Abstract
Background
While infection burden among patients with SLE is high, uncertainty exists about whether rates differ by immunosuppressive drug regimens. We compared infection rates among patients with SLE newly initiating immunosuppressive therapy either using mycophenolate mofetil (MMF), azathioprine (AZA), or cyclophosphamide (CYC).
Methods
Within the Medicaid Analytic eXtract (2000–10; 29 U.S. states), we identified adults with SLE starting MMF, AZA, or CYC treatment. We estimated propensity scores (PS) for receipt of MMF vs. AZA and MMF vs. CYC, based on sociodemographic, comorbidity, and medication use information. After 1:1 PS-matching, we estimated incidence rates (IR) of serious infections up to 6 months after drug initiation and used Cox regression to estimate hazard ratios (HR) of first infection and death. We performed primary intention-to-treat (ITT) and secondary as-treated analyses.
Results
We studied 1350 PS-matched pairs of MMF and AZA initiators and 674 pairs of MMF and CYC initiators. In 6-month ITT analyses, the IR per 100 person-years for first serious infection was 14.6 in MMF users and 15.2 in AZA users (HR of MMF vs. AZA 0.99, 95% CI 0.74–1.32). Comparing MMF to CYC, the IR per 100 person-years for first infection was 24.1 in MMF users and 24.6 in CYC; HR 0.95 (95% CI 0.69–1.32). There were no differences in mortality in either comparison. As-treated analyses yielded similar results.
Conclusion
In a nationwide, longitudinal study of Medicaid SLE patients at high risk of infection, rates of serious infection and mortality did not differ among new users of MMF, AZA, or CYC.
Serious infections are among the leading causes of hospitalization and mortality in patients with systemic lupus erythematosus (SLE) and lupus nephritis (LN).(1–5) Randomized controlled trials (RCT) and academic center-based cohort studies have described increased rates of infections related to immunosuppressive medications for SLE. However, these studies have been limited by small sample sizes, exclusions by disease severity and comorbidities, restrictions on concurrent medication use, short follow-up and, often, self-reported data.(6–17) In addition, RCTs are generally underpowered to estimate the comparative risk of adverse events such as infections with adequate precision.
In rheumatoid arthritis, large, population-based comparative safety studies have demonstrated elevated risks of serious infections associated with use of specific immunosuppressive medications.(18–28) Our prior work in the SLE Medicaid population highlighted an increased risk of serious infection among individuals who received immunosuppressive medications compared to those who did not.(29) Meta-analyses comparing the efficacy and safety of immunosuppressive medications in SLE patients from pooled RCT data reported similar numbers of infections for the drugs assessed.(30–32) However, serious infections were rare and the meta-analyses were limited by the same inclusion and exclusion criteria and oftentimes, short follow-up time in the original RCTs. The comparative risk of infection across individual immunosuppressive drugs among patients with SLE, however, has not been previously investigated in a population-based cohort.
In addition to hydroxychloroquine and corticosteroids, mycophenolate mofetil (MMF), azathioprine (AZA), and cyclophosphamide (CYC) are the most commonly used medications to treat patients with moderate-to-severe SLE. We aimed to assess whether there were any differences in the rates of serious infection among SLE patients initiating MMF compared to AZA and among those initiating MMF compared to CYC in a nationwide longitudinal cohort. We chose these comparisons because of the relatively interchangeable use of CYC and MMF for induction therapy and MMF and AZA for maintenance therapy among patients with severe SLE. Specifically, multiple meta-analyses of RCTs have found minimal if any conclusive differences between MMF and CYC for induction of remission and between MMF and AZA for maintenance of remission among patients with proliferative lupus nephritis.(30, 33–36) In addition, the most recent American College of Rheumatology guidelines for lupus nephritis supports interchangeable use of MMF and CYC for induction and MMF and AZA for maintenance therapy.(37, 38) However, given the lack of head-to-head studies of the comparative infection rates associated with these different immunosuppressive medications, it remains unclear which drug may confer increased risk and no information is currently available to inform treatment choice.
Methods
Data Source
We used insurance claims data from the Medicaid Analytic eXtract (MAX) database for Medicaid enrollees from the 29 most populated U.S. states, from January 1, 2000 through December 31, 2010. Medicaid is the largest public health insurance program in the U.S. covering more than 60 million racially and ethnically diverse, low-income individuals nationwide. The MAX database includes demographic information and longitudinal claims for covered healthcare services including the corresponding diagnosis and procedure codes and pharmacy dispensing details for all beneficiaries. De-identified data were obtained through a Data Use Agreement with the Centers for Medicare and Medicaid Services and an Institutional Review Board of Brigham and Women’s Hospital approved this study.
Study Cohort Selection and Exposure Definition
Our cohort included adults age 18–65 years, with prevalent SLE defined by ≥2 International Classification of Diseases, 9th Revision (ICD-9) codes for SLE (710.0) separated by ≥30 days. We then selected patients with a new prescription for MMF, AZA, or oral or intravenous CYC within 365 days of one of the ICD-9 codes for SLE, with continuous enrollment in Medicaid for the 6 months prior to the date of first prescription dispensing (the index date). For CYC, we additionally included Healthcare Common Procedure Coding system (HCPCS) J-codes for non-orally administered drugs and Current Procedural Terminology (CPT) codes for infusions. Patients were required to have no documented use of the drug of interest or the direct comparator drug during the 6 months prior to the index date and we excluded patients with HIV/AIDS, or organ or bone marrow transplantation. We additionally excluded patients who used tacrolimus because of significant imbalance between the comparator groups.
Outcome Definition
The primary outcome of interest was first serious infection, defined as a hospital discharge diagnosis ICD-9 code (in any position) for bacterial infection (bacteremia, cellulitis, pneumonia, pyelonephritis, osteomyelitis, septic arthritis, endocarditis), fungal (aspergillosis, cryptococcosis, histoplasmosis, pneumocystosis), viral (herpes zoster, cytomegalovirus, varicella zoster, influenza) and mycobacterial infection (tuberculosis, non-tuberculous mycobacteria). A validation study has demonstrated a mean positive predictive value (PPV) of 80% for bacterial infections.(39) The mean PPV for opportunistic infections was 76% and included hospitalization discharge diagnosis ICD-9 codes for tuberculosis (PPV 77%), atypical mycobacterial infection (PPV 70%), cryptococcosis (PPV 100%), and aspergillosis (67%).(39) We excluded candidiasis based on findings from this validation study (PPV 20%), and meningitis and encephalitis due to an inability to distinguish between infectious and SLE-related etiologies.(29, 39) We restricted our analyses to the most serious infections and therefore did not include infection diagnosis codes from outpatient encounters. We excluded common nosocomial infections including urinary tract and surgical site infections to focus on those that were more likely to result in hospitalization than to occur from hospitalization. We additionally examined only primary discharge diagnosis of serious infection to further restrict our definition to infections that were most likely the cause of the hospitalization. In secondary analyses that included all infection episodes rather than the time to the first such episode, we examined all serious infections separated by ≥30 days to minimize double counting of readmissions for the same infection. We also examined all-cause mortality using Social Security Death Index files linked to MAX. Cause of death information was not available.
Covariates
We assessed patient characteristics and other covariates during the 6 months prior to and including the index date. Demographic information included age at the index date, sex, U.S. geographic region (Northeast, Midwest, South, and West) and race/ethnicity. We used Medicaid’s categorizations for race/ethnicity: White, Black or African American, Hispanic or Latino, Asian (including Native Hawaiian or other Pacific Islander), Native American (including American Indian or Alaskan Native), and Other.(40) We defined area-level socioeconomic status using a previously validated composite score of seven U.S. Census variables.(41)
We used the SLE-specific risk adjustment index, which includes comorbidities relevant to SLE patients shown to be associated with all-cause mortality among U.S. Medicaid SLE patients.(42, 43) We defined lupus nephritis as ≥1 ICD-9 code for glomerulonephritis, proteinuria, or kidney failure on or after the SLE diagnosis. In addition to the SLE-specific risk adjustment index, we included diabetes mellitus, chronic kidney disease (using codes for renal disease or dialysis during the baseline period independent of the timing of the SLE code), alcoholism, smoking, obesity, hepatitis, chronic obstructive pulmonary disease, and malignancies.
We assessed the total number of medications prescribed and use of the following medications during the baseline 6 month period: hydroxychloroquine, chloroquine, sulfasalazine, methotrexate, leflunomide, cyclosporine, anti-TNFs, nonsteroidal antiinflammatory drugs, and corticosteroids. We included prior baseline use of CYC in the MMF vs. AZA cohort, and prior baseline use of AZA in the MMF vs. CYC cohort. In addition, we calculated the cumulative prednisone-equivalent corticosteroid dose dispensed during the 60 days prior to and including the index date and categorized the mean daily prednisone-equivalent dose as 0–5mg/day, >5–15mg/day, or >15mg/day.
To include health care utilization, we used number of outpatient, inpatient, and emergency department visits, and number of hospitalization days. As a proxy for disease severity, we used the number of SLE-related laboratory tests. We addressed preventive care by including vaccinations (influenza and pneumococcal) determined by ≥1 CPT code and pneumocystosis prophylaxis using dispensing data for trimethoprim-sulfamethoxazole, atovaquone, dapsone, or pentamidine.
Statistical Analyses
We assessed baseline characteristics of the study cohort with Chi-squared tests, Fisher’s exact tests, Wilcoxon rank sum tests, Mann-Whitney U tests and t-tests. To minimize confounding by indication, we used a propensity score (PS) matching method. Multivariable logistic regression models were constructed to estimate the respective probabilities of initiating MMF vs. AZA and of initiating MMF vs. CYC conditional on observed baseline covariates. We included all baseline demographic factors, comorbidities, medications, health care utilization factors, and the SLE-specific risk adjustment index, in addition to calendar year. We used nearest-neighbor-matching within a caliper of 0.025 on the PS at a fixed ratio of 1:1 for both the MMF vs. AZA and MMF vs. CYC comparisons.(44, 45)
In primary analyses for both drug pair comparisons, we determined rates of first serious infection and of all-cause mortality over 6- and 12-month periods beginning the day after the index date. The goal was to simulate an intention-to-treat (ITT) analysis at 6 and 12 months. We performed ITT analyses paralleling clinical trials to reduce the bias introduced by excluding nonadherers. Patients were censored at death, disenrollment from Medicaid, or end of follow-up. We calculated incidence rates (IR) for serious infections with 95% confidence intervals (CIs) for the PS-matched pairs of MMF vs. AZA and MMF vs. CYC new users. We used Cox proportional hazards models to estimate the hazard ratios (HR) with 95% CI of first serious infection between the PS-matched groups. Due to a residual imbalance in cumulative corticosteroid dose in both PS-matched drug comparisons, we additionally adjusted for cumulative corticosteroid dose in the 60 days preceding and including the index date in all models. We tested the proportional hazards assumption using an interaction term between the drug exposure and the log of follow-up time, but did not detect any such violations.(46)
In secondary analyses, we examined IRs and incidence rate ratios (IRRs) with 95% CI of all serious infections occurring after the index date using Poisson regression models. To assess potential differences in infection rates between oral and intravenous CYC therapy, we used Cox models to separately examine our outcome among PS-matched MMF vs. oral CYC and MMF vs. intravenous CYC users. We also conducted as-treated analyses for MMF vs. AZA and for MMF vs. CYC. For the MMF vs. CYC comparison, we examined oral and intravenous CYC separately as well as combined. For the MMF vs. AZA and the MMF vs. oral CYC comparisons we assessed rates of first serious infection beginning the day following the index date and censored at drug discontinuation, gaps greater than 30 days, switch to the comparator drug, death, disenrollment from Medicaid, or end of follow-up. Our ability to conduct the as-treated MMF vs. intravenous CYC comparison was limited by differences in protocols regarding frequency of use. We used the same criteria as for the oral comparison however we censored at gaps greater than 40 days rather than 30 to allow for 10 days leeway in scheduling of monthly infusions. We performed additional secondary analyses using matching weights instead of propensity scores both for the MMF vs. AZA comparison and for the MMF vs. CYC comparison. Matching weights allows for the preservation of sample size when potential paired comparators may be limited and may result in more efficient estimation and more precise variance calculations. (47) We used matching weights and weighted Cox models to compare serious infections and death in ITT and as-treated analyses. We conducted all analyses using SAS 9.3 (SAS Institute, Cary, N.C.).
Results
Cohort Selection and Patient Characteristics
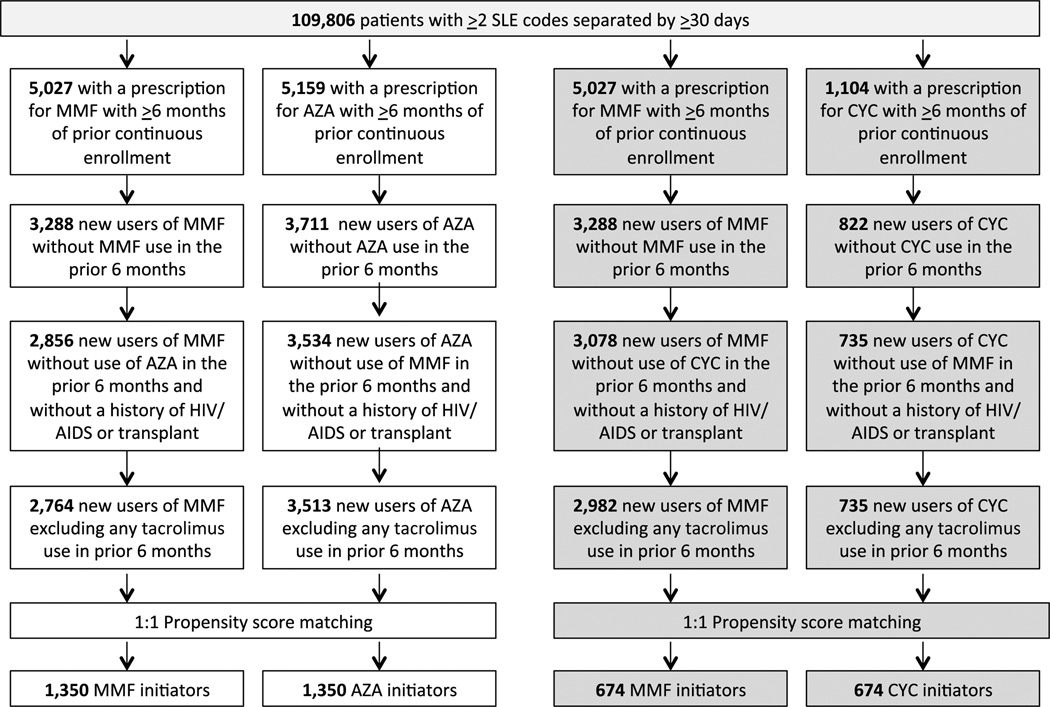
Prior to matching, we identified 2,764 MMF new users and 3,513 AZA new users who met our pre-specified inclusion and exclusion criteria (Supplemental Table 1). In the unmatched cohort, while demographic factors were similar, compared to AZA new users, MMF new users had higher SLE risk adjustment indices, a greater burden of kidney disease and specifically lupus nephritis, were taking more medications on average, received more preventive care, and had greater health care utilization. From this cohort, we identified 1,350 PS-matched pairs of MMF and AZA initiators (Figure 1). The mean ages were 35 years (SD 12) in both groups and the overall mean follow-up time was 3.7 years among MMF users and 4.0 years among AZA users (Table 1). The overall c-statistic was 0.85 for MMF vs.. AZA. There were no statistically significant differences between any of the characteristics including comorbidities, medication use, preventive care or health care utilization. Cumulative corticosteroid dose, however, was higher in the AZA group.
Figure 1.
Flowchart illustrating the selection of the two cohorts leading to 1,350 propensity score matched pairs of mycophenolate mofetil (MMF) and azathioprine (AZA) initiators and 674 propensity score matched pairs of MMF and cyclophosphamide (CYC) initiators
Table 1.
Baseline characteristics of mycophenolate mofetil (MMF) and azathioprine (AZA) and MMF and cyclophosphamide (CYC) propensity score matched cohorts of new users
| MMF v. AZA Propensity Score Matched Cohort |
MMF v. CYC Propensity Score Matched Cohort |
|||
|---|---|---|---|---|
| Baseline characteristics | MMF (N=1350) |
AZA (N=1350) |
MMF (N=674) |
CYC (N=674) |
| Years of follow-up – mean ±SD | 3.7 ± 3.0 | 4.0 ± 3.0 | 4.3 ± 3.3 | 4.1 ± 3.1 |
| Age – mean ±SD | 34.8 ± 11.6 | 34.5 ± 11.5 | 35.9 ± 12.1 | 35.7 ± 12.2 |
| Female - N (%) | 1234 (91.4) | 1245 (92.2) | 612 (90.8) | 607 (90.1) |
| Race/Ethnicity- N (%) | ||||
| White | 354 (26.2) | 342 (25.3) | 156 (23.2) | 166 (24.6) |
| Black | 611 (45.3) | 631 (46.7) | 338 (50.2) | 321 (47.6) |
| Asian | 76 (5.6) | 55 (4.1) | 29 (4.3) | 30 (4.5) |
| Hispanic | 261 (19.3) | 265 (19.6) | 131 (19.4) | 136 (20.2) |
| Native American | 20 (1.5) | 22 (1.6) | 11 (1.6) | NR |
| Other | 28 (2.1) | 35 (2.6) | NR | 12 (1.8) |
| Region- N (%) | ||||
| Northeast | 317 (23.5) | 306 (22.7) | 114 (16.9) | 120 (17.8) |
| South | 454 (33.6) | 466 (34.5) | 319 (47.3) | 300 (44.5) |
| Midwest | 304 (22.5) | 305 (22.6) | 124 (18.4) | 132 (19.6) |
| West | 275 (20.4) | 273 (20.2) | 117 (17.4) | 122 (18.1) |
| Area-level SES*-mean ±SD | 1.3 ± 1.6 | 1.3 ± 1.7 | 1.0 ± 1.6 | 1.0 ± 1.7 |
|
SLE risk adjustment index- mean ±SD |
1.4 ± 2.0 | 1.5 ± 2.4 | 2.9 ± 3.2 | 2.8 ± 2.6 |
| Diabetes mellitus | 147 (10.9) | 146 (10.8) | 116 (17.2) | 107 (15.9) |
| Chronic kidney disease | 19 (1.4) | 22 (1.6) | 19 (2.8) | 19 (2.8) |
| Smoking | 71 (5.3) | 70 (5.2) | 49 (7.3) | 55 (8.2) |
| Hepatitis | 58 (4.3) | 65 (4.8) | 136 (20.2) | 134 (19.9) |
| Chronic lung disease | 164 (12.2) | 164 (12.2) | 38 (5.6) | 40 (5.9) |
| Obesity | 39 (2.9) | 42 (1.6) | 33 (4.9) | 29 (4.3) |
| Lupus nephritis# | 410 (30.4) | 427 (31.6) | 368 (54.6) | 363 (53.9) |
| Cancer | 41 (3.0) | 42 (3.1) | 53 (7.9) | 60 (8.9) |
| Health care utilization+ - median (IQR) | ||||
| Outpatient visits | 4 (1, 8) | 4 (0, 9) | 6 (2, 10) | 6 (3, 10) |
| Emergency Dept. visits | 0 (0, 2) | 0 (0, 2) | 1 (0, 2) | 1 (0, 3) |
| Hospitalizations | 0 (0, 1) | 0 (0, 1) | 1 (0, 2) | 1 (0, 2) |
| Hospitalized days | 0 (0, 5) | 0 (0, 7) | 2 (0, 13) | 4 (0, 14.5) |
| Medications | ||||
| Number of medications –mean ±SD |
12.6 ± 7.9 | 13.2 ± 12.3 | 16.4 ± 9.6 | 16.2 ± 10.5 |
| Hydroxychloroquine – N (%) | 720 (54.0) | 735 (54.4) | 203 (30.1) | 229 (34.0) |
| Cyclophosphamide | 61 (4.5) | 55 (4.1) | N/A | N/A |
| Methotrexate – N (%) | 99 (7.3) | 97 (7.2) | 35 (5.2) | 40 (5.9) |
| Azathioprine – N (%) | N/A | N/A | 73 (10.8) | 70 (10.4) |
| Corticosteroids – N (%) | 1043 (77.3) | 1054 (78.1) | 549 (81.5) | 548 (81.3) |
| Cumulative 60-day median prednisone-equivalent dose (mg) (IQR) |
224.2 (0, 830.0) | 310 (0, 1018.2) | 330 (0, 1100.0) | 460 (0,1620.0) |
| Median daily prednisone- equivalent dose (mg/d)++ (IQR) |
3.7 (0, 13.8) | 5.2 (0, 17.0) | 5.5 (0, 18.3) | 7.7 (0, 27.0) |
| Mean daily prednisone- equivalent dose (mg/d) ++ | ||||
| 0–5 mg – N (%) | 733 (54.3) | 668 (49.5) | 324 (48.1) | 299 (44.4) |
| >5–15 mg – N (%) | 298 (22.1) | 290 (21.5) | 152 (22.6) | 118 (17.5) |
| >15 mg – N (%) | 319 (23.6) | 392 (29.0) | 198 (29.4) | 257 (38.1) |
| NSAIDs- N (%) | 378 (28.9) | 379 (28.1) | 173 (25.7) | 177 (26.3) |
| COXIBs – N(%) | 100 (7.4) | 125 (9.3) | 59 (8.8) | 59 (8.8) |
| Preventive Care- N (%) | ||||
| PCP prophylaxis## | 166 (12.3) | 174 (12.9) | 163 (24.2) | 147 (21.8) |
| Influenza vaccine | 48 (3.6) | 45 (3.3) | 23 (3.4) | 28 (4.2) |
| Pneumococcal vaccine | 18 (1.3) | 16 (1.2) | NR | 15 (2.2) |
All characteristics, unless otherwise specified, were assessed during the 6-month period prior to and including the index date. Chi-squared tests, Fisher’s exact tests, Wilcoxon rank sum tests, Mann-Whitney U tests, and t-tests were used to compare groups (MMF v. AZA and MMF v. CYC) and all p-values were >0.05 with the exception of corticosteroid dose.
IQR = interquartile range, 25%, 75%
In accordance with Federal policies concerning data privacy, cell size <11 were not reported (NR). Alcoholism, substance abuse, and other immunosuppressive drugs were also included in the propensity score but excluded from the table as one or more groups were NR.
SES = socioeconomic status based on a composite index of U.S. Census variables
Lupus nephritis defined as ≥1 ICD-9 code for glomerulonephritis, proteinuria or renal failure on or after SLE diagnosis
Baseline mean number of SLE-related laboratory tests was assessed and was balanced between the two groups.
Determined during the 60-days prior to and including the index date based on prednisone-equivalent doses.
Includes trimethoprim-sulfamethoxazole, atovaquone, dapsone and pentamidine
Prior to matching, for our MMF vs. CYC comparison, we identified 2,982 MMF new users and 735 CYC new users (Supplemental Table 1). Compared to MMF new users, CYC new users had higher SLE risk adjustment indices, less frequent hydroxychloroquine use, increased hospitalizations and higher cumulative median prednisone doses. We then identified 674 PS-matched pairs of MMF and CYC initiators. The mean ages were 36 (SD 12) in both groups and the mean follow-up time was 4.3 (SD 3.3) years for MMF and 4.1 (SD 3.1) years for CYC (Table 1). There were 364 MMF users who were common to both the MMF vs. AZA cohort and to the MMF vs. CYC cohort. Among the 674 CYC users, 314 (46.6%) received oral therapy and 360 (53.4%) received intravenous therapy. The only statistically significant difference was higher cumulative corticosteroid dose among CYC vs. MMF initiators and, hence, we additionally adjusted for this variable in our analyses. The overall c-statistic was 0.78 for MMF vs. CYC. Overall the MMF vs. CYC cohort had a similar distribution of demographic characteristics including age and race/ethnicity compared to the MMF vs. AZA cohort. However, the MMF vs. CYC cohort had greater comorbidities, particularly lupus nephritis, higher SLE risk adjustment index, increased medication use and median corticosteroid dose, and slightly higher health care utilization.
We additionally examined median daily prednisone-equivalent dose from the index date until the outcome of interest (first serious infection) for each of the drug comparisons. For the MMF vs. AZA cohort, the median daily prednisone-equivalent dose for MMF new users was 4.10 mg/day and for AZA, 4.02 mg/day. For the MMF vs. CYC comparison, the median daily prednisone-equivalent dose for MMF new users was 4.60 mg/day and for CYC, 5.05 mg/day. We did not additionally adjust for corticosteroid use following the index date for three central reasons. First, the median daily dose was similar across the comparator drugs. Second, we adjusted for baseline corticosteroid use both through inclusion in the propensity scores and additionally in our Cox proportional hazard models. Third, corticosteroid use following the index date may be an intermediate variable that lies on the causal pathway between the drug of interest and serious infections and adjusting by this potential mediator would bias our estimate of risk associated with the drugs of interest.(48, 49)
Serious Infection Rates
MMF vs. AZA
In primary 6-month ITT analysis, the IR of first hospitalized infection per 100 person-years was 14.6 (95% CI 11.6–17.6) among MMF new users and 15.2 (95% CI 12.9–18.3) among AZA new users (Table 2). Greater than 90 percent of infections in both MMF and AZA new users were bacterial. There was no significant difference in the time to infection between the two groups (HR 0.99, 95% CI 0.74–1.32). Similarly, we found no difference in infection rates in the 12-month ITT analysis, or when only considering hospitalizations for which infection was the primary discharge diagnosis.
Table 2.
Primary intention-to-treat (ITT) analyses at 6-months and 12-months for mycophenolate mofetil versus azathioprine for rates of first serious infection and death
| Mycophenolate mofetil (N=1350) |
Azathioprine (N=1350) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person- years |
IR (95% CI) |
HR (95% CI) |
Cases | Person- years |
IR (95% CI) |
HR | |
| 6-month ITT | ||||||||
| First serious infection* | 90 | 617.3 | 14.6 (11.6– 17.6) |
0.99 (0.74– 1.32) |
93 | 613.2 | 15.2 (12.1– 18.3) |
ref. |
|
First serious infection, primary diagnosis** |
51 | 627.7 | 8.1 (5.9– 10.4) |
0.85 (0.59– 1.20) |
61 | 621.3 | 9.8 (7.4– 12.3) |
ref. |
| Death | 13 | 639.4 | 2.0 (0.9– 3.1) |
0.90 (0.42– 1.91) |
14 | 635.8 | 2.2 (1.0–3.4) | ref. |
| 12-month ITT | ||||||||
| First serious infection* | 144 | 1122.4 | 12.8 (10.7– 14.9) |
1.05 (0.83– 1.33) |
139 | 1121.8 | 12.4 (10.3– 14.5) |
ref. |
|
First serious infection, primary diagnosis** |
89 | 1152.5 | 7.7 (6.1– 9.3) |
1.03 (0.77– 1.38) |
88 | 1147.8 | 7.7 (6.1–9.3) | ref. |
| Death | 22 | 1195.6 | 1.8 (1.1– 2.6) |
1.13 (0.61– 2.09) |
19 | 1195.6 | 1.6 (8.8–2.3) | ref. |
Cox proportional hazard models adjusted for cumulative corticosteroid dose in the 60 days prior to and including the index date and categorized as a mean daily dose of 0–5mg, >5–15mg and >15mg
95% CI = 95% confidence interval, IR = incidence rate per 100 person-years, HR = hazard ratio
Determined by any discharge diagnosis code for serious infection
Determined by the primary discharge diagnosis for serious infection
In secondary analyses including all serious infections following the index date, the IRs per 100 person-years of all serious infections were 17.4 (95% CI 14.1–20.6) among MMF users and 17.7 (95% CI 13.4–21.0) among AZA users, respectively (Table 3); these rates did not differ (IRR 1.01, 95% CI 0.85–1.20). In as-treated analyses, follow-up time was similar to the 6-month ITT analysis and results were consistent with the primary analyses (Supplemental Table 2). Additional secondary analyses using matching weights instead of propensity scores (MMF N=2764, AZA N=3513) yielded hazard ratios in line with the primary analyses for ITT and as-treated analyses; there were no significant differences by drug for serious infection (Supplemental Table 3).
Table 3.
Incidence rates (IR) and incidence rate ratios (IRR) for all serious infections at 6 and 12 months comparing propensity score matched mycophenolate mofetil to azathioprine new users
| Mycophenolate mofetil (N=1350) | Azathioprine (N=1350) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person- years |
IR (95% CI) | IRR (95% CI) | Cases | Person- years |
IR (95% CI) | IRR | |
| 6-month ITT | 111 | 639.4 | 17.3 (14.1–20.6) | 1.01 (0.85–1.20) | 113 | 635.8 | 17.8 (13.5–21.0) | ref. |
| 12-month ITT | 193 | 1190.9 | 16.2 (13.9–18.5) | 1.02 (0.87–1.20) | 195 | 1195.6 | 16.3 (14.0–18.6) | ref. |
Poisson regression models adjusted for cumulative corticosteroid dose in the 60 days prior to the index date and categorized as a mean daily dose of 0–5mg, >5–15mg and >15mg
Cases include all serious infection discharge diagnosis codes separated by ≥30 days
ITT= intention-to-treat analysis, IR = incidence rate per 100 person-years, IRR=incidence rate ratio, 95% CI = 95% confidence interval
MMF vs. CYC
In 6-month ITT analyses comparing MMF vs. CYC, the IRs of first hospitalized infection per 100 person-years were 24.1 (95% CI 18.6–29.7) among MMF initiators and 24.6 (95% CI 19.0–30.2) among CYC initiators, respectively (Table 4). Similar to the MMF vs. AZA comparison, greater than 90 percent of infections among both MMF and CYC users were bacterial. When considering CYC as the reference exposure, there was no significant difference in rates (HR 0.95, 95% CI 0.69–1.32). We obtained parallel results without any significant differences with our 12-month ITT analyses. Similarly, we found no difference when only first primary discharge diagnosis of infection was included (HR 0.98, 95% CI 0.65–1.47).
Table 4.
Primary intention-to-treat (ITT) analyses at 6-months and 12-months for mycophenolate mofetil versus cyclophosphamide for rates of first serious infection and death
| Mycophenolate mofetil (N=674) |
Cyclophosphamide (N=674) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person- years |
IR (95% CI) |
HR (95% CI) |
Cases | Person- years |
IR (95% CI) | HR | |
| 6-month ITT | ||||||||
| First serious infection* | 72 | 298.3 | 24.1 (18.6– 29.7) |
0.95 (0.69– 1.32) |
74 | 301.3 | 24.6 (19.0– 30.2) |
ref. |
|
First serious infection, primary diagnosis** |
47 | 306.5 | 15.3 (11.0– 19.7) |
0.98 (0.65– 1.47) |
47 | 309.4 | 15.2 (10.8– 19.5) |
ref. |
| Death | 15 | 317.9 | 4.7 (2.3– 7.1) |
0.95 (0.46– 1.95) |
15 | 322.0 | 4.7 (2.3–7.0) | ref. |
| 12-month ITT | ||||||||
| First serious infection* | 103 | 547.3 | 18.8 (15.2– 22.5) |
0.94 (0.72– 1.23) |
108 | 547.4 | 19.7 (16.0– 23.4) |
ref. |
|
First serious infection, primary diagnosis** |
65 | 569.9 | 11.3 (8.7– 14.2) |
0.96 (0.68– 1.35) |
67 | 568.2 | 11.8 (9.0– 14.6) |
ref. |
| Death | 18 | 601.3 | 3.0 (1.6–4.4) | 0.71 (0.39– 1.3) |
25 | 602.7 | 4.1 (2.5–5.8) | ref. |
Cox proportional hazard models adjusted for cumulative corticosteroid dose in the 60 days prior to and including the index date and categorized as a mean daily dose of 0–5mg, >5–15mg and >15mg.
95% CI = 95% confidence interval, IR = incidence rate per 100 person-years, HR = hazard ratio
Determined by any discharge diagnosis code for serious infection
Determined by the primary discharge diagnosis for serious infection
In secondary analyses of all serious infections, the respective IRs per 100 person-years were 28.9 (95% CI 23.0–34.8) among MMF users and 29.5 (95% CI 23.6–35.4) among CYC users (Table 5), again without any significant difference between these groups (IRR 0.94, 95% CI 0.74–1.19). To understand differences in risk of infection by route of administration of CYC, we separately compared matched pairs of MMF vs. intravenous CYC users and MMF vs. oral CYC users. While the IR of serious infections was higher among oral CYC users compared to intravenous users, the 95% CIs overlapped (Supplemental Table 4a). The HRs for both MMF vs. CYC comparisons were not statistically significant and the 95% CIs overlapped suggesting no difference in serious infection risk (Supplemental Tables 4b and 4c). As-treated analyses (Supplemental Table 5), also showed no difference in serious infection risk for MMF vs. oral CYC or intravenous CYC, or for the two combined. Additional secondary analyses using matching weights (MMF N=2982, CYC N=735), revealed similar HR estimates and no statistically significant differences in serious infections (Supplemental Table 6).
Table 5.
Incidence rates (IR) and incidence rate ratios (IRR) of all serious infections at 6 and 12 months comparing propensity score matched mycophenolate mofetil to cyclophosphamide new users
| Mycophenolate mofetil (N=674) | Cyclophosphamide (N=674) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person- years |
IR (95% CI) | IRR (95% CI) | Cases | Person- years |
IR (95% CI) | IRR | |
| 6-month ITT | 92 | 318.0 | 29.5 (23.6–35.4) | 0.94 (0.74–1.19) | 95 | 322.0 | 28.9 (23.0–34.8) | ref. |
| 12-month ITT | 142 | 601.3 | 23.6 (19.7–27.5) | 0.96 (0.77–1.19) (0.82–1.27) |
144 | 602.7 | 23.9 (20.0–27.8) | ref. |
Poisson regression models adjusted for cumulative corticosteroid dose in the 60 days prior to the index date and categorized as a mean daily dose of 0–5mg, >5–15mg and >15mg
Cases include all serious infection discharge diagnosis codes separated by ≥30 days
ITT= intention-to-treat analysis, IR = incidence rate per 100 person-years, IRR=incidence rate ratio, 95% CI = 95% confidence interval
Mortality Rates
For the MMF vs. AZA comparison in the primary 6-month ITT analyses, there were 13 deaths among MMF users and 14 among AZA users (HR 0.90, 95% CI 0.42–1.91) (Table 2). In the MMF vs. CYC cohort, we observed 15 deaths in each group (HR 0.95, 95% CI 0.46–1.95) (Table 4). In 12-month ITT analyses for both comparator groups, and in as-treated analyses, we similarly found no significant differences in mortality by drug category. Secondary analyses using matching weights supported these findings of no statistically significant differences in mortality for either comparison (Supplemental Tables 3 & 6).
Discussion
In a large, national comparative safety study of Medicaid beneficiaries with SLE, we found no significant differences in the respective rates of serious infections or mortality among new users of AZA or CYC when compared with otherwise similar patients who initiated treatment with MMF. These results are consistent with those of meta-analyses of RCTs that have suggested that the safety of MMF vs. AZA and MMF vs. CYC in terms of risk of serious infection may be comparable.(37, 38) However, these meta-analyses had pooled studies focused on treatment efficacy and thus the numbers of adverse events were small and difficult to meaningfully compare between groups. Similarly, individual clinical trials of MMF vs. AZA and MMF vs. CYC have demonstrated variability in serious infection rates between arms, but the actual numbers of infections were small and, therefore, differences may have represented Type 1 errors.(50) Cohort studies have shown elevated rates of serious infections among patients receiving CYC and an association of certain infections such as herpes zoster with MMF use.(10, 11) Comparisons of adverse events, as extrapolated from meta-analyses, have shown similar rates of infection and gastrointestinal upset between MMF and AZA users, but increased leukopenia among AZA users.(34) Similarly, leukopenia was found to be more common among CYC compared to MMF users in a meta-analysis of induction therapy.(31) However even with these pooled meta-analyses, adverse events were infrequent, making comparisons challenging to interpret. In addition, inclusion and exclusion criteria from the RCTs used in these meta-analyses limit the overall generalizability of the findings.
In the Medicaid population, previously shown to have a significant burden of SLE and of adverse outcomes, we found high rates of serious infections associated with use of all three of the immunosuppressive medications examined.(43, 51) The serious infection incidence rates observed for the MMF vs. AZA cohort are slightly higher, but in line with those we reported in a prior study among overall Medicaid beneficiaries with SLE.(29) The incidence rates for the MMF vs. CYC cohort are comparable to those we observed among Medicaid beneficiaries with lupus nephritis. Therefore, while no differences were observed in our analyses between the specific immunosuppressive drug comparisons, the high burden of serious infections among SLE patients receiving these medications overall is important to consider.
To our knowledge, this is the largest head-to-head population-based comparative effectiveness study to examine rates of infections and death associated with these commonly and often interchangeably used SLE medications. The sample size for both drug comparisons is larger than any study to date, including meta-analyses of RCT data. This large sample size and the relatively long follow-up in a real-world population treated in typical care settings have enabled us to study an infrequent outcome among all patients with an uncommon disease. It is still possible however, that even with this sample size we were underpowered to detect more subtle differences in infection risk between the drugs. However, in secondary analyses using matching weights, which allowed us to preserve a larger sample size of new users and thus improved our power, we similarly did not detect significant differences in serious infections between the drugs. Medicaid, public insurance for low-income individuals in the U.S. with a high burden of chronic diseases and their complications, is an important group to study to better understand and treat adverse disease and drug manifestations. The overall burden of comorbidities and adverse outcomes in this low-income population is high and therefore the absolute rates of serious infections in each group are likely higher than in other non-Medicaid SLE populations. However, a larger effect size is also expected among groups with increased underlying risk and therefore our finding of no significant difference when comparing each of our groups would likely hold among less vulnerable populations.
In light of the lack of random treatment assignment, the design of this observational study attempts to address a number of potential biases using contemporary pharmacoepidemiologic methods. The use of a new user design with active comparators reduces the possibility of immortal time bias and the depletion of the susceptibles and allows for appropriate adjustment of baseline confounders.(52) The time-varying nature of infections, with the possibility of highest risk at the time of initial use, is also best captured with this new user design. The use of active comparators matched on the PS, simultaneously accounting for a number of covariates including sociodemographic factors, health care utilization, comorbidities, prior drug use, and preventive care, enabled us to minimize potential confounding by indication as it relates to these observed characteristics.(53) The PS-score matched head-to-head comparisons of patients with comparable disease severity were designed to best account for the possibility that lupus severity itself could be associated with infection risk.
A challenge of using claims data is the lack of information about SLE disease activity, severity and duration. We addressed this using proxy measures such as health care utilization and laboratory test frequency, as well as the SLE risk adjustment index, and by using active comparator groups. However, residual confounding from other more granular factors that could not be comprehensively assessed in this database, such as laboratory values and imaging findings, is possible. While the medications we examined may be relatively interchangeably used, there may be a preference, for example, for AZA over MMF and for MMF over CYC for women of reproductive age. Some instances of prior use of intravenous CYC may have been missed if it was administered during a hospitalization due to potential incomplete reporting of this information during inpatient encounters. While this study utilizes the largest sample size to date to compare overall rates of infection, it lacked power to examine specific infection subtypes, as well as the effect of immunosuppressive dosages on these rates. This study compares risk of infection in the short term (6–12 months) but further studies are needed to examine whether longer term use of these drugs alters this risk. In addition, we limited our outcome to serious infections that required hospitalization to improve specificity and also to reduce surveillance bias. However, physicians may have had different thresholds to hospitalize patients and therefore some bias may remain. While the positive predictive value of the validated codes used for serious infections is consistent with those used in most administrative studies, not all cases may be identified. While it is unlikely that this would preferentially affect individuals receiving one drug compared to another, it may lead to non-differential bias and bias toward the null. Additionally, while we found no differences in all-cause mortality in either drug comparison, we lacked cause of death information and therefore were unable to examine infection-specific mortality.
This longitudinal cohort study among Medicaid beneficiaries has the potential to guide clinical decisions concerning these agents, particularly for patients with similar characteristics and moderate-to-severe SLE, who are at high risk for adverse outcomes. We demonstrated comparable rates of serious infection and death among matched MMF and AZA initiators and MMF and CYC initiators with SLE in the first 6 to 12 months of use. Based on these findings, concerns about differential infection risks may not need to influence physician and patient choice between MMF vs. AZA and MMF vs. CYC, even in a population highly susceptible to adverse outcomes.
Supplementary Material
Acknowledgments
Funding: This study was funded by the Lupus Foundation Career Development Award and the Rheumatology Research Foundation Investigator Award (Dr. Feldman) and by NIH NIAMS R01 AR057327 and K24066109 (Dr. Costenbader). The funding sources played no role in the study design, acquisition, analysis or interpretation of data or presentation of results.
Candace Feldman is currently funded by the Rheumatology Research Foundation Investigator Award. She received research support from Pfizer and consulted for Merck/EMD Serono for work unrelated to this manuscript.
Wolfgang Winkelmayer reports having served as an advisor or consultant, unrelated to the topic of this manuscript, to ACUMEN, Amgen, Bayer, Keryx, Medtronic, Mitsubishi-Tanabe, and Rockwell Pharma.
Jessica Franklin consults with Aetion, Inc., a software company, and receives research support from Merck and PCORI.
Karen Costenbader receives funding from NIH NIAMS R01 AR057327 and K24066109.
Seoyoung Kim has received research support through grants to Brigham and Women’s Hospital from Pfizer, AstraZeneca, Lilly, Bristol-Myers Squibb and Genentech.
Footnotes
Financial Disclosures:
Francisco Marty has no disclosures relevant to this work.
Hongshu Guan has no disclosures relevant to this work.
Daniel Solomon has no disclosures relevant to this work.
Author Contributions:
Candace Feldman: 1a,b,c, 2, 3
Francisco Marty: 1a, 2, 3
Wolfgang Winkelmayer: 1a, 2, 3
Hongshu Guan: 1c, 2, 3
Jessica Franklin: 1a,c, 2, 3
Daniel Solomon: 1a, 2, 3
Karen Costenbader: 1a,b,c, 2, 3
Seoyoung Kim: 1a,c, 2, 3
References
- 1.Feng X, Zou Y, Pan W, Wang X, Wu M, Zhang M, et al. Prognostic indicators of hospitalized patients with systemic lupus erythematosus: a large retrospective multicenter study in China. J Rheumatol. 2011;38(7):1289–1295. doi: 10.3899/jrheum.101088. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Dhillon N, Pope J. All-cause hospitalizations in systemic lupus erythematosus from a large Canadian referral centre. Rheumatology (Oxford) 2013 doi: 10.1093/rheumatology/kes391. [DOI] [PubMed] [Google Scholar]
- 3.Sciascia S, Ceberio L, Garcia-Fernandez C, Roccatello D, Karim Y, Cuadrado MJ. Systemic lupus erythematosus and infections: clinical importance of conventional and upcoming biomarkers. Autoimmun Rev. 2012;12(2):157–163. doi: 10.1016/j.autrev.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Juarez M, Misischia R, Alarcon GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Rheum Dis Clin North Am. 2003;29(1):163–184. doi: 10.1016/s0889-857x(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 5.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 6.Petri M, Genovese M. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus Cohort. J Rheumatol. 1992;19(10):1559–1565. [PubMed] [Google Scholar]
- 7.Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11(4):234–239. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 8.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 9.Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton) 2005;10(5):504–510. doi: 10.1111/j.1440-1797.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 10.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39(9):1475–1482. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarty E, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2012 doi: 10.1177/0961203312470186. [DOI] [PubMed] [Google Scholar]
- 12.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contreras G, Tozman E, Nahar N, Metz D. Maintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus. 2005;14(Suppl 1):s33–s38. doi: 10.1191/0961203305lu2115oa. [DOI] [PubMed] [Google Scholar]
- 14.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343(16):1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 15.Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005;16(4):1076–1084. doi: 10.1681/ASN.2004080686. [DOI] [PubMed] [Google Scholar]
- 16.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–1895. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 17.Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon DH, Lunt M, Schneeweiss S. The risk of infection associated with tumor necrosis factor alpha antagonists: making sense of epidemiologic evidence. Arthritis Rheum. 2008;58(4):919–928. doi: 10.1002/art.23396. [DOI] [PubMed] [Google Scholar]
- 19.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52(11):3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 20.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. Jama. 2009;301(7):737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 21.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 22.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. Jama. 2011;306(21):2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Xie F, Chen L, Muntner P, Grijalva CG, Spettell C, et al. Use of a disease risk score to compare serious infections associated with anti-tumor necrosis factor therapy among high- versus lower-risk rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2012;64(10):1480–1489. doi: 10.1002/acr.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh S, Li L, Harrold LR, Bayliss EA, Curtis JR, Liu L, et al. Comparative safety of infliximab and etanercept on the risk of serious infections: does the association vary by patient characteristics? Pharmacoepidemiol Drug Saf. 2012;21(5):524–534. doi: 10.1002/pds.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, Xie F, Chen L, Baddley JW, Beukelman T, Saag KG, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70(8):1401–1406. doi: 10.1136/ard.2010.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane MA, McDonald JR, Zeringue AL, Caplan L, Curtis JR, Ranganathan P, et al. TNF-alpha antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore) 2011;90(2):139–145. doi: 10.1097/MD.0b013e318211106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol. 2009;62(3):321–327. 7 e1–7 e7. doi: 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis JR, Xi J, Patkar N, Xie A, Saag KG, Martin C. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56(12):4226–4227. doi: 10.1002/art.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67(6):1577–1585. doi: 10.1002/art.39070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson LK, Masson P, Craig JC, Roberts MA, Flanc RS, Strippoli GF, et al. Induction and maintenance treatment of proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2013;61(1):74–87. doi: 10.1053/j.ajkd.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 31.Kamanamool N, McEvoy M, Attia J, Ingsathit A, Ngamjanyaporn P, Thakkinstian A. Efficacy and adverse events of mycophenolate mofetil versus cyclophosphamide for induction therapy of lupus nephritis: systematic review and meta-analysis. Medicine (Baltimore) 2010;89(4):227–235. doi: 10.1097/MD.0b013e3181e93d00. [DOI] [PubMed] [Google Scholar]
- 32.Touma Z, Gladman DD, Urowitz MB, Beyene J, Uleryk EM, Shah PS. Mycophenolate mofetil for induction treatment of lupus nephritis: a systematic review and metaanalysis. J Rheumatol. 2011;38(1):69–78. doi: 10.3899/jrheum.100130. [DOI] [PubMed] [Google Scholar]
- 33.Maneiro JR, Lopez-Canoa N, Salgado E, Gomez-Reino JJ. Maintenance therapy of lupus nephritis with mycophenolate or azathioprine: systematic review and meta-analysis. Rheumatology (Oxford) 2014;53(5):834–838. doi: 10.1093/rheumatology/ket429. [DOI] [PubMed] [Google Scholar]
- 34.Feng L, Deng J, Huo DM, Wu QY, Liao YH. Mycophenolate mofetil versus azathioprine as maintenance therapy for lupus nephritis: a meta-analysis. Nephrology (Carlton) 2013;18(2):104–110. doi: 10.1111/nep.12006. [DOI] [PubMed] [Google Scholar]
- 35.Mak A, Cheak AA, Tan JY, Su HC, Ho RC, Lau CS. Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology (Oxford) 2009;48(8):944–952. doi: 10.1093/rheumatology/kep120. [DOI] [PubMed] [Google Scholar]
- 36.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Induction and maintenance therapy for lupus nephritis: a systematic review and meta-analysis. Lupus. 2010;19(6):703–710. doi: 10.1177/0961203309357763. [DOI] [PubMed] [Google Scholar]
- 37.Tian SY, Feldman BM, Beyene J, Brown PE, Uleryk EM, Silverman ED. Immunosuppressive therapies for the induction treatment of proliferative lupus nephritis: a systematic review and network metaanalysis. J Rheumatol. 2014;41(10):1998–2007. doi: 10.3899/jrheum.140050. [DOI] [PubMed] [Google Scholar]
- 38.Tian SY, Feldman BM, Beyene J, Brown PE, Uleryk EM, Silverman ED. Immunosuppressive Therapies for the Maintenance Treatment of Proliferative Lupus Nephritis: A Systematic Review and Network Metaanalysis. J Rheumatol. 2015;42(8):1392–1400. doi: 10.3899/jrheum.141650. [DOI] [PubMed] [Google Scholar]
- 39.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Chronic Conditions Data Warehouse. Medicaid Enrollment by Race, 1999–2007. Available from: http://www.ccwdata.org/summary-statistics/demographics/index.htm. [Google Scholar]
- 41.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37(6):1158–1163. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol. 2000;27(6):1408–1413. [PubMed] [Google Scholar]
- 43.Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: a Hispanic and asian paradox. Arthritis Rheumatol. 2015;67(3):752–760. doi: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26(16):3078–3094. doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 45.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 46.Kleinbaum DK, M . Evaluating the proportional hazards assumption. New York: Springer; 2012. [Google Scholar]
- 47.Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9(2):215–234. doi: 10.1515/ijb-2012-0030. [DOI] [PubMed] [Google Scholar]
- 48.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1(1):4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–441. doi: 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.