Keywords: prisoners, long-acting naltrexone, medical mobile treatment, criminal justice
1. Introduction
1.1. Opioid use disorder among jail and prison inmates
Opioid use disorders (OUDs) are a severe problem among jail and prison inmates. Inmates in the US, Canada, Australia, and many European and Asian nations have disproportionately higher rates of opioid use disorders than their general populations.1-5 In the US, there are over 1.5 million state and federal prisoners,6 of whom an estimated 12-15% have histories of OUD.7 Moreover, scarce resources are provided for corrections-based substance use treatment in many nations, and many inmates with OUDs remain untreated;5,8 and less than .1% receive agonist treatment.1,4,5,9,10 As a consequence, opioid use either continues or resumes rapidly after release from incarceration,1,5,11 placing newly released inmates at risk for death from drug overdose12-19 and for infections with human immunodeficiency virus (HIV) and hepatitis B and C.1,3,20 Opioid use among newly released inmates also has adverse public safety consequences, as it typically results in increased criminal activity20-22 and reincarceration.21,23,24
1.2. Opioid agonist pharmacotherapy in jail and prison settings
There is a growing body of evidence supporting the effectiveness of opioid agonist (methadone and buprenorphine) as compared to opioid antagonists (oral NTX, XR-NTX) pharmacotherapy in jail and prison settings for both inmates who were using opioids at initiation of maintenance treatment23,25-30 and inmates who were previously, but not currently, opioid-dependent.31-35 However, many American prison and jail administrators remain reluctant to offer opioid agonist pharmacotherapy in their facilities, largely because of their preference for drug-free interventions36-40 and concerns about diversion of medication, especially with buprenorphine.41
1.3. Long-Acting Naltrexone (XR-NTX)
The use of long-acting injectable naltrexone may be a promising form of treatment for pre-release prisoners. Naltrexone blocks the intoxicating and reinforcing effects of opioids, but has no opioid-like effects. When taken regularly, it reduces opiate-taking behavior. Naltrexone for extended-release injectable suspension, (XR-NTX) is supplied as a microsphere formulation of naltrexone for suspension and is to be administered by intramuscular (IM) gluteal injection every 4 weeks (once a month). In 2010, it was approved for the prevention of relapse to opioid dependence, following opioid detoxification. Administered as a monthly injection, XR-NTX eliminates the need for adherence to daily oral therapy, and thus has the potential to improve clinical outcomes for this indication. Moreover, monthly administration avoids the daily plasma concentration fluctuations associated with daily oral administration of naltrexone and its major metabolite, 6β-naltrexol. Its lower frequency of administration, the fact it has no opioid-like effects, and cannot be diverted by patients may make XR-NTX more acceptable to corrections officials than methadone or buprenorphine. The primary reason for failure of oral naltrexone treatment for both opioid addiction and alcoholism has been failure on the part of patients to adhere to the daily medication regimen.42,43 Long-acting naltrexone reduces the adherence problem as confirmed by studies showing blockade of injected opiates for over 30 days. Importantly, a sustained release medication may protect participants from overdose death within the critical one month post-release period.12,18 Moreover, because naltrexone has no abuse potential, and is not a controlled substance, there is greater flexibility in settings in which naltrexone can be prescribed, including correctional settings. Moreover, controlled environments offer an excellent opportunity to initiate long-acting, injectable naltrexone because individuals with OUD have a higher likelihood of being abstinent from opioids for the required length of time in the controlled correctional environment prior to initiating naltrexone treatment. Extended-release injectable naltrexone (XR-NTX)) has been found effective in reducing opiate use compared to control participants for community corrections populations in the US;44-46 jail inmates in the US47 and for Russian adults with heroin use disorder.48 Results from Russia are especially noteworthy given that in a nation with one of the highest rates of heroin use in the world, methadone and buprenorphine are not available.48
1.3.1. Long-acting naltrexone with criminal justice populations
Gordon et al.49 conducted a Phase 4 pilot, open-label study of long-acting injectable naltrexone (XR-NTX), with prisoners with pre-incarceration OUD. In this study, involving one XR-NTX injection in prison followed by 6 monthly injections in the community, XR-NTX was found to be feasible; all 27 participants received their first injection. XR-NTX was acceptable to correctional officials; it did not disrupt security and other prison routines and there was no concern about its diversion. However, an important challenge was that while 78% of participants received their first community injection, only 37% received their 5th and 6th injections. In a multi-site study of parolees/probationers, 308 participants were randomized to XR-NTX across five sites in which 95% received the first injection; 65% received their 5th and 59% received their 6th injections. XR-NTX adherence rates were higher in parolees and probationers and roughly equal to opioid agonist treatment but still dropped off by month 6.45 Also, most parole/probation participants were recruited from community treatment programs and were nearing completion of treatment at study entry, which meant that they were more likely to have more stable lives than newly released prisoners. These results and many stressors faced by newly released prisoners, noted below, suggest that enhancements to prison initiation of XR-NTX are needed in order to ensure continued adherence to XR-NTX treatment. Many of our XR-NTX prisoners left treatment because of the need for stable housing, legitimate employment, child care, securing health benefits, addressing medical and psychiatric issues, and meeting requirements regarding criminal justice supervision interfered with continued treatment.
1.4. Mobile treatment for chronic diseases
Mobile treatment for chronic diseases have been implemented in a variety of settings. Mobile treatment provides an opportunity to expand outreach to surmount barriers to traditional clinic treatment for chronic disease. A number of programs have implemented mobile services including for opioid addiction using LAAM,50 methadone,51-53 HIV education and testing,54 HIV treatment,55 for mental health services56 and for cancer information and support.57,58 In two MAT studies that are most relevant to the current trial which implemented LAAM and Methadone, findings were supportive of mobile treatment with a risky opioid dependent population. In the Kou et. al.50 study out of 163 referrals to mobile LAAM treatment, 114 (70%) entered the program, 84% were retained for at least 90 days, and a 31% reduction in heroin-positive urine tests was reported. More importantly, the Greenfield et al.52 study indicated patients in the methadone mobile treatment group were retained in treatment for a median of 15.5 months compared to a median of 3.9 months for the patients at the fixed sites – and it is well known that, regardless of type of treatment, greater treatment duration is associated with reduced substance use and criminal activity.
As emphasized by Hall et al.,53 we need additional strategies that state and local governments can use to increase opioid treatment participation by broadening its reach to different types of patients such as pre-release prisoners, community corrections populations which are often socially disenfranchised and have high opioid use. Furthermore, the stigmatization of methadone and the difficulty for certain individuals to enter and continue treatment based on restrictions for individuals in the criminal justice system38,39,59 might make medical long-acting treatment more appealing to criminal justice professionals that have to monitor the movement and activities of parolees and home detention individuals in the community.
2. Methods
2.1 Study design
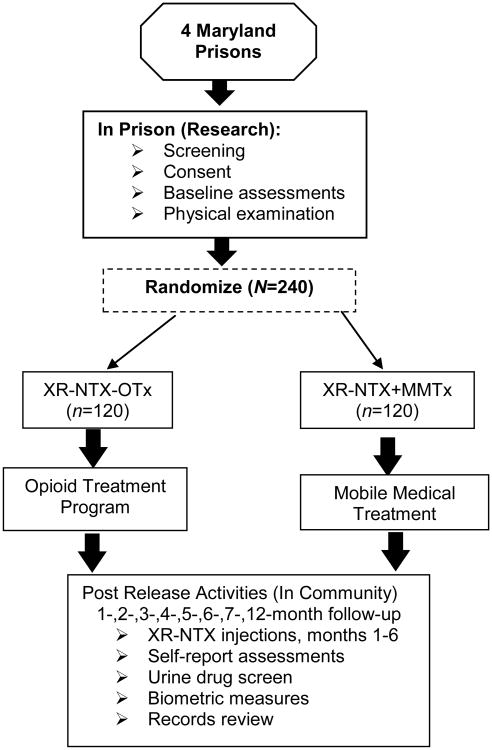
The study is a parallel two-group randomized controlled trial in which 240 incarcerated men and women will be randomly assigned within gender to one of two conditions: XR-NTX-OTx: One injection of XR-NTX in prison, followed by 6 monthly injections post-release at a community opioid treatment program (n=60 men and 60 women); or 2) XR-NTX+ MMTx: One injection of XR-NTX in prison followed by 6 monthly injections post-release at the patient's place of residence utilizing mobile medical treatment (n=60 men and 60 women) (see Figure 1). All participants will be confirmed opioid-free prior to long-acting injection, and evaluated at 1-, 2-, 3-, 4-, 5-, 6-, 7- (safety visit) and 12-months after release from prison.
Fig. 1.
Study design. XR-NTX= extended release naltrexone; OTx=opioid treatment program; MMTx=mobile medical treatment.
2.2 Research questions, outcomes and hypotheses
The primary research question is to compare the two study conditions in terms of: a) XR-NTX treatment adherence; b) opioid use; c) criminal activity; d) re-arrest; e) reincarceration; and f) HIV risk-behaviors (i. needle use; and ii. risky sexual behaviors).
The secondary research question is to determine if the number of months of post-release XR-NTX treatment is related to outcomes (a)-(f) (see above), and if so, is there a point at which XR-NTX-OTx v. XR-NTX-MMTx treatment success equilibrates. Such a finding could be potentially important because it would be informative about the needed length of XR-NTX treatment.
Based on preliminary data from the research team's current studies that individuals receiving long-acting naltrexone at a community treatment or opioid clinic, adherence rates begin to decrease by month four,32,49,60 it is anticipated XR-NTX+MMTx condition will have superior outcomes compared to the XR-NTX+OTx condition in terms of outcomes: a) XR-NTX treatment adherence; b) opioid use; c) criminal activity; d) re-arrest; e) reincarceration; and f) HIV risk-behaviors (i. needle use; ii. risky sexual behaviors). We are not aware of RCTs using mobile medical treatment for the provision of XR-NTX. However, based on studies utilizing mobile treatment for HIV services, and methadone, which increased access, engagement, and retention, we believe the provision of medical treatment, provided at a patient's place of residence, is expected to yield better adherence, subsequently improving outcomes mentioned above. If we find a point of equilibration it will tell us how many months we will need to provide mobile treatment (XR-NTX injections) and at what point it impacts on outcomes-significant given the cost of the medication. It is difficult to provide firm conjectures on this latter question given the lack of research in this area. However, based on our previous experience (cited above) there are many barriers that prisoners face as they reenter society such as employment, housing, child care issues, mental health issues, and transportation which may impede beginning and continuing treatment. By addressing these barriers by providing treatment at place of residence may assist in reducing the adherence barrier.
2.3 Study sites
2.3.1. Prisons
Male and female inmates recruited for participation will be drawn from four prisons in Baltimore City and Baltimore County. The five facilities are administered by the Maryland Division Department of Correction (DOC), and are staffed by administrative and custodial (correctional officers) personnel and by case managers, who provide referral services and are responsible for preparing reports concerning inmates' institutional progress and adjustment. The Maryland Department of Public Safety and Correctional Services (MDPSCS) and the study PI have an extensive history of collaborating on pharmacotherapy studies for opioid-dependent prisoners.
2.3.2. Community treatment clinic
Long-acting naltrexone will be provided by Glenwood Life Counseling Center (GLCC) personnel, either at GLCC or the participant's place of residence. Glenwood Life Counseling Center (GLCC) has been in continuous operation for 41 years. It is a State of Maryland- and CARF-certified outpatient drug treatment program that treats over 600 patients. GLCC provides individual and group drug abuse counseling, HIV assessment and risk-reduction counseling, and a limited amount of family-based therapy.
2.4 Inclusion/Exclusion criteria
Eligible prisoners must meet the following criteria: (1) adult male or female inmate at one of the four designated prisons who will be eligible for release within 30 days; (2) history of opiate use disorder [meeting DSM-5 criteria at the time of incarceration]; (3) suitability for XR-NTX treatment as determined by medical evaluation; (4) currently opioid-free by history, with negative urine for all opioids and no signs of opiate withdrawal at recruitment/study entry; (5) willingness to enroll in XR-NTX treatment in prison [not currently in or planning to pursue agonist (methadone, buprenorphine) treatment at release]; and (6) planning to live in Baltimore City. Inmates not meeting the OUD criterion will be eligible if they were treated in an opioid agonist treatment program during the year before incarceration.
Prisoners with one or more of the following conditions will be excluded from the study: (1) Active medical illness that may make participation hazardous (e.g., unstable diabetes, heart disease). Adequately treated medical conditions are acceptable; (2) Untreated psychiatric disorder that may make participation hazardous (e.g., untreated psychosis, bipolar disorder with mania). Adequately treated psychiatric disorders and appropriate psychotropic medications will be allowed; (3) History of allergic reaction to XR-NTX; (4) Current chronic pain diagnosis for which opioids are prescribed; (5) creatinine above normal limits; (6) pregnancy (for women); (7) suicidal ideation (within the past 6-months); (8) Body Mass Index (BMI) > 40 and/or (9) unadjudicated charges that may result in transfer to another facility and/or additional prison time. Individuals who would not have recent suicidal ideation (past 6 months) and have psychiatric conditions that are treated will be included
2.5 Recruitment, informed consent, screening, randomization
The study will employ the following procedures. Maryland Department of Correction (DOC) personnel will schedule project orientation appointments with research staff for the Baltimore area prison inmates with less than 60-90 days to serve. Group orientation sessions will be conducted at each prison in a private room, in which a research assistant (RA) will explain study procedures and eligibility requirements. Potentially interested inmates will then meet individually with research staff in a private room for an in-depth discussion of the purposes, procedures, risks, and benefits, of study participation, and to make a preliminary determination of eligibility, subject to confirmation during the physical examination (see below). Immediately after providing informed consent and completing a release of information form (ROI), each potential participant is scheduled for administration of the baseline measures. Following baseline assessment, which is used, in part, to confirm eligibility regarding histories of opioid addiction and nature and severity of medical and psychiatric problems, each potential participant will meet with the project medical staff for a medical history, physical examination, and laboratory tests to confirm eligibility and suitability for long-acting naltrexone administration, and to discuss the potential risks and benefits of study participation. Individuals who do not meet medical eligibility based on physical examination or do not wish to initiate long-acting naltrexone following a discussion of this treatment with the study physician will not be enrolled into the study. Potential participants who are deemed medically eligible and remain interested in participating in the study will then be randomly assigned to one of the two treatment conditions. Finally, because of the possibility of coercion when working with prisoners, the study RA will emphasize that the decision to participate or not will not affect the prisoner's status, institutional privileges, or release date.
Participants will be assigned to one of two conditions (XR-NTX-OTx or XR-NTX+MMTx) using a random permutation procedure, such that, within gender, for each block of 2, 4, or 6 participants, half will be assigned at random to the XR-NTX-OTx Condition and half to the XR-NTX+MMTx condition, ensuring that both male and female participants have an equal chance of being assigned to either condition. Random block sizes will be used in order to thwart any attempt by an interested observer, such as a staff member, to deduce the random assignment procedure.62 (Sealed envelopes will be prepared for the study physician based on this block randomization procedure so that he can explain the condition to which a participant has been assigned. He will open the designated envelope and inform the participant to which one of the two conditions s/he has been assigned. This assignment procedure will be performed by the study physician so that, immediately after assignment to treatment condition, consent to medication initiation can be obtained from participants.
2.6 Data management
RAs complete baseline study assessments and follow-up assessments using direct data entry or on paper (based on internet access in the prison). Any forms with paper responses from the participant will be uploaded to the study site Data Management Unit within 48 hours.
3. Regulatory Affairs and Data and Safety Monitoring
3.1 Approvals and certification
The Friends Research Institute's Institutional Review Board (IRB) approved the study. The US Office of Human Research Protections (OHRP) also approved the study protocol. The study was registered at ClinicalTrials.gov (NCT NCT02867124). A federal Certificate of Confidentiality (CoC) was obtained to protect the confidentiality of the participants' data. In addition, we received approval from the Maryland Department of Public Safety and Correction Services (MDPSCS) Research Committee. It should be noted that the MDPSCS does not have an IRB.
3.2 Data and safety monitoring
The study is being monitored by a Data and Safety Monitoring Board (DSMB). The Friends IRB, DSMB, and NIDA (the study sponsor) monitor recruitment, retention, and study safety. All Serious Adverse Events are reported to the IRB, DSMB, and NIDA medical monitor regardless of their possible relationship to study procedures.
4. Interventions
4.1. Pre-release and post-release extended- release XR-NTX
Participants will receive one injection prior to release. Long-acting, injectable naltrexone will be administered by intramuscular injection to the buttocks (alternating sides monthly), at a volume of 4cc (380mg of naltrexone), Long-acting naltrexone has the advantage of being FDA-approved for treatment of alcohol and opioid dependence, hence commercially available with strong safety data. Participants who deny opioid use in the past 10 days and who provide a specimen that tests negative for opioids on an instant urine test will receive a naloxone challenge test. Naloxone is a short-acting opioid antagonist. This test consists of an intravenous (or intramuscular for participants' without venous access) injection of a 0.8 mg of short-acting naloxone followed by a 20-minute observation period. A positive test will cause the temporary (up to 40 minutes) appearance of opioid withdrawal symptoms. Following a negative naloxone test, participants will be administered a low dose of oral naltrexone (12.5 mg) to further determine whether they will be able to tolerate depot naltrexone. If the participant has withdrawal symptoms in response to either the naloxone test or the oral naltrexone dose, the study physician will treat these symptoms with other medications. The medical staff of all five prisons are highly experienced in treating opioid withdrawal, will be fully aware of the ongoing study, and will be able to provide symptomatic treatment to participants in the unlikely event that it is needed when study staff are not on the premises. Such issues may consist of the following: 1) withdrawal; and/or 2) injection site reaction. Prior to discharge, participants will receive an information card about naltrexone to carry with them at all times which will alert any medical providers about the characteristics of naltrexone. One week before anticipated release from prison, each participant will have an exit interview with the study's RA. The RA will provide each such participant with a card with the address of GLCC outlining their schedule for their six monthly injections in the community. In addition, those randomized to XR-NTX+MMTx will receive a pamphlet from the study RA detailing the procedures and operations of receiving medication at their place of residence.
4.2 Study Arm:- XR-NTX-OTx
The participants randomized to XR-NTX-OTx will go to the community opioid treatment program only to receive injections under the direction of the study's medically responsible investigator and the study nurse from prison in order to ensure continuity of care for those receiving naltrexone in the community. The study RA will be in close contact upon release and will provide the participant with reminder calls and/or visits prior to each long-acting naltrexone appointment. Participants will also be encouraged to access individual and group drug abuse counseling, HIV assessment and risk-reduction counseling, and family-based therapy on an as needed basis.
4.3 Study Arm: XR-NTX+MMTx
For those randomized to receive XR-NTX at their place of residence, specific procedures will be implemented. First, the study nurse will be accompanied by the study RA so two staff members are always physically present. The study RA will confirm the place of residence prior to release from prison using our locator form. Contact will be made with the participant immediately following prison release and subsequently one week before each scheduled injection to verify his/her home address as many newly released inmates have difficulty acquiring stable housing. At the place of residence, the nurse will administer XR-NTX and follow the same procedures as they would at the clinic. Those participants randomized to the XR-NTX+MMTx condition will no longer receive XR-NTX if they become incarcerated in jail or prison (if incarcerated less than 37 days, they will still be eligible receive their injection). Participants who enter a semi-controlled environment, such as residential or inpatient treatment, will still be eligible to receive their injection if the program continues to allow them to receive it. We have fostered a relationship with many of the Baltimore City treatment programs and we will coordinate with them to continue to provide XR-NTX whenever possible. Individuals that are homeless or become homeless during the course of treatment will be offered the opportunity to receive XR-NTX at GLCC or a study field office. Based on our previous prison studies with medication we anticipate no more than 4-6 participants will be homeless. Participants in the XR-NTX+MMTx who require additional medical care will be referred by the study nurse/physician based on their level of need, similar to as they would at a standard outpatient opioid treatment program. Participants will also be encouraged to access individual and group substance use counseling, HIV assessment and risk-reduction counseling, and family-based therapy on an as needed basis.
5. Assessments
Assessment of participant characteristics and/or performance will involve a multidimensional set of instruments administered by trained research interviewers. Sources of information will include: (1) self-report; (2) official records; (3) urine drug screening results; and (4) treatment program records. Assessment at baseline will provide information on participant characteristics and pre-incarceration histories of substance use, substance use treatment, crime, incarceration, and HIV risk behavior (See Table 1). Participants in both conditions will be paid $50 for each follow-up visit ($400 total). Participants will not be paid for baseline assessments in prison due to the fact that such payments may be viewed as coercion.
Table 1. Data Collection Schedule.
| Measures | In Prison | Following Release from Prison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 mo | 2 mo | 3 mo | 4 mo | 5 mo | 6 mo | 7mo | 12mo | |
| XR-NTX adherence (clinic records) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| opioid use (ASI, TLFB, urine drug screen) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Criminal activituy (ASI, TLFB) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Re-arrest (official records) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Re-incarceration (official records) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| HIV risk behaviors (RAB) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Opioid overdose (OOS) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Opioid craving (VAS) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Biometric Measures (for inclusion/exclusion criteria, patient monitoring and AE determination) | |||||||||
| History & Physical | ✓ | ✓ | ✓ | ||||||
| Liver Function Tests, Hepatitis Profile | ✓ | ✓ | ✓ | ✓ | |||||
| Vital signs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Concommitant medication | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Pregnancy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HIV | ✓ | ✓ | ✓ | ||||||
| Urine toxicology | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Adverse Events | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
Note: XR-NTX=long-acting naltrexone; ASI=Addicition Severity Index; TLFB=Time line follow back; RAB=Risk Assessment Battery; OOS=Opioid Overdose Scale; VAS=Visual Analog Scale
5.1. XR-NTX Adherence
Data on post-release treatment status will be obtained from the community treatment program records. Treatment status will be measured at each follow-up point by whether or not a participant received an injection of XR-NTX at each follow-up point (yes v. no). We will also collect data on whether a person entered outpatient treatment (yes v. no) and the type of services utilized. Treatment duration for individuals who end treatment will be calculated based on the last date of clinic attendance (if a participant fails to receive his/her injection by day 37 s/he would be considered a drop-out). Thirty seven days is used in our current XR-NTX studies as this is how long the medication typically protects the participant. We will also collect data on whether or not patients entered other types of substance use treatment. Follow-up assessments will collect self-report data on reasons why participants entered, did not enter, or dropped out.
5.2. Addiction Severity Index (ASI) with Timeline Follow-Back (TLFB)
The ASI is a standardized 40-60 minute clinical research instrument widely used in addiction research to quantify problem areas of substance-using populations.61,62 This instrument has excellent inter-rater and test-retest reliability, as well as discriminant and concurrent validity61,62 We will also collect data on substance use frequency and criminal activity to cover the entirety of the follow-up periods post-baseline using a timeline follow-back procedure.
5.3. Risk Assessment of Battery (RAB)
This self-administered questionnaire, designed to identify individuals engaging in acts that could transmit HIV and other infectious diseases,63 contains 45-items consisting of two subscales: a drug risk subscale and a sexual behavior risk subscale, each with their own score, which also combined to yield an overall risk score.
5.4. Opiate Overdose Scale
This self-administered questionnaire will ask participants to report the number of opiate overdoses where they did and did not receive medical attention. The questionnaire, administered at baseline, will cover the period prior to the instant incarceration while the questionnaire follow-up in the community, will cover post-release months one-12.
5.5. Visual Analogue Scale (VAS)
Participants are asked to place a mark across the line at the point that corresponds to their immediate craving for opioids. Anchors included 0 mm—‘no cravings’ to 100 mm—‘most extreme cravings possible’.64,65 Participants will be assessed at baseline prior to randomization and at each follow-up visit and asked about peak cravings during the preceding 24 hours. Mean scores of opioid craving will be calculated at each follow-up.
5.6. Biological Assays
Urine will be tested on-site with CLIA waived QuikScreen cups using an immunochromatographic assay for rapid (2-5 minute) qualitative results based on SAMHSA-standard cutoffs for alcohol (20mg/dL or 0.02% BAC), amphetamine/methamphetamine (1,000 ng/ml), cannabis (50 ng/ml), cocaine/benzoylecgonine (150 ng/ml), opiates (2000 ng/ml), morphine (300 ng/mL), and oxycodone (100ng/mL). Fentanyl will be tested for forensic use only. In addition, we will test for methadone (300ng/ml) and buprenorphine (10ng/ml). Results will be used as outcome measures of heroin and other opioid use as well as to check on the validity of self-reported drug use information. Urine samples will not be obtained on the approximately 10% of participants who we expect to be re-incarcerated. Those participants in methadone or buprenorphine treatment who screen positive only on their respective treatment medication will be counted as negative for their urine drug screening results.
5.7. Biometric Measures
All participants will complete the following biometric measures for inclusion/exclusion criteria, patient monitoring, and serious adverse event (SAE) and adverse event (AE) reporting:1) history and physical; 2) liver function tests, hepatitis profile; 3) vital signs; 4) concomitant medications; 5) pregnancy, 6) HIV (baseline, 6,7), 7) Urine toxicology (all intervals); and 8) adverse events (all intervals).
5.8. Official record information on criminal activity and supervision
As in our previous research,66 official record data will be obtained from the MDPSCS for each follow-up period. Data will include type (e.g., charges involved) and number of arrests, convictions, and incarcerations; the number and length of time of each imposed disciplinary period; and reports of participants' behavior while under correctional supervision. Criminal record data will be used to assess the validity of self-report criminal activity data.
6. Outcomes
The primary outcomes are as follows: a) XR-NTX treatment adherence (number of injections completed); b) opioid use (number of opioid positive and self-report days); c) criminal activity (self-report days); d) re-arrest (yes vs. no and time to first arrest); e) reincarceration (yes vs. no); and f) HIV risk-behaviors (i. needle use; ii. risky sexual behaviors). In addition, we will attempt to determine if the number of months of post-release XR-NTX treatment is related to outcome (a-f above), and if so, is there a point at which XR-NTX v. Non-XR-NTX equilibrates (the time point at which the trajectories for use in the two treatment groups cross).
7. Statistical Analysis
A generalized linear mixed model will be used for the analysis of all outcomes. A Poisson distribution will be assumed for the count criterion variables (days of opioid, days of crime, and needle use and risky sexual behaviors). A binomial distribution will be assumed for the dichotomous criterion variables (positive opioid urine drug screen). We will use Cox regression models for time to re-arrest and re-incarceration criterion variables. All data will be analyzed on an intent-to-treat approach and imputation with an inclusive strategy that uses control variables will be used to estimate any missing data. Supplementary analyses will examine within treatment arm the frequency of each adverse event (AE), and opioid overdose.
7.1 Sample Size, Power, and Effect Size
We plan to recruit 240 participants and randomize them equally into each of the two study arms. Assuming α=05 and N=216 due to 10% attrition, an effect size of f2=.037 in the population associated with a Treatment Condition (Arm 1 vs. Arm 2) with effect sizes ranging from a minimum f2=.046 to a maximum f2=.072 would provide 80% power. These effect sizes fall toward the “small-to-medium” range, with f2=02 considered a “small” effect and f2 = .15 a “medium” effect.67
8. Design Considerations
We designed this effectiveness study based on the disparate injection adherence and retention differences seen in the Lee et al.45 and the Gordon et al.49 studies in which parolees and probationers had greater treatment adherence than newly-released prisoners, respectively. Thus, our interest was not in whether XR-NTX worked in this population, but to address the unanswered question of whether mobile medical services (XR-NTX) will increase medication adherence in the newly-released population, thereby reducing opioid use, HIV risk behaviors, re-arrest, and reincarceration.
9. Conclusion
Initiating clinical trials in prisoner populations involves several additional steps. The IRB must contain a prisoner advocate, the protocol must also be approved by the OHRP, a federal Certificate of Confidentiality should be obtained, and the cooperation and approval of the state department of corrections and prison personnel is necessary. Thus, implementing such treatment within a correctional setting is far from an easy task. It is important that treatment, corrections, and research personnel need to collaborate continually to develop, implement, and evaluate such new interventions effectively.68 It's crucial that all agencies agree on the basic design and implementation of the study, particularly details regarding logistics and space, and ensuring that study intervention, recruitment, and assessment do not interfere with ongoing routines at the prison. Such studies are an important step in introducing therapies for OUD in prisoners and newly paroled prisoners re-entering society. Finally, it is recommended that researchers, treatment providers, and corrections officials should not be limited to reporting outcomes on the efficacy and effectiveness of their interventions, but on the unique challenges they faced and how they overcame these barriers and obstacles. These efforts are valuable in that they serve as a guide for subsequent corrections-treatment-research partnerships. Although research, treatment, and corrections agencies personnel may have different priorities and agenda, they can agree that opioid addiction and its adverse consequences are serious public health problems that can be reduced with careful planning and collaboration. The use of long-acting injectable naltrexone may be a promising form of treatment for pre-release prisoners. Finally, as many individuals in the criminal justice system drop out of treatment, this study will assess whether treatment at their place of residence will improve adherence and positively affect treatment outcomes.
Acknowledgments
We would like to thank the Maryland Department of Public Safety and Correctional Services (DPSCS) and Ms. Sandra Davis-Hart, Director of Treatment for DPSCS and Glenwood Life Counseling Center (GLCC). In addition, we would like to thank Dr. Tish Wiley, NIDA.
Funding: This study is being funded by the National Institute on Drug Abuse grant number 1R01DA040636-01 (PI: Gordon).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dolan K, Khoei EM, Brentari C, Stevens A. Prisons and drugs: A global review of incarceration, drug use and drug services. Oxford: Beckley Foundation; 2007. p. 12. [Google Scholar]
- 2.Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101(2):181–191. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- 3.Kanato M. Drug use and health among prison inmates. Current opinion in psychiatry. 2008;21(3):252–254. doi: 10.1097/YCO.0b013e3282fc985c. [DOI] [PubMed] [Google Scholar]
- 4.Kastelic A, Pont J, Stöver H. Opioid Substitution Treatment in Custodial Settings A Practical Guide. BIS-Verlag; Oldenburg: 2008. [Google Scholar]
- 5.Kinlock TW, Gordon MS, Schwartz RP. Incarcerated populations. In: Ruiz P, Strain E, editors. Lowinson & Ruiz's Substance Abuse: A Comprehensive Textbook. Fifth. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 881–891. [Google Scholar]
- 6.Carson EA, Golinelli D. Prisoners in 2012: Advance counts. Washington, D.C.: Department of Justice, Bureau of Justice Statistics; 2013. [Google Scholar]
- 7.Mumola CJ, Karberg JC. Drug Use and Dependence, State and Federal Prisoners, 2004. Washington, D.C.: U.S. Department of Justice, Bureau of Justice Statistics (NCJ 213530); 2006. [Google Scholar]
- 8.Kinlock TW, Gordon MS, Shabazz H. Pharmacotherapy for incarcerated individuals with histories of heroin addiction. Hauppauge, NY: Nova Science Publishers Inc.; In Press. [Google Scholar]
- 9.Fox AD. Opioid Addiction and Criminal Justice Systems: Opportunities to Break the Cycle of Incarceration. SGIM Forum. 2015;38(1) [Google Scholar]
- 10.Taxman FS, Perdoni ML, Harrison LD. Drug treatment services for adult offenders: the state of the state. Journal of substance abuse treatment. 2007;32(3):239–254. doi: 10.1016/j.jsat.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strang J, Gossop M, Heuston J, Green J, Whiteley C, Maden A. Persistence of drug use during imprisonment: relationship of drug type, recency of use and severity of dependence to use of heroin, cocaine and amphetamine in prison. Addiction. 2006;101(8):1125–1132. doi: 10.1111/j.1360-0443.2006.01475.x. [DOI] [PubMed] [Google Scholar]
- 12.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binswanger IA, Merrill JO, Krueger PM, White MC, Booth RE, Elmore JG. Gender differences in chronic medical, psychiatric, and substance-dependence disorders among jail inmates. Am J Public Health. 2010;100(3):476–482. doi: 10.2105/AJPH.2008.149591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binswanger IA, Blatchford PJ, Lindsay RG, Stern MF. Risk factors for all-cause, overdose and early deaths after release from prison in Washington state. Drug Alcohol Depend. 2011;117(1):1–6. doi: 10.1016/j.drugalcdep.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996-99. Addiction. 2003;98(2):185–190. doi: 10.1046/j.1360-0443.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 16.Farrell M, Marsden J. Acute risk of drug-related death among newly released prisoners in England and Wales. Addiction. 2008;103(2):251–255. doi: 10.1111/j.1360-0443.2007.02081.x. [DOI] [PubMed] [Google Scholar]
- 17.Krinsky CS, Lathrop SL, Brown P, Nolte KB. Drugs, detention, and death: a study of the mortality of recently released prisoners. Am J Forensic Med Pathol. 2009;30(1):6–9. doi: 10.1097/PAF.0b013e3181873784. [DOI] [PubMed] [Google Scholar]
- 18.Merrall EL, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105(9):1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart LM, Henderson CJ, Hobbs MS, Ridout SC, Knuiman MW. Risk of death in prisoners after release from jail. Aust N Z J Public Health. 2004;28(1):32–36. doi: 10.1111/j.1467-842x.2004.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 20.Inciardi JA. The War on Drugs IV: The continuing saga of the mysteries and miseries of intoxication, addiction, crime and public policy. Boston, MA: Allyn and Bacon; 2008. [Google Scholar]
- 21.Hough M. Drug user treatment within a criminal justice context. Subst Use Misuse. 2002;37(8-10):985–996. doi: 10.1081/ja-120004162. [DOI] [PubMed] [Google Scholar]
- 22.Kinlock TW, O'Grady KE, Hanlon TE. The effects of drug treatment on institutional behavior. The Prison Journal. 2003;83:257–276. [Google Scholar]
- 23.Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100(6):820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 24.Metz V, Matzenauer C, Kammerer K, et al. Evaluation of Opioid-Dependent Prisoners in Oral Opioid Maintenance Therapy. Heroin Addiction and Related Clinical Problems. 2010;12(1):5–16. [Google Scholar]
- 25.Dolan KA, Shearer J, MacDonald M, Mattick RP, Hall W, Wodak AD. A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system. Drug Alcohol Depend. 2003;72(1):59–65. doi: 10.1016/s0376-8716(03)00187-x. [DOI] [PubMed] [Google Scholar]
- 26.Hedrich D, Alves P, Farrell M, Stover H, Moller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction. 2012;107(3):501–517. doi: 10.1111/j.1360-0443.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- 27.Magura S, Rosenblum A, Lewis C, Joseph H. The effectiveness of in-jail methadone maintenance. Journal of Drug Issues. 1993;23:75–99. [Google Scholar]
- 28.Magura S, Lee JD, Hershberger J, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99(1-3):222–230. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover H, Michels Drug use and opioid substitution treatment for prisoners. Harm Reduct J. 2010;7:17. doi: 10.1186/1477-7517-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasino V, Swanson AJ, Nolan J, Shuman HI. The Key Extended Entry Program (KEEP): a methadone treatment program for opiate-dependent inmates. Mt Sinai J Med. 2001;68(1):14–20. [PubMed] [Google Scholar]
- 31.Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. N Engl J Med. 1969;280(25):1372–1375. doi: 10.1056/NEJM196906192802502. [DOI] [PubMed] [Google Scholar]
- 32.Gordon MS, Kinlock TW, Schwartz RP, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction. 2008;103(8):1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinlock TW, Gordon MS, Schwartz RP, O'Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91(2-3):220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinlock TW, Gordon MS, Schwartz RP, O'Grady KE. A study of methadone maintenance for male prisoners: 3-month postrelease outcomes. Criminal justice and behavior. 2008;35(1):34–47. doi: 10.1177/0093854807309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinlock TW, Sears EA, O'Grady KE, Callaman JM, Brown BS. Treatment retention and changes in motivation among drug court probationers. Journal of Offender Rehabilitation. 2009;48:1–18. [Google Scholar]
- 36.Friedmann PD, Hoskinson R, Gordon M, et al. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abus. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105(1-2):83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich JD, Boutwell AE, Shield DC, et al. Attitudes and practices regarding the use of methadone in US state and federal prisons. J Urban Health. 2005a;82(3):411–419. doi: 10.1093/jurban/jti072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rich JD, McKenzie M, Shield DC, et al. Linkage with methadone treatment upon release from incarceration: a promising opportunity. J Addict Dis. 2005b;24(3):49–59. doi: 10.1300/J069v24n03_04. [DOI] [PubMed] [Google Scholar]
- 40.Zaller N, McKenzie M, Friedmann PD, Green TC, McGowan S, Rich JD. Initiation of buprenorphine during incarceration and retention in treatment upon release. Journal of substance abuse treatment. 2013;45(2):222–226. doi: 10.1016/j.jsat.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wish ED, Artigiani E, Billing A, et al. The emerging buprenorphine epidemic in the United States. J Addict Dis. 2012;31(1):3–7. doi: 10.1080/10550887.2011.642757. [DOI] [PubMed] [Google Scholar]
- 42.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2006;(1):CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Pettinati HM, Volpicelli JR, Pierce JD, Jr, O'Brien CP. Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis. 2000;19(1):71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- 44.Coviello DM, Cornish JW, Lynch KG, et al. A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Subst Abus. 2012;33(1):48–59. doi: 10.1080/08897077.2011.609438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornish JW, Langleben DD, Nordeck CD, et al. Six-Month Depot Naltrexone Treatment Reduces Relapse in Parolees Formerly Addicted to Opioids. Poster presented at 51st Annual Meeting of the American College of Neuropsychopharmacology; December 2-6, 2012; Hollywood, Florida. 2012. [Google Scholar]
- 47.Lee JD, McDonald R, Grossman E, et al. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110(6):1008–1014. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- 48.Krupitsky E, Zvartau E, Woody G. Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr Psychiatry Rep. 2010;12(5):448–453. doi: 10.1007/s11920-010-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon MS, Kinlock TW, Vocci FJ, Fitzgerald TT, Memisoglu A, Silverman B. A Phase 4, Pilot, Open-Label Study of VIVITROL(R) (Extended-Release Naltrexone XR-NTX) for Prisoners. Journal of substance abuse treatment. 2015;59:52–58. doi: 10.1016/j.jsat.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Kuo I, Brady J, Butler C, et al. Feasibility of referring drug users from a needle exchange program into an addiction treatment program: experience with a mobile treatment van and LAAM maintenance. Journal of substance abuse treatment. 2003;24(1):67–74. doi: 10.1016/s0740-5472(02)00343-4. [DOI] [PubMed] [Google Scholar]
- 51.Buning EC, Van Brussel GH, Van Santen G. The ‘methadone by bus’ project in Amsterdam. Br J Addict. 1990;85(10):1247–1250. doi: 10.1111/j.1360-0443.1990.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 52.Greenfield L, Brady JV, Besteman KJ, De Smet A. Patient retention in mobile and fixed-site methadone maintenance treatment. Drug Alcohol Depend. 1996;42(2):125–131. doi: 10.1016/0376-8716(96)01273-2. [DOI] [PubMed] [Google Scholar]
- 53.Hall G, Neighbors CJ, Iheoma J, et al. Mobile opioid agonist treatment and public funding expands treatment for disenfranchised opioid-dependent individuals. Journal of substance abuse treatment. 2014;46(4):511–515. doi: 10.1016/j.jsat.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor CA, Patsdaughter CA, Grindel CG, Taveira PF, Steinberg JL. A mobile HIV education and testing program: bringing services to hard-to-reach populations. AIDS Patient Care STDS. 1998;12(12):931–937. doi: 10.1089/apc.1998.12.931. [DOI] [PubMed] [Google Scholar]
- 55.Dube C, Nozaki I, Hayakawa T, Kakimoto K, Yamada N, Simpungwe JB. Expansion of antiretroviral treatment to rural health centre level by a mobile service in Mumbwa district, Zambia. Bull World Health Organ. 2010;88(10):788–791. doi: 10.2471/BLT.09.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suggett J, Lloyd C, Meehan T, King R. Is there a difference? A comparative study of Mobile Intensive Treatment Team and Continuing Care Team consumers' clinical and other characteristics. Advances in Mental Health. 2012;10(3):293–297. [Google Scholar]
- 57.Foster C, Scott I, Addington-Hall J. Who visits mobile UK services providing cancer information and support in the community? Eur J Cancer Care (Engl) 2010;19(2):221–226. doi: 10.1111/j.1365-2354.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 58.Iredale R, Hilgart J, Hayward J. Patient perceptions of a mobile cancer support unit in South Wales. Eur J Cancer Care (Engl) 2011;20(4):555–560. doi: 10.1111/j.1365-2354.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 59.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag. 2009;5(4):219–227. doi: 10.5055/jom.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O'Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depend. 2014;142:33–40. doi: 10.1016/j.drugalcdep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. Journal of substance abuse treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 62.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Metzger D, Woody GE, McLellan AT, Navaline H, O'Brien CP. International Conference on AIDS. Measuring risk behavior among IDUs: validation of the risk for AIDS behavior (RAB) scale. Paper presented at: Int Conf AIDS; Jul 19-24, 1992; University of Pennsylvania, Philadelphia. [Google Scholar]
- 64.Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol. 1985;46(4):267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- 65.Childress AR, McLellan AT, O'Brien CP. Conditioned responses in a methadone population. A comparison of laboratory, clinic, and natural settings. Journal of substance abuse treatment. 1986;3(3):173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz RP, Jaffe JH, O'Grady KE, et al. Interim methadone treatment: impact on arrests. Drug Alcohol Depend. 2009;103(3):148–154. doi: 10.1016/j.drugalcdep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen J. Statistical power analysis for the behavioral sciences (Second edition) Hillsdale, NJ: LEA; 1988. [Google Scholar]
- 68.Gordon MS, Kinlock TW, Miller PM. Medication-assisted treatment research with criminal justice populations: challenges of implementation. Behavioral sciences & the law. 2011;29(6):829–845. doi: 10.1002/bsl.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]