Abstract
Background
The Patient Navigation in Medically Underserved Areas study objectives are to assess if navigation improves: 1) care uptake and time to diagnosis; and 2) outcomes depending on patients’ residential medically underserved area (MUA) status. Secondary objectives include the efficacy of navigation across 1) different points of the care continuum among patients diagnosed with breast cancer; and 2) multiple regular screening episodes among patients who did not obtain breast cancer diagnoses.
Design/Methods
Our randomized controlled trial was implemented in three community hospitals in South Chicago. Eligible participants were: 1) female, 2) 18+ years old, 3) not pregnant, 4) referred from a primary care provider for a screening or diagnostic mammogram based on an abnormal clinical breast exam. Participants were randomized to 1) control care or 2) receive longitudinal navigation, through treatment if diagnosed with cancer or across multiple years if asymptomatic, by a lay health worker. Participants’ residential areas were identified as: 1) established MUA (before 1998), 2) new MUA (after 1998), 3) eligible/but not designated as MUA, and 4) affluent/ineligible for MUA. Primary outcomes include days to initially recommended care after randomization and days to diagnosis for women with abnormal results. Secondary outcomes concern days to treatment initiation following a diagnosis and receipt of subsequent screening following normal/benign results.
Discussion
This intervention aims to assess the efficacy of patient navigation on breast cancer care uptake across the continuum. If effective, the program may improve rates of early cancer detection and breast cancer morbidity.
1. Introduction
Underserved women disproportionately die from breast cancer [1, 2] In 1990, Dr. Harold Freeman introduced patient navigation as a potential solution to reduce disparities through addressing patient-level barriers and optimizing coordination of care [3, 4]. In 2005, the Patient Navigation Research Program (PNRP) was implemented throughout the country to assess the potential of navigation to improve cancer care uptake and outcomes [5]. One PNRP site was Chicago [6, 7], which exhibited increasing disparities due to differential access to technological advances in breast cancer care [8–10]. PNRP and other efficacy studies have subsequently demonstrated navigation is effective for improving breast cancer care uptake and time to diagnostic resolution [11–22].
Gaps however exist. First, with the exception of the Denver PNRP [17], extant individual- level RCTs to evaluate the efficacy of navigation have been relatively small (<250 participants total) [19, 18, 21, 20] or have relied on self-report outcomes [22]. There is a need for more large individual-level RCTs, especially those relying on medical records, to confirm the effects of navigation. Second, most studies have not reported assessing if and how intervention efficacy may depend on macro-level factors. This gap is surprising, given navigation programs are more likely to be located within less-resourced settings, including Medically Underserved Area designated communities [23]. Such communities frequently have high percentages of racial/ethnic minorities and exhibit multiple levels of disadvantage, including high rates of poverty and limited healthcare access. These communities may thus be particularly in need of and benefit from navigation services [23], although such differential effects have been understudied. There is a need to examine how the efficacy of navigation varies depending on such contextual factors.
To address these needs, we conducted the Patient Navigation in Medically Underserved Areas (PNMUA) study. PNMUA design leveraged the following strengths in response to gaps in the literature: large sample size; electronic medical record-confirmed outcomes; and a priori plan to assess effect modification of MUA designation on intervention efficacy. The main objectives were to assess: 1) if navigation improved recommended breast cancer care uptake (screening or diagnostic mammography) and time to diagnosis following an abnormal mammogram; and, 2) if navigation effects depended on patients’ residential MUA status. Our study had an additional vantage point due to its longitudinal nature. Most navigation studies concerning the full cancer care continuum have been qualitative in nature [12]. Little is known about navigation’s efficacy throughout the continuum among women diagnosed with breast cancer (i.e., screening, diagnostic care, and treatment) as well as among women who do not receive cancer diagnoses (i.e., multiple episodes of screening). Thus, we also planned secondary analyses to assess the efficacy of navigation across: 1) different points of the care continuum among patients diagnosed with breast cancer; and 2) multiple regular screening episodes among patients who did not obtain breast cancer diagnoses.
2. Study Design and Methods
2.1 Overview
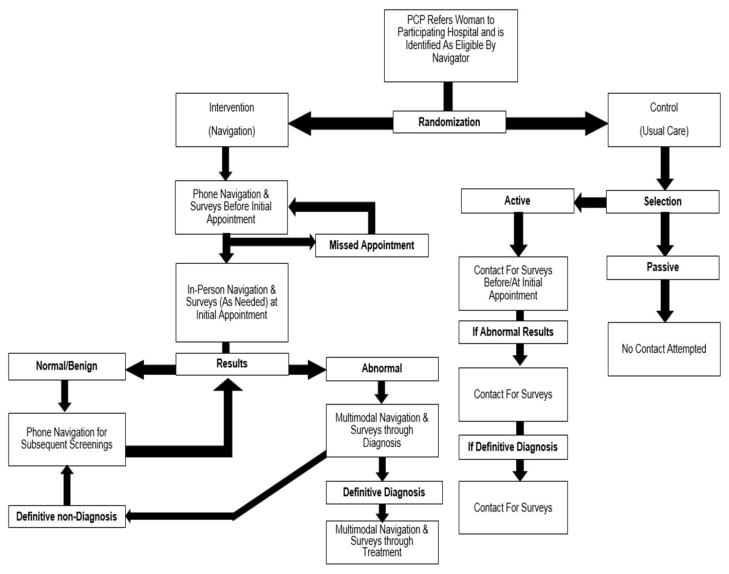
PNMUA is a large individual-level RCT, which ultimately included 9506 women (3754 navigated, 575 active control, 5177 passive control). As described above, the primary objectives were to assess the efficacy of navigation and how it might vary by macro-level factors (i.e., MUA designation). The primary predictor was study arms – intervention (navigation) and control (usual care) groups. Primary outcomes were uptake of initial recommended breast cancer care and time to diagnostic resolution. Secondary objectives were to examine the efficacy throughout the continuum among women ultimately diagnosed and ultimately not diagnosed with breast cancer. Figure 1 depicts a simplified overview of study processes described below, including randomization and study arm-specific interactions between navigators and participants.
Figure 1.
Overview of PNMUA study processes.
2.2 Conceptual framework and Hypotheses
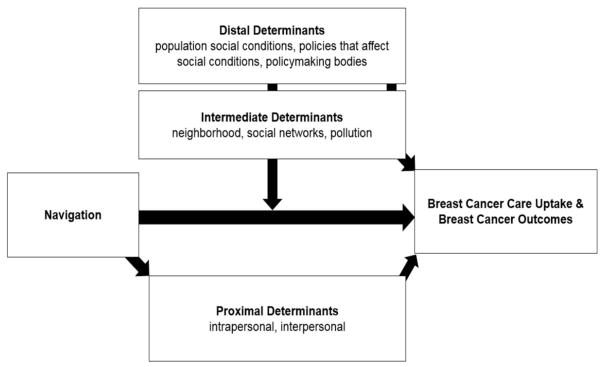
PNMUA draws from the conceptual model adopted by the eight National Institutes for Health-funded Centers for Population Health and Health Disparities (CPHHD) [24]. The model is a multilevel, transdisciplinary approach that takes into account three primary determinants for understanding how population and individual risk factors interact. First, the distal determinants are considered fundamental causes of inequities and are reflected at the population level (e.g., population social conditions, policies that affect social conditions, policymaking bodies). Second, the intermediate determinants are immediate social contexts, physical contexts, and social relationships in which the distal effects are experienced (e.g., neighborhood, social networks, pollution). Third, the proximal determinants refer to individual characteristics (e.g., demographic, intrapersonal, interpersonal).
In the context of PNMUA, patient navigators were expected to improve health outcomes through addressing the proximal determinants of health. Further, the trial selected the sites for the intervention in such a way that we could examine moderating effects of macro-level factors, specifically one at the intermediate determinant level, on our proximal-level intervention. Figure 2 depicts these conceptualizations.
Figure 2.
Conceptual Framework for PNMUA.
We had two primary hypotheses. First, study arms were expected to differ in breast cancer care uptake and diagnostic resolution, theoretically due to the intervention’s effect on proximal determinants. Navigated women were hypothesized to be more likely to undergo screening and diagnostic care in a timely fashion, because navigators are able to diminish/remove barriers to care (e.g., cancer worry; lack of childcare; lack of transportation) relative to women randomized to standard care. This hypothesis was informed by a growing body of studies evaluating navigation’s effects on cancer care uptake and outcomes [12–14]. Second, macro-level, intermediate determinants – specifically residential MUA status - were theorized to serve as effect modifiers. Based on earlier findings related to MUA status and neighborhood characteristics overall [25–27], we hypothesized that navigated women would have greater breast cancer care uptake and shorter time to diagnostic resolution in neighborhoods designated MUAs and neighborhoods that were eligible but not designated as MUAs, but not necessarily in neighborhoods that were not ineligible to be MUAs.
2.3 Settings
PNMUA was conducted from June 2011 through June 2014 in three hospitals (A, B, and C) in South Side of Chicago, Illinois. All three hospitals were situated in MUA designated areas that exhibit high levels of concentrated poverty and racial segregation [28].
2.4 Navigator Staffing and Training
A total of eight navigators were hired as paid employees at one of the three hospitals. Navigators were lay health workers and residents within the communities in which their hospital was located. The navigators were hospital employees giving them access to the medical record data. Prior to interacting with patients, navigators attended a three-day training conducted by Dr. Calhoun, that covered 10 modules taught using adult learning strategies [5]. Pre/posttest assessment was used to evaluate the navigators’ knowledge. A posttest score of 70% on each module was required for passing, and navigators were required to repeat the modules until they received a passing score [5].
2.5 Ethics Committee Approval
All study materials and processes were in accordance with the Code of Ethics of the World Medical Association and were approved by the University of Illinois at Chicago Institutional Review Board. Approved materials and processes included informed consent, intervention activities, and surveys completed during navigator-participant interactions; waivers of informed consent for control participants; and HIPAA waivers of authorization for medical record abstraction. More detailed information pertaining to informed consent and patient details are described in the context of study processes, as per Sections 2.6 and 2.7.
2.6 Eligibility
Navigators daily worked in hospitals to identify eligible participants from hospital lists of new patients who had been referred for a mammography appointment. Eligibility criteria included: 1) female patients, 2) age 18 or older, 3) who were not pregnant, 4) with initial referrals from a primary care provider for a screening mammography or a diagnostic mammography based on an abnormal clinical breast exam. Our age criterion is considerably different than screening guidelines for women of average risk [29, 30], because PNMUA was designed to assess the efficacy of navigation for all women who may undergo diagnostic mammography, including younger, symptomatic women. We note that this population in particular needs to be represented in trials such as this one, given their higher risk of breast cancer morbidity and mortality [31]. Patients were excluded if they had not been referred for screening or diagnostic mammography from primary care. To avoid confounding, patients who were already in process of diagnostic services following an abnormal screening mammogram when the study began also were excluded from participation.
2.7 Randomization
We employed a post-randomization consent design, wherein women were assigned to study arms before informed consent was obtained. All eligible patients were randomly assigned to either the intervention group (patient navigation) or the control group (usual care) using computerized patient identification numbers generated in SAS at the beginning of the study. Randomization was 1:1 control/navigation for hospitals A and B. Due to the large number of women at hospital C, randomization was 3:1 control/navigation. Treatment assignment was masked to health care providers and investigators.
2.8 Intervention arm (Navigation)
Navigators served as proactive patient advocates to assist patients in overcoming proximal barriers to accessing care.
First contact
Navigators made up to a maximum of 10 attempts by phone to initiate contact before the initial provider-referred appointment within two days of women’s scheduled appointments. For those successfully contacted by phone prior to the initial mammography appointment, navigators first offered a brief description of the study, obtained informed consent, and administered baseline surveys. Subsequently, navigators utilized a “teach back” method, wherein navigators provided information about the upcoming appointments (e.g., procedures, time/date and location of appointment) and encouraged patients to report their understanding of this information and ask questions, if they had any questions, about their upcoming appointments. Patients were also provided with contact information for navigators and were able to initiate contact at any point they chose during the study. Navigators identified and addressed specific proximal or patient-level barriers women had concerning their upcoming appointments (e.g., healthcare access, knowledge, worry). If women were successfully contacted two days prior to the appointment, the navigator called again to remind the patient about the appointment and re-assessed any potential barriers to compliance. For patients who were not able to be contacted by phone, a navigator met the patient at her appointment, explained the study, obtained written informed consent for interested patients, and administered baseline surveys. Women were subsequently provided with contact information for navigators and were able to initiate contact at any point they chose during the study.
Initial recommended mammography visit
For all navigated women, at the appointment, the navigator provided an abbreviated version of services described above, wherein they assessed if the patient had any questions, provided education and information, and discussed how the results of the exam would be communicated to the patient. If the patient had any questions for the clinical staff, the navigator assisted the patient in writing down the list of questions to ask the appropriate clinical provider. In addition, navigators tracked women’s adherence to this initial appointment and contacted patients who missed this appointment to identify and address any barriers specific to these appointments. In addition, the navigator worked to ensure that the system delivered results to the patient and the patient understood the results in terms of the treatment protocol that they should follow (e.g., annual rescreening, follow-up with diagnostic testing, or treatment initiation).
Subsequent contact
Navigation services provided during subsequent contacts were identical to that described above (e.g., teach back method, assessment of barriers). The timing of contact depended on women’s mammography results. For women who attended their initial appointment and obtained a normal or benign result (BI-RADS 1 or 2), navigators provided phone- and mail-based reminders concerning recommended follow-up screening within a few months of the recommended date for them to obtain that care (e.g., a year or two).
Navigators attempted to contact women immediately if they received an abnormal result (BI-RADS 0, 3, 4, 5). In addition to navigation services, navigators administered surveys pertaining to diagnostic care (e.g., perceived self-efficacy during care; communication about abnormal results). Contact was largely phone-based, although some participants received in- person assistance, depending on their specific needs. Women with abnormal results were tracked and contact was attempted and maintained until diagnostic resolution was achieved. Contact was conducted by mail, phone, and in person, as was preferred by the patient. For women with a definitive non-cancer diagnosis or false positive result, navigators provided phone- and mail-based reminders concerning recommended follow-up screening identical to what is described above.
For women who received a definitive breast cancer diagnosis, women were followed and navigated throughout the entirety of their treatment. Similar to diagnostic care, women undergoing treatment maintained contact with navigators by mail, phone, and in person, depending on their preferences. Navigators continued to provide health education; address financial barriers to treatment, such as assisted with applying for Medicaid/Medicare; provide psychosocial support; remind patients of their appointments; help patients reschedule missed appointments; address interpersonal factors resulting in patients’ refusal of treatment; and to identify and address if treatment options were not offered. In addition to navigation services, navigators administered surveys pertaining to treatment care (e.g., communication about treatment; recommendation and attainment of different treatment options).
2.9 Control arm (Usual Care)
Women randomized into the control arm received usual care. Most women in the control arm were “passive controls” and did not interact with study staff throughout initial or subsequent screening, diagnosis, or treatment-related care. The only available data for these patients are electronic medical record data. A subset of women randomized into the control arm (10%) were randomly selected into an “active control” group, based on appointment date and type of mammography appointment to complete study surveys. Study staff tried to contact this subset up to 10 times to complete survey questionnaires. Contact was first attempted to obtain consent and administer surveys prior to the initial appointment. Among those who did not interact with study staff prior to attending appointments, surveys were administered during the same day or shortly after attending the initial appointment. Follow-up surveys were also administered to active control women who obtained abnormal results and were referred for follow-up diagnostic care and treatment subsequent to a definitive cancer diagnosis.
2.10 Macro-level factors (MUA status)
Patients in the three hospitals, although predominantly from the hospital neighborhoods, were from all over the Chicago land area. Patient residential addresses were obtained from medical records and were then geocoded using a Geographic Information System (GIS) software, ArcMap 10.1. Census tract numbers were appended to the residential addresses and used to obtain relevant 2007–2011 American Community Survey data concerning neighborhood socioeconomic status and racial/ethnic composition factors [32].
Regarding MUA status, we first identified eligible areas that were designated MUAs by accessing publically available HRSA data for the calendar year 2009, when the program began [33]. Second, the MUA Illinois administrator provided the study team with Index of Medical Underservice (IMUA) scores for all Rational Service Areas in Chicago. The IMU considers four variables: ratio of primary care medical physicians per 1000 residents; infant mortality rate; percentage of the population with incomes below the poverty level; and, percentage of the population aged 65 years or older. The IMU score ranges from 0 (completely underserved) to 100 (least underserved). Areas eligible for MUA designation should have an IMU value of 62 or less. We used this criterion to identify areas that would be eligible, but were not currently designated for MUA status. Through these methods, we were able to classify participants’ residential tracts as: 1) poor, eligible for MUA and received MUA status during the earlier period (before 1998), 2) poor, eligible for MUA and received MUA status during the later period (after 1998), 3) poor, eligible for MUA but not designated as MUA, and 4) affluent, thus not eligible for MUA.
2.11 Study Management
The Principal Investigator (Dr. Calhoun) and key co-investigators (e.g., Drs. Darnell, Kim, Molina) were responsible for protocol development and implementation and dissemination of study findings in manuscripts and conference presentations. An advisory board comprising community partners with years of working experience in breast cancer within South Chicago consulted with the study team quarterly during the first year of the project and annually thereafter. Leadership from participating hospitals provided feedback on intervention development and implementation throughout the study via monthly meetings or more, as was needed. Simultaneously, the Principal Investigator (Dr. Calhoun) and a staff member with years of experience in patient navigation oversaw intervention implementation and engaged in quality assurance with regard to navigation services provided and data entered by navigators (study logs; surveys). With regard to database management, staff at the primary academic institution, the University of Illinois at Chicago, developed and managed a complete study database that leveraged diverse data sources (medical records; publically available ecological data; survey data; see Table 1). The Principal Investigator and 2–3 co-investigators oversaw database management via bimonthly meetings with data entry and biostatistician staff.
Table 1.
PNMUA measures.
| Measures | Data Source | Variable Type | Point of Data Collection/Abstraction | |||
|---|---|---|---|---|---|---|
| Pre-/At Initial Care | Diagnostic Care | Treatment | End of study1 | |||
| Study arm (navigated; control) | Study logs2 | Primary Predictor | X | |||
| Time to mammography appointment (days from randomization to appointment) | MR3 | X | ||||
| Time to diagnosis after abnormal result (days from abnormal result to definitive diagnosis) | X | |||||
| MUA designation (eligible/designate d before 1998; eligible/designated after 1998; eligible/not designated; ineligible) | HRSA[33]4 | Effect Modifier | X | |||
| Adherence to screening, diagnostic care, and treatment guidelines (composite score)5 | MR3 | Secondary Outcome | X | |||
| Receipt of multiple mammograms every 1–2 years (yes/no) | MR3 | X | ||||
| Hospital Site | Study logs2 | Potential Covariates | X | |||
| Number of navigator contacts | Study logs2 | X | ||||
| Number and types of barriers[5] | Study logs2 | X | ||||
| Census tract factors (e.g. poverty; ethnic composition) | ACS[32 ]6 | X | ||||
| Demographics (e.g., age, marital status, ethnicity, income, education)[34] | Survey; MR3 | X | X | |||
| Healthcare access (insurance, PCP)[34] | X | X | ||||
| Cancer history (e.g., family, personal) | X | X | ||||
| Healthcare history (e.g., lifetime mammography/Pap test use)[34] | Survey | X | ||||
| Healthcare distrust[35] | X | |||||
| Shared decision making preferences7 | X | X | X | |||
| Patient-provider communication about breast cancer (e.g., provider recommendations, adherence to recommendation)7 | X | X | X | |||
| Fatalism[36] | X | X | ||||
| Healthcare self-efficacy[37] | X | X | ||||
| Patient satisfaction[5] | X | X | ||||
Full medical record abstraction for all individuals who were randomized to the trial across all three participating hospitals occurred at the end of the study.
Study logs were maintained and managed by navigators under the auspices a staff member with years of management in patient navigation programs.
Medical records from the three participating sites.
Health Resources and Services Administration data for 2011.
This composite variable will be the sum of multiple guidelines and conventions regarding breast cancer care, including uptake of a mammogram every 1–2 years after entry into the study, if 50–74 years old[29–30]; receipt of a diagnosis within 60 days of an abnormal screen or symptomatic presentation[38]; initiation of treatment within 60 days of a definitive cancer diagnosis[38]; and treatment adherence according to the National Comprehensive Cancer Network’s guidelines[39]. Women aged <50 or >75 years old, the range will be 0–3. For women aged 50–74 years old, the range will be 0–4.
American Community Survey data 2007–2011.
Items developed and pilot-tested during this trial.
2.12 Measures
Table 1 depicts the measures that were used, the data sources for the measures, variable type, and when the particular measure was collected/abstracted during the course of the study.
2.13 Planned Statistical Analyses
Table 2 re-iterates our primary objectives and links them to the type of model will be used as well as the outcome and predictors that will be included.
| Primary objectives To assess if: | Outcomes | Predictors | Covariates1 | Analysis Type |
|---|---|---|---|---|
| navigation improves recommended breast cancer care uptake (screening or diagnostic mammography) and time to diagnosis following an abnormal mammogram | Time to mammography appointment (days) | Study Arm2 | Hospital site, age, race/ethnicity, insurance status, and neighborhood-level socioeconomic status (e.g., poverty) | Cox survival analysis |
| Time to diagnosis after abnormal result (days) | ||||
| navigation effects depend on patients’ residential MUA status | Time to mammography appointment (days) | Study Arm*MUA status3,4 | Cox survival analysis (overall & stratified)5 | |
| Time to diagnosis after abnormal result (days) | ||||
| Secondary objectives To assess the efficacy of navigation across | ||||
| different points of the care continuum among patients diagnosed with breast cancer | Adherence to screening, diagnostic care, and treatment guidelines (composite score)6 | Study Arm2 | Ordinal regression | |
| multiple regular screening episodes among patients who did not obtain breast cancer diagnoses. | Receipt of multiple mammograms every 1–2 years (yes/no) | Logistic Regression |
Listed covariates will be included in models. Simultaneously other variables listed in Table 2 may be also be included in models, depending on preliminary bivariate analyses.
Control/Usual Care group will be the referent group.
Affluent/ineligible MUA status will be the referent group.
The main effects of study arm and MUA status will be included in these models.
If interaction terms are significant, strata-specific Cox regression models will be run to assess study arm differences for the different types of MUA status groups.
For this outcome, given ranges differ by age ranges, analyses will be stratified by age group (50–74 years old or other).
2.14 Power analyses
We developed a simulation model to specify hypothesized results for the more complex primary objective, which concerns interactive effects of MUA status and study arm differences. The very conservative assumption was made that outcomes in the control group were equal to 0.40 for all three hospitals and that there was an interaction for treatment. Then, we simulated 10,000 sets of data were calculated for each to determine a significant effect for the various coefficients in the model. The results showed power greater than 0.80 for all parameters of the model for a two-tailed test. In general, Cox regression models are far more powerful than logistic regression because of the time-dependent nature of the data. For example, assuming a very low event rate of 0.10, a binary predictor correlated with covariates at 0.20, and a two- sided test, we are able to detect hazard ratios equal to or greater than 1.17.
2.15 Missingness and sensitivity analyses
With regard to the potential for missingness, outcome data for women who refused to participate in the study and women who dropped out of the study will be available from hospital electronic medical records and will be included in a standard intent to treat analysis. In the event of significant missingness, we plan to use Full Information Maximum Likelihood procedures [40] and conduct sensitivity analyses with available empirical data. Other sensitivity analyses will concern the timing of initial contact with participants (e.g., pre- versus at initial appointment) as well as the number of contacts with the navigator to evaluate dose-response associations.
3. Discussion
Patient navigation has emerged as an important strategy to reduce and eliminate disparities in screening, diagnosis, care and survivorship of breast cancer. PNMUA is situated to 1) add to the growing body of literature regarding the effectiveness of patient navigation in improving screening and time to diagnostic resolution and 2) contribute to important gaps in the literature related to macro-level factor and longitudinal patterns in recommended breast cancer care. We believe our study has made several important improvements over previous studies testing navigation. First, we adopt an individual-level RCT approach. As discussed above, there are a growing number of individual-level RCTs in the context of navigation and breast cancer. In response to some previous studies’ limitations, PNMUA is able to leverage a relatively large sample size and is able to access to medical record data. Second, we have the ability to examine the interactive effects of navigation and macro-level MUA factors. Third, our longitudinal study (2011–2014) allows us to assess efficacy across multiple episodes of cancer care – for both individuals diagnosed with breast cancer as well as those not ultimately diagnosed with breast cancer. Finally, our study is situated within South Chicago, an area that has been shown to have particularly poor breast cancer outcomes and mortality, which makes it well suited for intervention.
Limitations
We acknowledge several limitations to this study. The study draws from a sample of women accessing breast care at three hospitals located in MUAs of Chicago, thus limiting its generalizability to women in general who are accessing breast care. As with any longitudinal intervention study, some amount of attrition is expected due to voluntary drop out, illness, loss of contact, or seeking care at a different location. Further, while longitudinal in nature, four years is a relatively small time period in the context of breast cancer screening, especially current guidelines. Another potential limitation is the method of random assignment for women in the ‘active control’ group. Participants from both the intervention group and active control group interacted with navigators. Both groups received information about the PNMUA study and completed questionnaires with navigators prior to the initial appointment. Further, if ‘active controls’ obtained abnormal results or a definitive diagnosis, they would have completed surveys and thus interacted with navigators. While ‘passive controls’ allow for some ability to disentangle whether the effects of patient navigation are due to the intervention itself or attention from the interaction with navigators, the only data available for ‘passive controls’ is limited to medical record and neighborhood-level data. Also, there is the possibility of contamination between the control and intervention arms since all study participants were patients at the hospital sites. Contamination may lead to minimized differences in study outcomes between groups. In any study of health behavior, there is always the possibility that self-reported health behavior may be over or under reported. However, most of the data for this study was extracted from hospital electronic medical records.
Conclusion
In conclusion, results from the PNMUA study will provide crucial information about the effectiveness of patient navigation on improving adherence to screening and early detection through timely follow-up of abnormal test results as well as how this effectiveness potentially varies among women from neighborhoods varying in access to resources. Further, we will have preliminary data concerning efficacy across different episodes of cancer care uptake in terms of comparing time to care at screening, diagnosis, and treatment stages as well as adherence to longitudinal screening recommendations.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health-National Institute on Minority Health and Health Disparities (P60MD003424; P60MD003424-02S1) and the National Cancer Institute (P50CA106743; P50CA148143; U54CA203000; U54CA202995; U54CA202997).
We would like to thank Ms. Nerida M. Berrios, Dr. Micheal Berbaum, Dr. Ganga Vijayasiri, Dr. Richard Warnecke, Dr. Richard Campbell, Ms. Heather Pauls, and Ms. Ifeanyi Chukwudozie for their technical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ooi S, Martinez M, Li C. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127(3):729–38. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Clegg L, Ward E, Ries L, Wu X, Jamison P, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 3.Freeman H. Patient navigation: a community based strategy to reduce cancer disparities. Journal of Urban Health. 2006;83:139–41. doi: 10.1007/s11524-006-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman H, Rodriguez R. History and principles of patient navigation. Cancer. 2011;117:3537–40. doi: 10.1002/cncr.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freund K, Battaglia T, Calhoun E, Dudley D, Fiscella K, Paskett E, et al. The NCI Patient Navigation Research Program Methods, Protocol, and Measures. Cancer. 2008;113:3391–9. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markossian T, Darnell J, Calhoun E. Follow-up and timeliness after abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiology Biomarkers and Prevention. 2012;21:1691–700. doi: 10.1158/1055-9965.EPI-12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tejeda S, Darnell J, Cho Y, Stolley M, Markossian T, Calhoun E. Patient barriers to follow-up care for breast and cervical cancer abnormalities. Journal of Women’s Health. 2013;22:507–17. doi: 10.1089/jwh.2012.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansell D, Grabler P, Whitman S, Ferrans C, Burgess-Bishop J, Murray L, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009;20:1681–8. doi: 10.1007/s10552-009-9419-7. [DOI] [PubMed] [Google Scholar]
- 9.Hirschman J, Whitman S, Ansell D. The black: whtie disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control. 2007;18:323–33. doi: 10.1007/s10552-006-0102-y. [DOI] [PubMed] [Google Scholar]
- 10.Hunt B, Whitman S, Hurlbert M. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiology. 2013;38:118–23. doi: 10.1016/j.canep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Ko N, Darnel J, Calhoun E, Freund K, Wells K, Shapiro C, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Research Program. Journal of Clinical Oncology. 2014;32:2758–64. doi: 10.1200/JCO.2013.53.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paskett E, Harrop J, Wells K. Patient navigation: An update on the state of science. CA: A Cancer Journal for Clinicians. 2011;61:237–49. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells K, Battaglia T, Dudley D, Garcia R, Greene A, Calhoun E, et al. Patient navigation: State of the art or is science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson-White S, Conroy B, Slavish K, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nursing. 2010;33:127–40. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia T, Bak S, Heeren T, Chen C, Kalish R, Tringale S, et al. Boston Patient Navigation Research Program: The impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiology Biomarkers and Prevention. 2012;21:1645–54. doi: 10.1158/1055-9965.EPI-12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paskettt E, Katz M, Post D, Pennell M, Young G, Seiber J, et al. The Ohio Patient Navigation Research Program: does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiology Biomarkers and Prevention. 2012;21:1620–8. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raich P, Whitley E, Thorland W, Valverde P, Fiarclough D, Program DPNR. Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiology Biomarkers and Prevention. 2012;21:1629–38. doi: 10.1158/1055-9965.EPI-12-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante J, Chen P-H, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: A randomized controlled trial. Journal of Urban Health. 2007;85:114–25. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ell K, Vourlekis B, Lee P, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Preventive Medicine. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell A, Jo A, Crespi C, Sudan M, Bastani R. Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: results of a randomized trial. Cancer Causes Control. 2010;21:1931–40. doi: 10.1007/s10552-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dignan M, Burhansstipanov L, Hariton J, Harjo L, Rattler J, Lee R, et al. A comparison of two Native AMerican Navigator formats: face-to-face and telephone. Cancer Control: journal of the Mofitt Cancer Center. 2005;12:28–33. doi: 10.1177/1073274805012004S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall J, Mbah O, Ford J, Phelan-Emrick D, Ahmed S, Bone L, et al. Effect of patient navigation on breast cancer screening among African American Medicare beneficiaries: A randomized controlled trial. Journal of General Internal Medicine. 2016;31:68–76. doi: 10.1007/s11606-015-3484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedlund N, Risendal B, Pauls H, Valverde P, Whitley E, Esparaza A, et al. Dissemination of patient naivgation programs across the United States. Journal of Public Health Management and Practice. 2014;20:E15–E24. doi: 10.1097/PHH.0b013e3182a505ec. [DOI] [PubMed] [Google Scholar]
- 24.Warnecke R, Oh A, Breen N, Gehlert S, Paskett E, Tucker K, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Heath. 2008;98:1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett R, Cho Y, Weaver K, Ryu K, Campbell R, Dolecek T, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Annals of epidemiology. 2008;18:43–7. doi: 10.1016/j.annepidem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Zenk S, Tarlov E, Sun J. Spatial equity in facilities providing low-or no-fee screening mammography in Chicago neighborhoods. Journal of Urban Health. 2006;83:195–210. doi: 10.1007/s11524-005-9023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarlov E, Zenk S, Campbell R, Warnecke R, Block R. Characteristics of mammography facility locations and stage at breast cancer diagnosis in Chicago. Journal of Urban Health. 2009;86:196–213. doi: 10.1007/s11524-008-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miraftab FWD, Salo K. Cities and inequalities in a global and neoliberal world. Routledge; 2015. [Google Scholar]
- 29.USPSTF. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 30.USPSTF. Screening for breast cancer: topic page. 2013. [Google Scholar]
- 31.Martin N, Wingfield J. USPSTF screening recommendations for breast cancer: the potential impact on the African American community. Journal of Health Care for the Poor and Underserved. 2012;23:91–7. doi: 10.1353/hpu.2012.0072. [DOI] [PubMed] [Google Scholar]
- 32.American Community survey 5 year estimates: 2007–2011. 2014. [Google Scholar]
- 33.US Department of Health & Human Services. MUA find. Retrieved from: http://muafind.hrsa.gov.
- 34.CDC. Behavioral Risk Factor Surveillance System 2014 Codebook. [Google Scholar]
- 35.Shea J, Macro E, Dean L, McMurphy S, Schwartz J, Armstrong K. Development of a revised health care system distrust scale. Journal of General Internal Medicine. 2008;23:727–32. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powe B. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer nursing. 1995;18:385–92. [PubMed] [Google Scholar]
- 37.Wolf M, Chang C, Davis T, Makoul G. Development and validation of the Communication and Attitudinal Self-efficacy scale for cancer (CASE-Cancer) Patient Education and Counseling. 2005;57:333–41. doi: 10.1016/j.pec.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.DeGroff A, Royalty J, Howe W, Buckman DW, Gardner J, Poister T, Hayes N. When performance management works: A study of the National Breast and Cervical Cancer Early Detection Program. Cancer. 2014;S16:2566–2574. doi: 10.1002/cncr.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Comprehensive Cancer Network. NCCN Guidelines. Retrieved from: https://www.nccn.org/framework/
- 40.Graham J. Adding missing-data-relevant variables to FIML-based structural equation models. Structural Equation Modeling. 2003;10:80–100. [Google Scholar]