Abstract
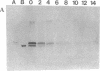
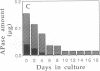
Induction of phosphatase activity is an important component of the plant cell response to phosphate deficiency. Suspension cell cultures of Brassica nigra contain two major inducible acid phosphatase (APase) isozymes; vacuolar phosphoenolpyruvate (PEP) APase and cell wall nonspecific APase. Polyclonal antibodies raised against purified PEP-APase crossreacted specifically with both isozymes. Furthermore, anti-(PEP-APase) IgG detected proteins from a wide range of higher plants, suggesting that the major plant APase isozymes have diverged from a common ancestral form. Quantification on immunoblots indicated that in B. nigra suspension cells experiencing transition from Pi sufficiency to deficiency or vice versa, the amount of total antigenic APase protein correlated closely with total enzyme activity. This was also shown in intact plant roots. Therefore, the activity was governed by the synthesis and degradation of APases. Increases in the amounts of both major APase isozymes occurred simultaneously following Pi deprivation of B. nigra suspension cells, suggesting the involvement of a common regulatory mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cordonnier M. M., Pratt L. H. Comparative Phytochrome Immunochemistry as Assayed by Antisera against Both Monocotyledonous and Dicotyledonous Phytochrome. Plant Physiol. 1982 Sep;70(3):912–916. doi: 10.1104/pp.70.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Duff S. M., Lefebvre D. D., Plaxton W. C. Purification, characterization, and subcellular localization of an acid phosphatase from black mustard cell-suspension cultures: comparison with phosphoenolpyruvate phosphatase. Arch Biochem Biophys. 1991 Apr;286(1):226–232. doi: 10.1016/0003-9861(91)90033-f. [DOI] [PubMed] [Google Scholar]
- Duff S. M., Moorhead G. B., Lefebvre D. D., Plaxton W. C. Phosphate Starvation Inducible ;Bypasses' of Adenylate and Phosphate Dependent Glycolytic Enzymes in Brassica nigra Suspension Cells. Plant Physiol. 1989 Aug;90(4):1275–1278. doi: 10.1104/pp.90.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Goldstein A. H., Baertlein D. A., McDaniel R. G. Phosphate Starvation Inducible Metabolism in Lycopersicon esculentum: I. Excretion of Acid Phosphatase by Tomato Plants and Suspension-Cultured Cells. Plant Physiol. 1988 Jul;87(3):711–715. doi: 10.1104/pp.87.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. H., Danon A., Baertlein D. A., McDaniel R. G. Phosphate Starvation Inducible Metabolism in Lycopersicon esculentum: II. Characterization of the Phosphate Starvation Inducible-Excreted Acid Phosphatase. Plant Physiol. 1988 Jul;87(3):716–720. doi: 10.1104/pp.87.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. H., Mayfield S. P., Danon A., Tibbot B. K. Phosphate Starvation Inducible Metabolism in Lycopersicon esculentum: III. Changes in Protein Secretion under Nutrient Stress. Plant Physiol. 1989 Sep;91(1):175–182. doi: 10.1104/pp.91.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Lefebvre D. D., Duff S. M., Fife C. A., Julien-Inalsingh C., Plaxton W. C. Response to Phosphate Deprivation in Brassica nigra Suspension Cells : Enhancement of Intracellular, Cell Surface, and Secreted Phosphatase Activities Compared to Increases in Pi-Absorption Rate. Plant Physiol. 1990 Jun;93(2):504–511. doi: 10.1104/pp.93.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. I. Purification and physical and immunological characterization. Arch Biochem Biophys. 1989 Feb 15;269(1):219–227. doi: 10.1016/0003-9861(89)90103-3. [DOI] [PubMed] [Google Scholar]
- Moorhead G. B., Plaxton W. C. Purification and characterization of cytosolic aldolase from carrot storage root. Biochem J. 1990 Jul 1;269(1):133–139. doi: 10.1042/bj2690133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. N., Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990 Jul;4(7):1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Martin J. B., Douce R. Relationship between the cytoplasm and the vacuole phosphate pool in Acer pseudoplatanus cells. Arch Biochem Biophys. 1983 Aug;225(1):143–148. doi: 10.1016/0003-9861(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Rogers D. T., Lemire J. M., Bostian K. A. Acid phosphatase polypeptides in Saccharomyces cerevisiae are encoded by a differentially regulated multigene family. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2157–2161. doi: 10.1073/pnas.79.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]