Abstract
IMPORTANCE
Atherosclerotic vertebrobasilar (VB) occlusive disease is a significant etiology of posterior circulation stroke, with regional hypoperfusion as an important potential contributor to stroke risk.
OBJECTIVE
To test the hypothesis that, among patients with symptomatic VB stenosis or occlusion, those with distal blood flow compromise as measured by large-vessel quantitative magnetic resonance angiography (QMRA) are at higher risk of subsequent posterior circulation stroke.
DESIGN, SETTING, AND PARTICIPANTS
A prospective, blinded, longitudinal cohort study was conducted at 5 academic hospital-based centers in the United States and Canada; 82 patients from inpatient and outpatient settings were enrolled. Participants with recent VB transient ischemic attack or stroke and 50% or more atherosclerotic stenosis or occlusion in vertebral and/or basilar arteries underwent large-vessel flow measurement in the VB territory using QMRA. Physicians performing follow-up assessments were blinded to QMRA flow status. Follow-up included monthly telephone calls for 12 months and biannual clinical visits (for a minimum of 12 months, and up to 24 months or the final visit). Enrollment took place from July 1, 2008, to July 31, 2013, with study completion on June 30, 2014; data analysis was performed from October 1, 2014, to April 10, 2015.
EXPOSURE
Standard medical management of stroke risk factors.
MAIN OUTCOMES AND MEASURES
The primary outcome was VB-territory stroke.
RESULTS
Of the 82 enrolled patients, 72 remained eligible after central review of their angiograms. Sixty-nine of 72 patients completed the minimum 12-month follow-up; median follow-up was 23 (interquartile range, 14–25) months. Distal flow status was low in 18 of the 72 participants (25%) included in the analysis and was significantly associated with risk for a subsequent VB stroke (P = .04), with 12- and 24-month event-free survival rates of 78% and 70%, respectively, in the low-flow group vs 96% and 87%, respectively, in the normal-flow group. The hazard ratio, adjusted for age and stroke risk factors, in the low distal flow status group was 11.55 (95% CI, 1.88–71.00; P = .008). Medical risk factor management at 6-month intervals was similar between patients with low and normal distal flow. Distal flow status remained significantly associated with risk even when controlling for the degree of stenosis and location.
CONCLUSIONS AND RELEVANCE
Distal flow status determined using a noninvasive and practical imaging tool is robustly associated with risk for subsequent stroke in patients with symptomatic atherosclerotic VB occlusive disease. Identification of high-risk patients has important implications for future investigation of more aggressive interventional or medical therapies.
Posterior circulation strokes account for up to 30% of all ischemic strokes, and atherosclerotic occlusive disease of the vertebrobasilar (VB) system is an important etiology, responsible for approximately one-third of the cases.1,2 Symptomatic atherosclerotic VB occlusive disease is associated with a high risk of recurrent stroke despite medical therapy, occurring in 10% to 15% of the patients within 2 years.3–8 Despite this prevalence and potentially devastating prognosis, the posterior circulation has largely been overlooked compared with the extensive emphasis on carotid artery territory stroke. This discrepancy may be related, at least in part, to the greater challenges of surgical interventions for the VB system compared with the carotid system.9,10 However, advances in endovascular treatment options for VB occlusive disease in recent years have kindled interest in interventional therapies. Endovascular angioplasty and stenting in the posterior circulation are now technically feasible but still carry a significant periprocedural risk11–13; therefore, such therapies are likely to benefit only selected patients at highest risk of recurrent ischemia.
The role of hemodynamic insufficiency in the etiology of stroke associated with VB stenosis and/or occlusion has long been purported, but there have been few data to directly support this mechanism. Although hemodynamic impairment as an important indicator of risk for stroke has been well demonstrated in the anterior circulation, the imaging techniques used rely primarily on assessment of tissue perfusion and have not translated easily to evaluation of the more-constrained posterior circulation territory.14 Measurement of large cerebral vessel flow, using phase-contrast quantitative magnetic resonance angiography (QMRA), is readily feasible in posterior circulation vessels and can provide a surrogate measure of hemodynamic status.15 Previous retrospective data have indicated that designation of flow compromise based on QMRA is an indicator for recurrent stroke risk in patients with atherosclerotic VB occlusive disease.15 Given the limitations of single-center retrospective data, the generalizability and confirmation of these findings required prospective, standardized evaluation. Thus, we now report the results of Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS), a prospective, observational multicenter study undertaken to test the hypothesis that patients with distal blood flow compromise as measured by QMRA are at higher risk of subsequent posterior circulation stroke.
Methods
Study Design and Participants
The details of the trial design and the baseline features of the study cohort have been published.16,17 VERiTAS was a longitudinal, prospective, multicenter observational study conducted at 5 academic hospital-based centers in the United States and Canada and was funded by the National Institute for Neurological Disorders and Stroke. The study protocol was approved by the institutional review boards at each site (University of Illinois at Chicago, Columbia University Medical Center, University of California at Los Angeles Medical Center, University Health Network–Toronto Western Hospital, Washington University in St Louis Medical Center), and written informed consent was provided by each participant; there was no financial compensation. Patients with posterior circulation transient ischemic attack or stroke within 60 days that was referable to 50% or more atherosclerotic stenosis of extracranial or intracranial VB arteries, up to and including occlusion, based on conventional digital subtraction angiography or computed tomographic angiography were enrolled. For purposes of enrollment, site determination of angiographic eligibility was used, but imaging was sent for subsequent central review to determine the individual’s ultimate inclusion in the analyzed cohort. Following initiation of the study, 2 additional exclusion criteria were instituted based on interim central review of the imaging data. First, patients with unilateral vertebral disease (stenosis or occlusion) were no longer enrolled owing to the preponderance of normal flow status among such patients, which endangered recruitment of a sufficient number of patients with low-flow status to test the study hypothesis. Second, individuals with solely unilateral vertebral occlusion were excluded from subsequent analyses owing to uncertainty in distinguishing the underlying etiology as dissection vs atherosclerotic disease given the disparate prognosis and stroke mechanisms of these 2 entities.
Study Assessments and Follow-up
Details of the baseline clinical and imaging evaluation and follow-up schedule have been reported.16 Briefly, all participants underwent standard neurologic evaluation, and data regarding the nature and frequency of cerebral ischemic events, medications at the time of enrollment, vascular risk factors, and available laboratory and standard imaging results were collected. Prespecified definitions for hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, renal dysfunction, smoking status, alcohol intake, and body mass index categories were applied.17
Participants underwent a magnetic resonance imaging protocol, including QMRA, within 14 days of enrollment using a standardized protocol on a 3-T magnetic resonance scanner. The QMRA portion of the study was performed using NOVA (Noninvasive Optimal Vessel Analysis, VasSol, Inc) software, as previously described16 (eMethods in the Supplement), with remote supervision by a certified NOVA technician to ensure correct identification of vessels and parameters for flow measurement on prespecified locations of each major cerebral artery, including the vertebral artery, basilar artery, and posterior cerebral arteries. The data were transferred automatically via secure, internet-based transfer to the clinical coordinating center (CCC) at the University of Illinois at Chicago for central review. The patient and participating site personnel, including the treating physician, remained blinded to the results of the imaging.
Patients underwent routine medical management at the discretion of the treating physician; the physicians were encouraged to adhere to published guidelines for vascular risk factor management and secondary stroke prevention.18–22 The local site coordinators contacted patients monthly by telephone for 12 months for determination of new neurologic events and documentation of medication changes. In the event of a suspected end point, arrangements were made for prompt (within 72 hours) evaluation of the patient by the local study physician. If the evaluation was not feasible (eg, owing to hospitalization elsewhere), medical records were obtained from treating physicians and the patient was evaluated as soon as possible. Scheduled in-person evaluations were performed at 6-month intervals for a minimum of 12 months and up to 24 months; patients who missed appointments were seen at the earliest possible time thereafter.
Imaging and End Point Assessment
For analysis, enrollment angiographic data were centrally reviewed by a blinded interventional neuroradiologist for final determination of the degree of stenosis using the Warfarin-Aspirin Symptomatic Intracranial Disease method.23 The QMRA data were reviewed centrally, and distal flow status was designated as low or normal based on a previously published15 algorithm defining flow compromise as more than a 20% reduction below the normative lower limits of posterior circulation vessel–specific flows. The algorithm stratifies patients based on the flow in the basilar artery as well as in nonfetal posterior cerebral arteries (eFigure in the Supplement). In so doing, the algorithm intrinsically incorporates any sources of collateral flow (eg, via the posterior communicating arteries) by their effect on the blood flow within these distal run-off vessels. In the event of borderline blood flow rates, additional criteria were used to designate flow status as previously described.15 All flow status designations were made by the blinded CCC NOVA reviewer and confirmed by the CCC project coordinator and study principal investigator (S.A.-H.).
The primary end point for the study was fatal and nonfatal ischemic stroke in the VB territory, defined as new neurologic symptoms or signs localizing to an area of the brain supplied by the VB arterial system lasting at least 24 hours or lasting less than 24 hours but associated with a new infarct on computed tomography or magnetic resonance imaging. All potential primary end points were submitted for adjudication and designated as definite, probable, possible, or no event based on prespecified criteria by an independent panel of 2 stroke neurologists (including S.E.K.) blinded to the patient’s hemodynamic data; both the occurrence of stroke and the territory of stroke were adjudicated. If the opinions differed, a third blinded stroke neurologist was consulted and the majority opinion prevailed. Only definite and probable events were included in the end point analyses.
Statistical Analysis
Sample size and power estimates of VERiTAS have been detailed.16 The clinical, angiographic, and hemodynamic features of the full cohort at enrollment have been reported.17 For analysis, participants were characterized as having low or normal distal flow status. Comparison of baseline demographic, clinical, and angioanatomic features of disease was performed using an unpaired 2-tailed t test or Wilcoxon rank sum test as appropriate for continuous variables and a χ2 test for categorical variables. In the primary analysis, the 2 groups were compared using Kaplan-Meier analysis with the log rank statistic. Cox proportional hazards regression modeling was used to assess the influence of the following potential risk variables in univariate analysis: age, sex, race, time lag to enrollment (≤21 days), nature of qualifying event (transient ischemic attack or stroke), hypertension, hyperlipidemia, diabetes mellitus, smoking, renal insufficiency, coronary artery disease, body mass index (BMI) (≥30; ie, obesity [calculated as weight in kilograms divided by height in meters squared]), physical activity (exercise enough to “break a sweat” at least twice per week), stenosis severity, and location. All variables at P < .25 in univariate analysis were included in a backward elimination, stepwise multivariate analysis. Variables with significance at P ≤ .05 were allowed to remain in the model. Interactions between flow status and other stroke risk indicator variables were checked. Based on clinical relevance, the severity and location of disease were subsequently added back to the final model; severity was classified as moderate (50%–69% stenosis), severe (70%–99% stenosis), or occlusion (100% stenosis) based on the worst disease severity; location was specified as basilar (including those with coincident vertebral disease) vs nonbasilar (those with exclusively vertebral disease). Medical management of disease was compared between the 2 groups based on available data at 6-, 12-, 18-, and 24-month follow-up visits (±3 months). The proportions of patients on target for medical management were compared using the χ2 test. On target was defined as follows: blood pressure less than 140/90 mm Hg (or 130/80 mm Hg for patients with diabetes), receiving a statin or other lipid-lowering medication, receiving an antithrombotic medication (antiplatelet or anticoagulant), hemoglobin A1c concentration less than 7%, and BMI less than 30. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Enrollment took place over a 5-year period from July 1,2008, to July 31,2013, with study completion on June 30,2014. Overall, 82 patients were initially enrolled; the CCC had the highest enrollment, but this represented less than half of the study participants (35 of 82 patients [43%]), and baseline characteristics did not differ significantly from the non-CCC-enrolled patients. Following central imaging review, 10 patients (12%) were excluded from the analyzed cohort for failing to meet eligibility criteria: 8 patients with unilateral vertebral occlusion, 1 patient with a VB fenestration misinterpreted as vertebral stenosis based on site review, and 1 patient with complete resolution of basilar occlusion without evidence of underlying atherosclerotic disease (Figure 1).
Figure 1. Flow Diagram of Study Participants.
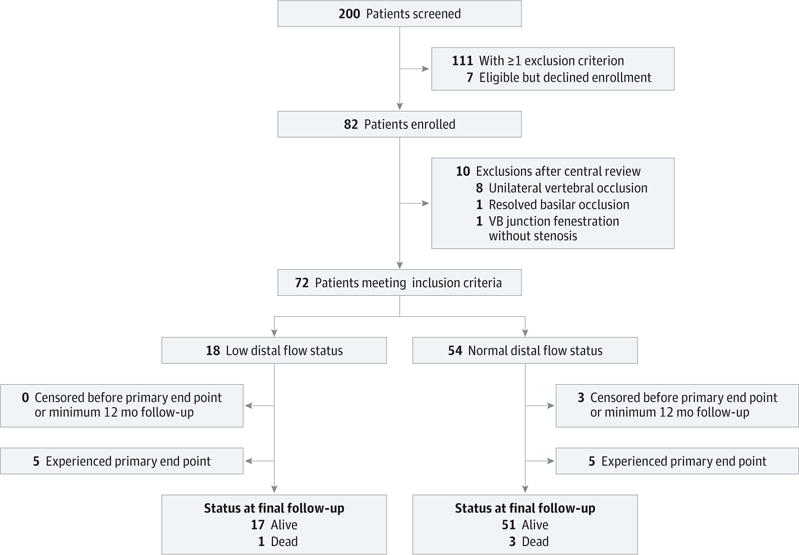
VB indicates vertebrobasilar.
Of the 72 patients included in the analysis, 18 (25%) met the criteria for low distal flow status. Most baseline risk factors did not differ significantly between the distal flow status groups (Table 1). The less frequent level of physical activity among the low distal flow status group was the only significant difference (P = .004) noted. The time lag from the qualifying event to enrollment was shorter in the low distal flow status group compared with the normal distal flow status group, but the difference did not reach significance (median, 15 vs 22 days; P = .22).
Table 1.
Patient Characteristics
| Characteristic | Low Distal Flow (n = 18) | Normal Distal Flow (n = 54) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 68 (9) | 65 (11) | .28 |
| Female sex, No. (%) | 8 (44) | 24 (44) | >.99 |
| Race, No. (%) | |||
| Black | 4 (22) | 14 (26) | .81 |
| White | 12 (67) | 37 (69) | |
| Other | 2 (11) | 3 (6) | |
| Ethnicity, No. (%) | |||
| Hispanic or Latino | 4 (22) | 4 (7) | >.99 |
| Not Hispanic or Latino | 14 (78) | 50 (93) | |
| Qualifying event, No. (%) | |||
| Stroke | 12 (67) | 40 (74) | .54 |
| TIA | 6 (33) | 14 (26) | |
| Prior posterior circulation event,a No. (%) | 11 (61) | 31 (57) | .78 |
| Days from qualifying event to enrollment, median (IQR) | 15 (4–24) | 22 (5–44) | .22 |
| Vascular risk factors, No. (%) | |||
| Hypertension | 17 (94) | 50 (93) | >.99 |
| Diabetes mellitus | 6 (33) | 17 (31) | .88 |
| Hyperlipidemia | 13 (72) | 45 (83) | .32 |
| Coronary artery disease | 5 (28) | 11 (20) | .53 |
| Chronic renal insufficiency or failure | 0 | 2 (4) | >.99 |
| Smoking | |||
| Never | 8 (44) | 23 (43) | .39 |
| Former | 6 (33) | 11 (20) | |
| Current | 4 (22) | 20 (37) | |
| Alcohol use | |||
| None | 11 (61) | 33 (61) | .39 |
| <1 Drink/d | 5 (28) | 19 (35) | |
| ≥1 Drink/d | 2 (11) | 2 (4) | |
| BMI, No. (%) | .59 | ||
| Normal | 2 (11) | 11 (20) | |
| Overweight | 8 (44) | 25 (46) | |
| Obese, class, 1–3 | 8 (44) | 18 (33) | |
| Physical activityb | 1 (6) | 23 (43) | .004 |
| Angiographic disease, No. (%) | |||
| Severe stenosis/occlusion, ≥70% | 16 (89) | 40 (74) | .33 |
| Stenosis location, basilar involved | 12 (67) | 38 (70) | .77 |
| Stenosis location, extracranial only | 1 (6) | 6 (11) | .67 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; TIA, transient ischemic attack.
Posterior circulation ischemic event (TIA or stroke) prior to enrollment event.
Enough exercise to raise a sweat at least twice per week.
The median follow-up duration was 23 (interquartile range, 14–25) months. For the primary analysis, the maximum follow-up included was 27 months to accommodate a 3-month time window around the planned maximum 24-month follow-up. The overall attrition rate before the prespecified minimum 12-month follow-up was 3 of 72 patients (4%): 1 patient died unrelated to a primary event at 2 months, 1 patient withdrew from the study at 8 months, and 1 patient underwent an early 1-year follow-up final study visit at 10 months. Medical management variables at 6,12,18, and 24 months based on the data available at each time point did not differ significantly between the 2 groups (eTable 1 in the Supplement).
During follow-up, the primary end point occurred in 5 of 18 patients (28%) with low distal flow status and 5 of 54 patients (9%) with normal distal flow status. This result translated into a significantly higher risk of subsequent stroke in the low distal flow status group (P = .04, log rank) (Figure 2); 12- and 24-month event-free survival rates of 78% and 70%, respectively, in the low-flow group and 96% and 87%, respectively, in the normal-flow group (eTable 2 in the Supplement). The univariate analysis of risk factors revealed 9 additional variables with significance at P < .25 (Table 2). In the final risk factor-adjusted multivariate model (Table 3), low distal flow status was strongly and significantly associated with VB territory stroke, with a hazard ratio (HR) of 11.55 (95% CI, 1.88–71.00; P = .008). No significant interactions between distal flow status and other variables were present, although the sample size was limited to detect such interactions. Disease severity and location did not attenuate the value of distal flow status as an indicator of risk when added to this multivariate model (flow status HR, 13.27 [95% CI, 2.01–89.66]; P = .007) (eTable 3 in the Supplement). An additional sensitivity analysis that included the 82 participants initially enrolled attenuated, but did not materially change, the results (HR, 3.93; 95% CI, 1.02–15.18).
Figure 2. Cumulative Hazard Curve for the Primary End Point of Vertebrobasilar Territory Stroke.
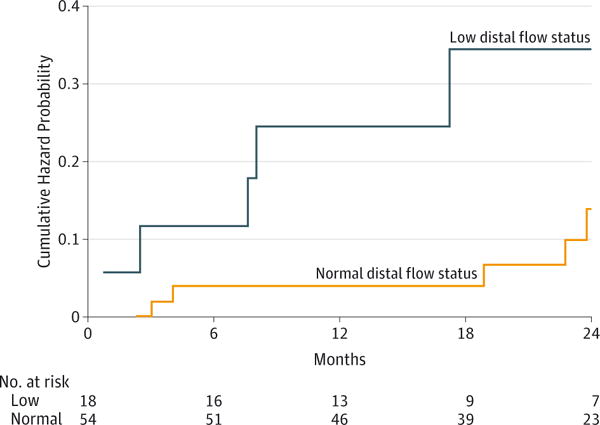
The number of participants at risk for each 6-month interval is indicated below the graph.
Table 2.
Univariate Analysis of Risk Factors for Primary End Point of Subsequent VB Territory Stroke
| Risk Factor3 | HR (95% CI) | P Value |
|---|---|---|
| Age | 0.92 (0.87–0.98) | .01 |
| Female sex | 2.06 (0.58–7.30) | .26 |
| Black race | 2.36 (0.66–8.38) | .19 |
| Low distal flow status | 3.41 (0.99–11.82) | .05 |
| Stroke as qualifying event | 0.89 (0.23–3.43) | .87 |
| Prior stroke or TIA | 3.13 (0.66–14.77) | .14 |
| Lag time, ≤21 db | 3.82 (0.81–18.00) | .09 |
| Diabetes mellitus | 6.20 (1.60–24.06) | .01 |
| Hyperlipidemia | 0.97 (0.21–4.57) | .97 |
| Coronary artery disease | 3.80 (1.10–13.17) | .04 |
| Smoking | 0.89 (0.23–3.42) | .86 |
| Alcohol use | 0.39 (0.08–1.85) | .24 |
| BMI ≥30 | 0.80 (0.21–3.09) | .73 |
| Physical activity | 0.22 (0.03–1.77) | .16 |
| Stenosis severity, ≥70% | 0.61 (0.16–2.36) | .47 |
| Stenosis location | ||
| Basilar involved | 1.15 (0.30–4.46) | .84 |
| Extracranial | 1.16 (0.15–9.22) | .89 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; TIA, transient ischemic attack; VB, vertebrobasilar.
Risk associated with hypertension and renal failure could not be evaluated owing to a lack of primary end points among patients without hypertension or with renal failure.
Lag time from qualifying event to enrollment.
Table 3.
Risk-Adjusted Multivariate Model of Predictors of Subsequent Stroke
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Low distal flow status | 11.55 (1.88–71.00) | .008 |
| Age | 0.80 (0.70–0.91) | .001 |
| Coronary artery disease | 10.47 (1.54–71.34) | .02 |
| Diabetes mellitus | 9.63 (1.66–55.76) | .01 |
| Physical activity | 0.06 (0.005–0.64) | .02 |
Abbreviation: HR, hazard ratio.
Discussion
The results of the VERiTAS study demonstrate that hemodynamic compromise assessed by large-vessel flow measurements is an independent indicator of risk for subsequent VB territory stroke in patients with VB atherosclerotic stenosis and/or occlusion. Although the limited sample size reduces the precision of the estimated cumulative risk of stroke, these prospective, multicenter, blinded results confirm and closely replicate the results of the smaller, single-center, retrospective study that found a very similar 24-month stroke-free survival rate of 71% in the low distal flow status group.15
Although the cohort, by the nature of study recruitment, represents a selected population, the overall demographics and clinical profile resemble those of other published prospective series with population-based and consecutive cohorts.24,25 Pooled data from such studies indicate a 90-day risk of stroke following presentation of 9.6%, which is higher than that observed in our overall cohort; however, our study examined stroke risk from the time of enrollment rather than from the time of the qualifying event given that the patients’ hemodynamic status based on QMRA could be assessed only after entry into the study. Because prior data support a high risk of early recurrence (potentially highest in the first 1 to 2 weeks after the initial event25,26), our median 21-day time lag to enrollment could account for the lower observed overall risk. Nonetheless, the observed risk in our overall group (eTable 2 in the Supplement) falls within the range reported in a recent meta-analysis8 of retrospective and prospective studies, at a mean of 9.6 per 100 person-years.
In our cohort, the low distal flow status group had a shorter lag time from the qualifying event to enrollment than did the normal distal flow status group, which could serve as a potential confounder given that the risk of recurrent ischemic events has been found25 to be highest soon after the presenting event. Median lag time, however, did not emerge as a significant indicator of stroke risk in univariate analysis and was confirmed to have no influence on the independent predictive value of distal flow status even when examined in the multivariate model. Other baseline vascular risk factors were similar between the groups. Medical management profiles during the study period did not differ significantly between the 2 groups based on available data. However, medical management was at the discretion of treating physicians, and laboratory tests documenting the efficacy of treatments, such as low- density lipoprotein cholesterol and hemoglobin A1c levels, were not study mandated and thus were recorded only when included as part of routine care. This lack of consistent data represents an inherent limitation of our observational study design, although the likelihood of differential management for the low vs normal distal flow status groups is minimal given that treating physicians were blinded to the patients’ hemodynamic status.
Other factors that emerged as independently associated with subsequent stroke in our cohort included coronary artery disease, diabetes mellitus, and physical activity. Diabetes mellitus and physical activity have been well described as risk factors for stroke.21 The definition of coronary artery disease in this cohort included a history of myocardial infarction,17 which is a known risk factor for recurrent stroke.27 In our cohort, the inverse association between age and risk of stroke was similar to previously reported results in prospectively monitored patients with carotid occlusion.28 More detailed analysis of these risk factors warrants investigation in larger populations of patients in future studies.
Prior series have observed extracranial vertebral artery stenosis to carry a more benign prognosis3; we did not find this feature to be an indicator of risk, but the low number of participants with isolated extracranial disease limits definitive conclusion. Basilar disease location has been reported as a risk factor8 but did not emerge as an independent indicator of risk in our analysis. Anatomical features of the disease, such as stenosis severity or location of disease, neither reliably indicated distal flow status, as previously published,17 nor served as independent indicators of stroke risk. Patients in our cohort were included based on central imaging review and confirmation of the degree of VB stenosis or occlusion; however, sensitivity analysis of the full cohort of 82 initially enrolled patients did not substantively alter the results of the multivariate analysis: flow status remained an independent indicator of stroke risk.
The etiology of stroke in the setting of atherosclerotic VB occlusive disease can be attributed to several potential mechanisms, including thromboembolism, concomitant small-vessel occlusive disease, or hypoperfusion. Reports3 demonstrating a high risk of early recurrence in symptomatic patients implicate unstable plaque and thromboembolism as an important mechanism. Our study, however, would tend to support hypoperfusion as a key underlying mechanism. Beyond the direct consequences of compromised distal flow, thrombus formation as a sequela of low distal flow may be a contributor in addition to an underlying low distal flow state potentiating stroke by reducing the washout of emboli.29 Regardless, the clinically relevant factor emerging from our data is that the presence of hemodynamic compromise provides valuable prognostic information. Our cohort was treated with standard medical therapy, which typically entailed antiplatelet monotherapy and statin use, but achieved optimal blood pressure targets in less than half of the patients (eTable 1 in the Supplement). More recent studies30 have demonstrated incremental benefits in stroke risk reduction with the use of dual antiplatelets. The aggressive medical management regimen of vascular risk factors in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial4 resulted in lower stroke rates in the medical arm compared with patients with similar profiles in the Warfarin-Aspirin Symptomatic Intracranial Disease trial,31 which had used standard medical management. Although these data indicate that the overall stroke risk in our cohort could be improved by intensive medical therapies, such therapies may be difficult or costly to routinely implement outside a trial setting and are unlikely to beneficially affect an underlying hypoperfusion state.
Revascularization strategies to augment blood flow represent one logical approach to consider for the flow-compromised patient. Endovascular angioplasty and stenting offer the potential to improve hemodynamic compromise, and successful distal flow augmentation following such interventions has been demonstrated.32 However, periprocedural risks are high with VB stenting, ranging up to 20% in recent prospective studies,12,33 particularly for basilar disease. Our data support targeting the high-risk, distal flow-compromised patients as the candidate population for future trials of endovascular intervention. Furthermore, our data indicate that patients with normal distal flow, who compose three-fourths of the patient population and are at low risk of subsequent stroke while receiving medical therapy, are unsuitable for potentially risk-prone procedures and should not undergo intervention.
Conclusions
This prospective observational study has demonstrated that distal flow status in the posterior circulation is a robust indicator of subsequent VB stroke risk in patients with symptomatic atherosclerotic VB occlusive disease. Thus, noninvasive large-vessel distal flow measurement using QMRA represents a novel and easily applicable method for risk stratification in such patients. These results have important implications for patient management, including identification of high-risk patients for potential future investigation of interventional or aggressive medical therapies.
Supplementary Material
Acknowledgments
Funding/Support: The study was funded by grant R01 NS059745 from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS). Additional funding and support was provided by the Dr Ralph and Marian Falk Research Trust Foundation. Material research support was provided by VasSol Inc (supplying NOVA technology and technical support).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Dr Amin-Hanjani had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Amin-Hanjani, Pandey, Richardson, Caplan, Derdeyn, Charbel.
Acquisition, analysis, or interpretation of data: Amin-Hanjani, Pandey, Rose-Finnell, Du, Richardson, Thulborn, Elkind, Zipfel, Liebeskind, Silver, Kasner, Aletich, Derdeyn, Gorelick, Charbel.
Drafting of the manuscript: Amin-Hanjani, Pandey, Richardson.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Pandey, Du, Richardson.
Obtained funding: Amin-Hanjani, Pandey, Richardson, Thulborn, Charbel.
Administrative, technical, or material support: Rose-Finnell, Du, Thulborn, Charbel.
Study supervision: Amin-Hanjani, Pandey, Richardson, Derdeyn, Charbel.
Conflict of Interest Disclosures: Dr Amin-Hanjani reported receiving material research support (no direct funds) from GE Healthcare and VasSol Inc for the VERiTAS study. Dr Liebeskind reported serving as a consultant for Covidien and Stryker. Dr Derdeyn reported serving as a consultant for Microvention, Penumbra, and Silk Road and as a member of the scientific advisory board for Pulse Therapeutics. Dr Gorelick reported serving as the founder and director/codirector of the Clinical Coordinating Center for the Lundbeck-sponsored Desmoteplase in Acute Ischemic Stroke trial. Dr Charbel reported having a financial interest in VasSol Inc. No other conflicts were reported.
Additional Contributions: We thank all members of the VERiTAS Study Group.
References
- 1.Caplan LR. Vertebrobasilar disease. Adv Neurol. 2003;92:131–140. [PubMed] [Google Scholar]
- 2.Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56(3):389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 3.Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. 2013;44(3):598–604. doi: 10.1161/STROKEAHA.112.669929. [DOI] [PubMed] [Google Scholar]
- 4.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi AI, Ziai WC, Yahia AM, et al. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52(5):1033–1039. [PubMed] [Google Scholar]
- 6.The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. Stroke. 1998;29(7):1389–1392. doi: 10.1161/01.str.29.7.1389. [DOI] [PubMed] [Google Scholar]
- 7.Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke. 1986;17(5):938–942. doi: 10.1161/01.str.17.5.938. [DOI] [PubMed] [Google Scholar]
- 8.Abuzinadah AR, Alanazy MH, Almekhlafi MA, et al. Stroke recurrence rates among patients with symptomatic intracranial vertebrobasilar stenoses: systematic review and meta-analysis [published online December 11, 2014] J Neurointerv Surg. doi: 10.1136/neurintsurg-2014-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ausman JI, Diaz FG, Vacca DF, Sadasivan B. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990;72(4):554–558. doi: 10.3171/jns.1990.72.4.0554. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins LN, Budny JL. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70(2):207–211. doi: 10.3171/jns.1989.70.2.0207. [DOI] [PubMed] [Google Scholar]
- 11.Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40(5):e340–e34. doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 12.Nahab F, Lynn MJ, Kasner SE, et al. NIH Multicenter Wingspan Intracranial Stent Registry Study Group Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72(23):2014–2019. doi: 10.1212/01.wnl.0b013e3181a1863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. VISSIT Trial Investigators Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313(12):1240–1248. doi: 10.1001/jama.2015.1693. [DOI] [PubMed] [Google Scholar]
- 14.Derdeyn CP, Grubb RL, Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53(2):251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- 15.Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36(6):1140–1145. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 16.Amin-Hanjani S, Rose-Finnell L, Richardson D, et al. VERiTAS Study Group Vertebrobasilar Flow Evaluation and Risk of Transient Ischaemic Attack and Stroke study (VERiTAS): rationale and design. Int J Stroke. 2010;5(6):499–505. doi: 10.1111/j.1747-4949.2010.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin-Hanjani S, Du X, Rose-Finnell L, et al. VERiTAS Study Group Hemodynamic features of symptomatic vertebrobasilar disease. Stroke. 2015;46(7):1850–1856. doi: 10.1161/STROKEAHA.115.009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes–2007. Diabetes Care. 2007;30(suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Adams R, Albers G, et al. American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 22.Adams RJ, Albers G, Alberts MJ, et al. American Heart Association; American Stroke Association Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39(5):1647–1652. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–646. [PMC free article] [PubMed] [Google Scholar]
- 24.Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain. 2009;132(Pt 4):982–988. doi: 10.1093/brain/awp026. [DOI] [PubMed] [Google Scholar]
- 25.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009;40(8):2732–2737. doi: 10.1161/STROKEAHA.109.553859. [DOI] [PubMed] [Google Scholar]
- 26.Kozak O, Tariq N, Suri MF, Taylor RA, Qureshi AI. High risk of recurrent ischemic events among patients with deferred intracranial angioplasty and stent placement for symptomatic intracranial atherosclerosis. Neurosurgery. 2011;69(2):334–342. doi: 10.1227/NEU.0b013e31821789ad. [DOI] [PubMed] [Google Scholar]
- 27.Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2009;80(9):1012–1018. doi: 10.1136/jnnp.2008.170456. [DOI] [PubMed] [Google Scholar]
- 28.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280(12):1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 29.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55(11):1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wang Y, Zhao X, et al. CHANCE Investigators Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 31.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 32.Guppy KH, Charbel FT, Corsten LA, Zhao M, Debrun G. Hemodynamic evaluation of basilar and vertebral artery angioplasty. Neurosurgery. 2002;51(2):327–333. [PubMed] [Google Scholar]
- 33.Fiorella D, Derdeyn CP, Lynn MJ, et al. SAMMPRIS Trial Investigators Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43(10):2682–2688. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


