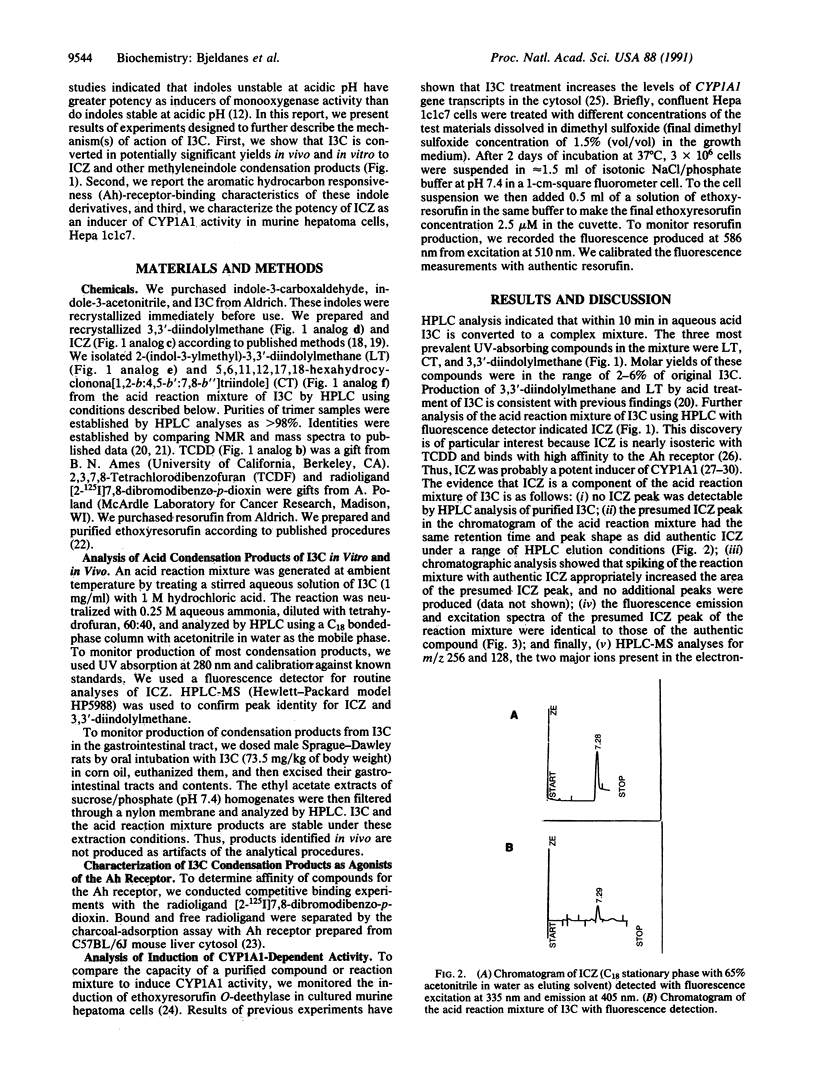
Abstract
Indole-3-carbinol (I3C) is a secondary plant metabolite produced in vegetables of the Brassica genus, including cabbage, cauliflower, and brussels sprouts. I3C is both an anti-initiator and a promoter of carcinogenesis. Consumption of I3C by humans and rodents can lead to marked increases in activities of cytochrome P-450-dependent monooxygenases and in a variety of phase II drug-metabolizing enzymes. We have reported previously that the enzyme-inducing activity of I3C is mediated through a mechanism requiring exposure of the compound to the low-pH environment of the stomach. We report here the aromatic hydrocarbon responsiveness-receptor Kd values (22 nM-90 nM), determined with C57BL/6J mouse liver cytosol and the in vitro- and in vivo-molar yields (0.1-6%) of the major acid condensation products of I3C. We also show that indolo[3,2-b]carbazole (ICZ) is produced from I3C in yields on the order of 0.01% in vitro and, after oral intubation, in vivo. ICZ has a Kd of 190 pM for aromatic hydrocarbon responsiveness-receptor binding and an EC50 of 269 nM for induction of cytochrome P4501A1, as measured by ethoxyresorufin O-deethylase activity in murine hepatoma Hepa 1c1c7 cells. The binding affinity of ICZ is only a factor of 3.7 x 10(-2) lower than that of the highly toxic environmental contaminant and cancer promoter 2,3,7,8-tetrachlorodibenzo-p-dioxin. ICZ and related condensation products appear responsible for the enzyme-inducing effects of dietary I3C.
Full text
PDF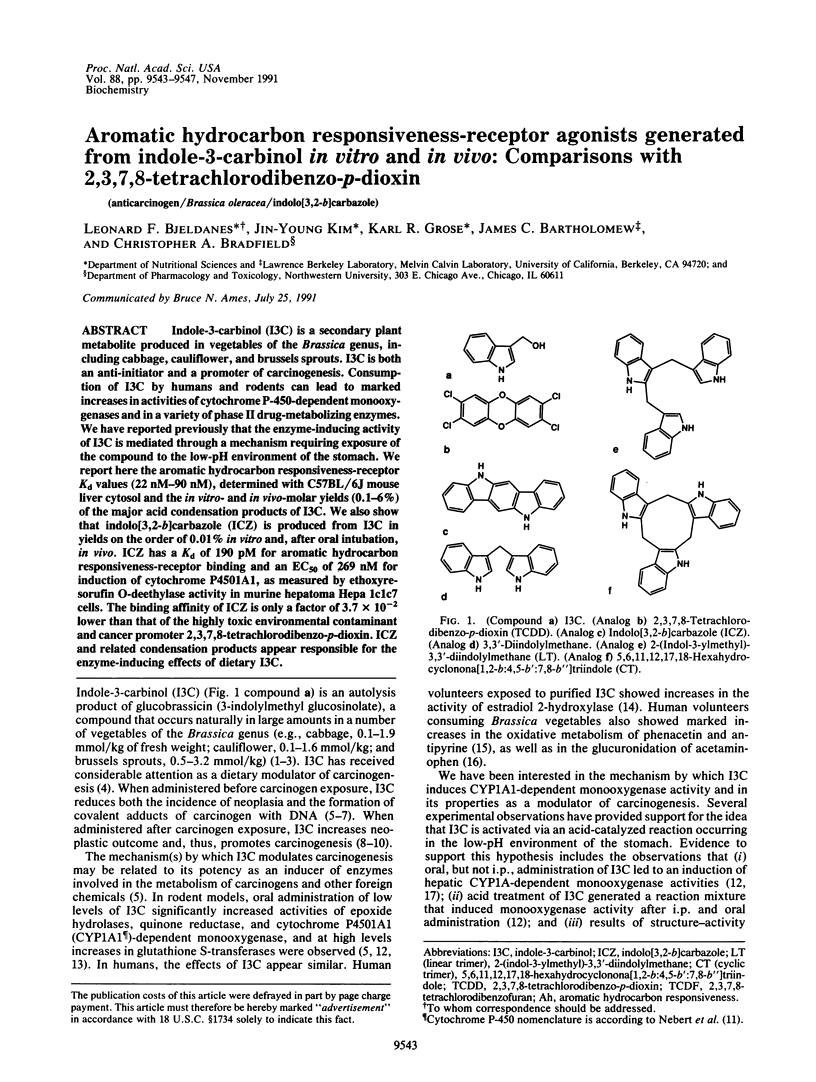

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Hendricks J. D., Shelton D. W., Nixon J. E., Pawlowski N. E. Enhancement of carcinogenesis by the natural anticarcinogen indole-3-carbinol. J Natl Cancer Inst. 1987 May;78(5):931–934. [PubMed] [Google Scholar]
- Birt D. F., Walker B., Tibbels M. G., Bresnick E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis. 1986 Jun;7(6):959–963. doi: 10.1093/carcin/7.6.959. [DOI] [PubMed] [Google Scholar]
- Bradfield C. A., Bjeldanes L. F. Effect of dietary indole-3-carbinol on intestinal and hepatic monooxygenase, glutathione S-transferase and epoxide hydrolase activities in the rat. Food Chem Toxicol. 1984 Dec;22(12):977–982. doi: 10.1016/0278-6915(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Bradfield C. A., Bjeldanes L. F. Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987;21(3):311–323. doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- Bradfield C. A., Kende A. S., Poland A. Kinetic and equilibrium studies of Ah receptor-ligand binding: use of [125I]2-iodo-7,8-dibromodibenzo-p-dioxin. Mol Pharmacol. 1988 Aug;34(2):229–237. [PubMed] [Google Scholar]
- Bradfield C. A., Poland A. A competitive binding assay for 2,3,7,8-tetrachlorodibenzo-p-dioxin and related ligands of the Ah receptor. Mol Pharmacol. 1988 Nov;34(5):682–688. [PubMed] [Google Scholar]
- Burke M. D., Orrenius S. The effect of albumin on the metabolism of ethoxyresorufin through O-deethylation and sulphate-conjugation using isolated rat hepatocytes. Biochem Pharmacol. 1978;27(11):1533–1538. doi: 10.1016/0006-2952(78)90481-1. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J., Berry D. L., Gleason G. L., Kishore G. S., Slaga T. J. Time-dependent inhibition by 2,3,7,8-tetrachlorodibenzo-p-dioxin of skin tumorigenesis with polycyclic hydrocarbons. Cancer Res. 1980 May;40(5):1580–1587. [PubMed] [Google Scholar]
- Fenwick G. R., Heaney R. K., Mullin W. J. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Gillner M., Bergman J., Cambillau C., Fernström B., Gustafsson J. A. Interactions of indoles with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol Pharmacol. 1985 Oct;28(4):357–363. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Induction of mRNA specific for cytochrome P1-450 in wild type and variant mouse hepatoma cells. J Biol Chem. 1983 Sep 10;258(17):10390–10394. [PubMed] [Google Scholar]
- Linden J. Calculating the dissociation constant of an unlabeled compound from the concentration required to displace radiolabel binding by 50%. J Cyclic Nucleotide Res. 1982;8(3):163–172. [PubMed] [Google Scholar]
- McDanell R., McLean A. E., Hanley A. B., Heaney R. K., Fenwick G. R. Chemical and biological properties of indole glucosinolates (glucobrassicins): a review. Food Chem Toxicol. 1988 Jan;26(1):59–70. doi: 10.1016/0278-6915(88)90042-7. [DOI] [PubMed] [Google Scholar]
- Michnovicz J. J., Bradlow H. L. Induction of estradiol metabolism by dietary indole-3-carbinol in humans. J Natl Cancer Inst. 1990 Jun 6;82(11):947–949. doi: 10.1093/jnci/82.11.947. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Eisen H. J., Negishi M., Lang M. A., Hjelmeland L. M., Okey A. B. Genetic mechanisms controlling the induction of polysubstrate monooxygenase (P-450) activities. Annu Rev Pharmacol Toxicol. 1981;21:431–462. doi: 10.1146/annurev.pa.21.040181.002243. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jones J. E. Regulation of the mammalian cytochrome P1-450 (CYP1A1) gene. Int J Biochem. 1989;21(3):243–252. doi: 10.1016/0020-711x(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Pantuck E. J., Pantuck C. B., Anderson K. E., Wattenberg L. W., Conney A. H., Kappas A. Effect of brussels sprouts and cabbage on drug conjugation. Clin Pharmacol Ther. 1984 Feb;35(2):161–169. doi: 10.1038/clpt.1984.22. [DOI] [PubMed] [Google Scholar]
- Pantuck E. J., Pantuck C. B., Garland W. A., Min B. H., Wattenberg L. W., Anderson K. E., Kappas A., Conney A. H. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979 Jan;25(1):88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- Pence B. C., Buddingh F., Yang S. P. Multiple dietary factors in the enhancement of dimethylhydrazine carcinogenesis: main effect of indole-3-carbinol. J Natl Cancer Inst. 1986 Jul;77(1):269–276. [PubMed] [Google Scholar]
- Pirkle J. L., Wolfe W. H., Patterson D. G., Needham L. L., Michalek J. E., Miner J. C., Peterson M. R., Phillips D. L. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. J Toxicol Environ Health. 1989;27(2):165–171. doi: 10.1080/15287398909531288. [DOI] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J., Keys B., Safe S., Newman M. S. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol Lett. 1986 Nov;34(1):67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Raverty W. D., Thomson R. H., King T. J. Metabolites from the sponge Pachymatisma johnstoni; L-6-bromo-hypaphorine, a new amino-acid (and its crystal structure). J Chem Soc Perkin 1. 1977;(10):1204–1211. [PubMed] [Google Scholar]
- Salbe A. D., Bjeldanes L. F. Effect of diet and route of administration on the DNA binding of aflatoxin B1 in the rat. Carcinogenesis. 1989 Apr;10(4):629–634. doi: 10.1093/carcin/10.4.629. [DOI] [PubMed] [Google Scholar]
- Shertzer H. G. Indole-3-carbinol and indole-3-acetonitrile influence on hepatic microsomal metabolism. Toxicol Appl Pharmacol. 1982 Jun 30;64(2):353–361. doi: 10.1016/0041-008x(82)90229-0. [DOI] [PubMed] [Google Scholar]
- Shertzer H. G. Indole-3-carbinol protects against covalent binding of benzo[a]pyrene and N-nitrosodimethylamine metabolites to mouse liver macromolecules. Chem Biol Interact. 1984 Jan;48(1):81–90. doi: 10.1016/0009-2797(84)90008-5. [DOI] [PubMed] [Google Scholar]
- Vang O., Jensen M. B., Autrup H. Induction of cytochrome P450IA1 in rat colon and liver by indole-3-carbinol and 5,6-benzoflavone. Carcinogenesis. 1990 Aug;11(8):1259–1263. doi: 10.1093/carcin/11.8.1259. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W., Loub W. D. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978 May;38(5):1410–1413. [PubMed] [Google Scholar]
- d'Argy R., Bergman J., Dencker L. Effects of immunosuppressive chemicals on lymphoid development in foetal thymus organ cultures. Pharmacol Toxicol. 1989 Jan;64(1):33–38. doi: 10.1111/j.1600-0773.1989.tb00596.x. [DOI] [PubMed] [Google Scholar]


