Abstract
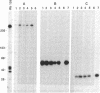
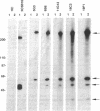
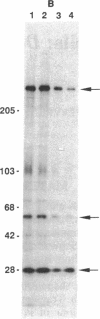
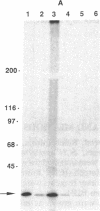
Identification of continuous epitopes in the target antigens of Plasmodium falciparum transmission-blocking antibodies is likely to facilitate the production of a subunit peptide vaccine. Two such epitopes shared among several sexual-stage antigens were identified with murine monoclonal antibodies. An epitope recognized by four monoclonal antibodies capable of blocking infectivity of gametocytes in the mosquitoes is shared among three antigens (230, 48/45 doublet, and 27 kDa). These antigens are synthesized at different times during the development and maturation of gametocytes, and the blocking epitope appears conserved among parasites from diverse geographical locations. Immune response against such a unique epitope (continuous, cross-reacting, and conserved) is likely to be boosted by natural infection. The 27-kDa protein is reported here as a target of malaria transmission-blocking monoclonal antibodies, and the cross-reacting epitope represents an attractive candidate for a transmission-blocking vaccine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzofsky J. A., Cease K. B., Cornette J. L., Spouge J. L., Margalit H., Berkower I. J., Good M. F., Miller L. H., DeLisi C. Protein antigenic structures recognized by T cells: potential applications to vaccine design. Immunol Rev. 1987 Aug;98:9–52. doi: 10.1111/j.1600-065x.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Bruce M. C., Alano P., Duthie S., Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990 Apr;100(Pt 2):191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Creasey A., Byrne K., Read D., Alano P., Fenton B. Plasmodium falciparum: an abundant stage-specific protein expressed during early gametocyte development. Exp Parasitol. 1989 Aug;69(2):140–149. doi: 10.1016/0014-4894(89)90182-3. [DOI] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Quakyi I. A., Good M. F. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1989 Jan 1;169(1):135–147. doi: 10.1084/jem.169.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Kumar N., Quakyi I., Good M., Mendis K., Graves P., Miller L. Immunity to sexual stages of malaria parasites. Prog Allergy. 1988;41:193–214. [PubMed] [Google Scholar]
- Carter R., Nijhout M. M. Control of gamete formation (exflagellation) in malaria parasites. Science. 1977 Jan 28;195(4276):407–409. doi: 10.1126/science.12566. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fries H. C., Lamers M. B., van Deursen J., Ponnudurai T., Meuwissen J. H. Biosynthesis of the 25-kDa protein in the macrogametes/zygotes of Plasmodium falciparum. Exp Parasitol. 1990 Aug;71(2):229–235. doi: 10.1016/0014-4894(90)90025-8. [DOI] [PubMed] [Google Scholar]
- Good M. F., Kumar S., Miller L. H. The real difficulties for malaria sporozoite vaccine development: nonresponsiveness and antigenic variation. Immunol Today. 1988 Nov;9(11):351–355. doi: 10.1016/0167-5699(88)91336-9. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988 Mar;10(2):209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Grotendorst C. A., Kumar N., Carter R., Kaushal D. C. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infect Immun. 1984 Sep;45(3):775–777. doi: 10.1128/iai.45.3.775-777.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Kaushal D. C., Carter R. Radioiodination of parasite antigens with 1,3,4,6-tetrachloro-3 alpha, 6 alpha-diphenylglycoluril (IODOGEN): studies with zygotes of Plasmodium gallinaceum. J Protozool. 1982 Feb;29(1):114–117. doi: 10.1111/j.1550-7408.1982.tb02891.x. [DOI] [PubMed] [Google Scholar]
- Ifediba T., Vanderberg J. P. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981 Nov 26;294(5839):364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Quakyi I. A., Syin C., Raum M. G., Keister D. B., Coligan J. E., McCutchan T. F., Miller L. H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988 May 5;333(6168):74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- Kumar N., Carter R. Biosynthesis of the target antigens of antibodies blocking transmission of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Nov;13(3):333–342. doi: 10.1016/0166-6851(84)90124-5. [DOI] [PubMed] [Google Scholar]
- Kumar N., Carter R. Biosynthesis of two stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinetes. Mol Biochem Parasitol. 1985 Feb;14(2):127–139. doi: 10.1016/0166-6851(85)90032-5. [DOI] [PubMed] [Google Scholar]
- Kumar N. Phase separation in Triton X-114 of antigens of transmission blocking immunity in Plasmodium gallinaceum. Mol Biochem Parasitol. 1985 Dec;17(3):343–358. doi: 10.1016/0166-6851(85)90008-8. [DOI] [PubMed] [Google Scholar]
- Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987 May;9(3):321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Nijhout M. M., Carter R. Gamete development in malaria parasites: bicarbonate-dependent stimulation by pH in vitro. Parasitology. 1978 Feb;76(1):39–53. doi: 10.1017/s0031182000047375. [DOI] [PubMed] [Google Scholar]
- Nijhout M. M. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1979 Aug;48(1):75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- Ong C. S., Zhang K. Y., Eida S. J., Graves P. M., Dow C., Looker M., Rogers N. C., Chiodini P. L., Targett G. A. The primary antibody response of malaria patients to Plasmodium falciparum sexual stage antigens which are potential transmission blocking vaccine candidates. Parasite Immunol. 1990 Sep;12(5):447–456. doi: 10.1111/j.1365-3024.1990.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Carter R., Rener J., Kumar N., Good M. F., Miller L. H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987 Dec 15;139(12):4213–4217. [PubMed] [Google Scholar]
- Quakyi I. A., Otoo L. N., Pombo D., Sugars L. Y., Menon A., De Groot A. S., Johnson A., Alling D., Miller L. H., Good M. F. Differential non-responsiveness in humans of candidate Plasmodium falciparum vaccine antigens. Am J Trop Med Hyg. 1989 Aug;41(2):125–134. [PubMed] [Google Scholar]
- Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J Exp Med. 1983 Sep 1;158(3):976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. E. Sexual development of malarial parasites. Adv Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. N., Ponnudurai T., Beckers P. J., Verhave J. P., Smits M. A., Meuwissen J. H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985 Nov 1;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., van Deursen J., Brakenhoff R. H., Lensen T. H., Ponnudurai T., Meuwissen J. H. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol. 1986 Aug;20(2):155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]