Abstract
The HisJ protein from Escherichia coli and related Gram negative bacteria is the periplasmic component of a bacterial ATP‐cassette (ABC) transporter system. Together these proteins form a transmembrane complex that can take up L‐histidine from the environment and translocate it into the cytosol. We have studied the specificity of HisJ for binding L‐His and many related naturally occurring compounds. Our data confirm that L‐His is the preferred ligand, but that 1‐methyl‐L‐His and 3‐methyl‐L‐His can also bind, while the dipeptide carnosine binds weakly and D‐histidine and the histidine degradation products, histamine, urocanic acid and imidazole do not bind. L‐Arg, homo‐L‐Arg, and post‐translationally modified methylated Arg‐analogs also bind with reasonable avidity, with the exception of symmetric dimethylated‐L‐Arg. In contrast, L‐Lys and L‐Orn have considerably weaker interactions with HisJ and methylated and acetylated Lys variants show relatively poor binding. It was also observed that the carboxylate group of these amino acids and their variants was very important for proper recognition of the ligand. Taken together our results are a key step towards designing HisJ as a specific protein‐based reagentless biosensor.
Keywords: HisJ, periplasmic protein, ligand binding, biosensor
Introduction
The uptake and efflux of a vast variety of substrates in living systems, from bacteria to humans, is often achieved using ATP‐cassette (ABC) transporters, consisting of membrane spanning proteins and soluble periplasmic binding proteins (PBP).1 The translocation of substrates across the cytoplasmic membrane in these systems takes place at the expense of the conversion of ATP to ADP.1 Bacterial ATP‐cassettes associated with the inner membranes consist of two transmembrane domains (TMD) creating the pathway for transport across the membrane, and a dimeric nucleotide binding domain (NBD) protein which binds and hydrolyzes ATP to ADP and energizes the cellular uptake.2, 3
The soluble PBPs, in spite of having diverse sizes, molecular weights and substrate specificities, all show a conserved three dimensional fold.4 Essentially, this conserved fold consists of two lobes, connected by a hinge region and the substrate binding takes place in the cleft between these two. Depending on the nature and the number of strands at the hinge region the PBPs have been classified into three major groups, I, II, and III. Those PBPs having three or two β‐sheet strands connecting the N‐ and the C‐lobes, are classified as Class I and II PBPs respectively, whereas PBPs with a single α‐helical connecter between the N‐ and the C‐lobes are classified as Class III proteins.4, 5, 6, 7 The latter are usually associated with the binding of metal ions, metal‐siderophore complexes, or heme. The Class I and II PBPs on the other hand play a role in the transport of amino acids, specific carbohydrates, phosphate and sulfate anions, and so forth.6 The PBPs are sometimes also called substrate binding proteins (SBPs).8
HisJ is the periplasmic component of the ATP‐cassette (ABC) transporter and it is associated with high affinity histidine binding and translocation.9 Environmental histidine can spontaneously diffuse into the periplasmic space of Gram negative bacteria through the pores of outer membrane porins, such as OmpF and LamB. Subsequently, it binds with HisJ with very high affinity (nM Kd) and translocation of the L‐histidine into the cytosol takes place using the inner membrane ATPase‐permease complex HisQMP2.9, 10, 11, 12 The Lysine, Arginine, Ornithine Binding protein (LAOBP) is a closely related PBP which shares very high sequence identity (70%) with HisJ and it binds L‐lysine, L‐arginine and L‐ornithine in the periplasm with similar high affinity.13, 14, 15 Interestingly, LAOBP also uses the same inner membrane ATPase‐permease complex as HisJ to transport its ligands into the cytosol.13 Previous work has shown that L‐His binds with high affinity (nm) with HisJ and that L‐Lys, L‐Arg and L‐Orn have a high affinity for LAOBP.14, 15 Additionally, it has also been demonstrated that these two proteins can bind with each other's preferred ligands with μM affinities.14, 15 The ligand flexibility and the ability of both these PBPs to interact with the same inner membrane ATPase‐permease, could be due to tandem duplication of the genes responsible for expression of HisJ and LAOBP (hisJ and argT, respectively),13 where the high affinity and specificity for respective ligands displayed by these proteins are thought to have arisen through divergent evolution.13
The X‐ray crystal structures for S. typhimurium and E. coli HisJ in the apo‐form as well as bound to L‐His have already been reported.16, 17 The structures show that the HisJ protein consists of two domains (D1 and D2) connected by two β‐strands where binding to histidine leads to inter‐domain closure (closed liganded form) as expected for a Class II PBP.16, 17 The structural data also show that HisJ forms a high affinity interaction with L‐His using 12 residues through the formation of hydrogen bonds, salt bridges and van der Waals interactions.16, 17 Recently our laboratory has also analyzed the solution state structure of E. coli apo‐HisJ using NMR spectroscopy.12 From our previous study, it has been deduced that D1 of HisJ plays a more crucial role in the formation of initial high‐affinity protein–ligand interactions, which is then communicated to D2 and leads to further protein–ligand interactions and stabilization of the closed liganded form.12
On the other hand, the crystal structures for LAOBP from S. typhimurium, bound to its high affinity (L‐lysine, L‐arginine, and L‐ornithine) and low affinity (L‐histidine) ligands, are also available.18 The structural analysis reported for LAOBP shows that irrespective of the bound ligand (high or low affinity), it has a very similar “closed” conformation as indicated by a very low r.m.s.d value when the high affinity liganded and low affinity liganded structures are overlayed.18 Moreover, when the crystal structure of HisJ bound to histidine is compared to the “closed” histidine‐bound structure of LAOBP, it shows that these two different proteins (HisJ and LAOBP) in the histidine‐bound form have very similar “closed” conformations.16 Taken together, this information indicates that a common closed form for these two proteins (HisJ and LAOBP) is performing similar functions.
The closed conformation of these PBPs plays a crucial role in substrate transport, as this form of the proteins interacts with the inner membrane ATPase‐permease complex, transmitting a signal that triggers hydrolysis of ATP and consequent passage of the ligand to the cytosol.19 Interestingly, the existence of a “closed” unliganded conformation of the HisJ protein, due to dynamic interconversions of conformations, has been reported as well.20, 21, 22 Using molecular dynamics simulations we have also reported a similar observation recently for HisJ.23 Moreover, it is known that both the unliganded and ligand‐bound HisJ interact with similar affinities to the inner membrane ATP‐cassette, HisQMP2.24 The same report also proposed that HisJ may always be associated with the inner membrane permease complex, where the unliganded form interacts with HisQMP2 in an open conformation.24 Ligand binding to the HisJ bound to HisQMP2 leads to domain closure for HisJ, which then in turn triggers a high rate of ATP hydrolysis and hence substrate transport.24
Because of the large ligand‐mediated conformational changes observed in the type I and type II PBP superfamily proteins, these proteins are considered as excellent starting points for the design of highly sensitive reagentless biosensors.25 For example, various mutant forms of HisJ have been constructed to generate a fluorescent biosensor that can monitor the level of L‐His in serum as an indicator for the deficiency of the enzyme histidine ammonia lyase which converts L‐histidine into urocanic acid.26 In addition, it is also known that reengineering of the binding pocket can improve the specificity of such proteins.27 However, before such work can be completed the ligand‐binding properties of the wild type protein need to be characterized and analyzed in detail, to ascertain that other naturally occurring compounds do not interfere with the binding. It is well known that results obtained with a biosensor designed to measure the level of a relatively rare metabolite can be unreliable when other non‐desired metabolites of higher abundance can bind as well.28
Although there is exhaustive structural data available for the binding of both high and low affinity ligands to LAOBP, as well as some information for HisJ binding to its high affinity ligand L‐His,12, 16, 17, 19 to date there have been no detailed reports for HisJ's interactions with L‐Lys, L‐Arg and L‐Orn. Hence in this work we have initially investigated the structural changes that accompany the addition of L‐Lys, L‐Arg, and L‐Orn to apo‐HisJ using 1H,15N HSQC NMR titration experiments and we compare these data with titration data obtained for L‐His.12 We have also used an NMR fingerprint screening approach and isothermal titration calorimetry (ITC) experiments to study the binding and determine the dissociation constants for various naturally occurring analogs, enantiomers, methylated, or acylated versions or other naturally occurring metabolites of histidine, arginine, and lysine. For comparison with the ITC experiments, we also performed differential scanning calorimetry (DSC) experiments of HisJ in the presence of different ligands to determine the thermal stability of the apo‐ and ligand‐bound protein under our experimental conditions.
Results
NMR titration experiments with his, arg, lys and orn
Extensive structural information is available for the binding of L‐His to HisJ and LAOBP. Interestingly, in a previous study where the Cα atoms for the residues 5–235 of LAOBP were superimposed for all four liganded crystal structures, it was shown that the overall conformation of the protein in the ligand‐bound state is essentially the same.18 In addition, very small global r.m.s.d values were also obtained when the crystal structure of HisJ bound to L‐His is superimposed on the structure of the LAOBP bound to L‐His.16 Taken together these two analyses indicate that both HisJ and LAOBP, having 70% sequence identity, form essentially the same “closed” conformation with all four ligands.
In our 1H,15N HSQC NMR titration experiments with the HisJ protein we observe that all the resonances are affected in a similar manner by additions of L‐His, L‐Arg, L‐Lys, and L‐Orn. Interestingly, resonances arising from multiple residues from different parts of the protein are affected upon ligand addition, suggesting an overall conformational change for the protein rather than a highly localized binding event. Our NMR data obtained for L‐His bound to HisJ must represent the L‐histidine bound closed conformation of the HisJ protein that was seen in the published crystal structure (PDB code:1HSL). This closed state correlates with the domain closure upon L‐His binding as well as representing the overall folding. Therefore, we did observe changes in the chemical shift in many residues, even those that are far away from the binding site. When we compared this data with the resonance shifts measured for L‐Arg, L‐Lys, and L‐Orn bound to HisJ we see very similar patterns. However due to the differences of the charge and the size of the side chains, the peak positions for the different ligand bound forms of HisJ were not identical (Figs. 1 and 2), in spite of the overall pattern being comparable. We therefore conclude from the data presented in Figure 1, that the global fold of HisJ bound to all four ligands is very similar if not identical, and resembles the closed state observed in the crystal structure for the histidine bound HisJ complex (PDB code:1HSL).
Figure 1.
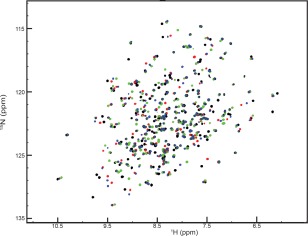
1H,15N (TROSY) HSQC overlay spectra of 2D,15N HisJ in the presence of an excess of 16 times l‐His (black), l‐Arg (red), l‐Lys (green), and l‐Orn (blue).
Figure 2.
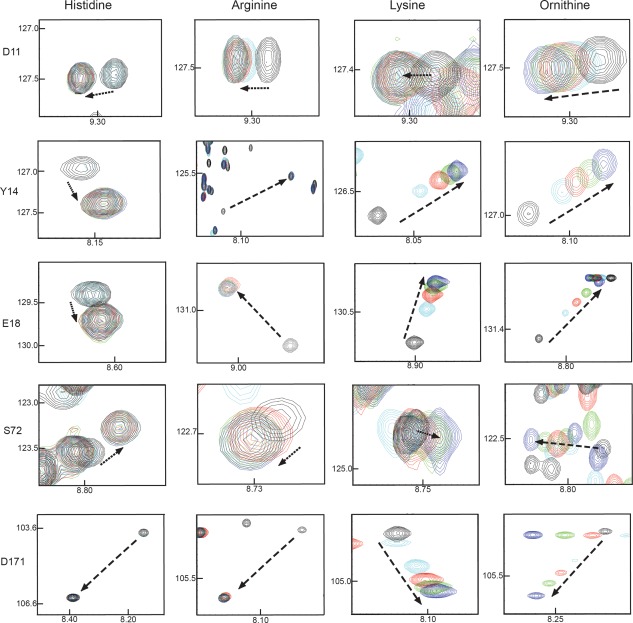
NMR titration data recorded for the (a) D11, (b) Y14, (c) E18, (d) S72, and (e) D171 residues from HisJ in the presence of excess l‐His, l‐Arg, l‐Lys, and l‐Orn, respectively. For each representative data apo‐HisJ appears in black, whereas 0.5, 1.0, 2.0, and 16 times excess ligands are shown in cyan, red, green and blue, respectively. The arrow indicates the movement (fast exchange) of apo‐HisJ peaks for the specific residues upon successive ligand addition.
From previous work it is known that 12 residues make up the entire binding pocket in the crystal structures of the four ligand‐bound forms of the LAOBP protein.29 However, sidechains of four residues, D11, Y14, S72, and D171, undergo the most dramatic structural changes between the apo‐ and liganded forms.30 We therefore decided to focus our analysis of the NMR spectra mostly on these residues of HisJ along with E18, which has been predicted to form a salt bridge with the amino group in the side chain of lysine.17 LAOBP complexed with either L‐Arg or L‐Lys or L‐Orn showed that efficient ligand interactions required a conformational change for the backbone and sidechain of D11. On the other hand it appears that Y14 of HisJ is the most critical residue for the binding of histidine as it is interacting with seven different atoms of its side chain. The crystal structure of HisJ bound to L‐His also shows that residue S72 forms several interactions with the COO−, as well as with the Cα atoms of L‐His.
All of the aforementioned five residues showed slow chemical exchange on the NMR time scale during successive additions of L‐His and L‐Arg (Fig. 2), whereas, a fast chemical exchange process was observed during titrations with L‐Lys and L‐Orn for these residues (Fig. 2). Assuming that the on‐rates for all amino acids are identical, these data indicate that the binding of L‐Lys and L‐Orn is weaker than the binding of L‐His and L‐Arg. Lastly, it should be noted that the NMR titration data presented in this article show that the observable differences in chemical shift perturbation (CSP) values from all five residues, except for D171, are all part of the larger D1 domain of HisJ. This is an important observation given that our previous report has shown that upon ligand binding the D1 lobe initiates domain closure. Hence, this lobe of the protein seems to play a more fundamental role in ligand binding.12
Ligand binding: NMR screening
NMR ligand‐titration experiments provide a straightforward tool to determine and characterize nm to µM binding affinities. The same technique also makes it possible to detect extremely weak protein‐protein interactions in the range of 10−1 to 10−2 M, using 1H,15N HSQC NMR spectra.29, 31 Hence, for proteins that are stable and that can be produced with very high yield in E. coli, like HisJ, one can use NMR spectroscopy as a convenient screening approach, to identify protein–ligand interactions. However, such a simple “fingerprint” experiment at one‐ligand concentration does not provide information about the K d. As was expected our NMR screening experiments show that all four known ligands (histidine, lysine, arginine, and ornithine) bind with HisJ. In addition, we also observed that a few amino acid analogs, and post‐translationally modified amino acid derivatives could also bind with HisJ (see Table 1). Nonetheless, the NMR screening results also showed that several compounds (e.g., l‐histidine amide, d‐histidine, histamine, cis‐urocanic acid, trans‐urocanic acid, imidazole, 1,2,4‐triazole, l‐glycine, l‐arginine amide, l‐lysine amide and 2,6‐diaminopimelic acid) did not bind at all to HisJ (Table 1, Supporting Information Figs. S2–S6). Clearly these compounds all lack certain chemical features, such as the free carboxylate group, that seems to be essential for recognition by HisJ. On the other hand l‐histidine, N‐acetyl l‐histidine, 1‐methyl l‐histidine, 3‐methyl l‐histidine, l‐arginine, homo‐l‐arginine, N G‐methyl‐l‐arginine, N G, NG′‐dimethyl‐l‐arginine, l‐lysine, and l‐ornithine all were shown to bind to HisJ. Hence in subsequent experiments the binding of these compounds identified in the screening was assessed in a more quantitative manner by ITC and DSC experiments.
Table 1.
Thermodynamic Parameters Obtained for ITC Titration and Screening Data Obtained from Fingerprint‐NMR Experiments for HisJ and Various Ligands at pH 7.0
| Ligand | K d | N | ΔH (Kcal mol−1) | TΔS (Kcal mol−1) | NMR |
|---|---|---|---|---|---|
| l‐histidine | 64 ± 10 nM | 0.9 ± 0.5 | −11.9 ± 0.6 | −0.006 | Slow |
| N‐acetyl l‐histidine | 27 ± 9 μM | 0.9 ± 0.0 | −1.5 ± 0.3 | 0.015 | + |
| l‐histidine amide | No bindinga | – | – | – | + |
| 1‐methyl‐l‐histidine | 18 ± 1 μM | 1.6 ± 0.2 | −0.7 ± 0.04 | 0.019 | + |
| 3‐methyl‐l‐histidine | 3 ± 0.7 μM | 0.5 ± 0.07 | −7.21 ± 0.06 | 0.001 | + |
| d‐histidine | No binding | – | – | – | – |
| β‐(1, 2, 4‐triazol‐3‐yl) dl‐alanine | No binding | – | – | – | + |
| Carnosine | No binding | – | – | – | + |
| Histamine | No binding | – | – | – | – |
| Cis‐Urocanic acid | No binding | – | – | – | – |
| Trans‐Urocanic acid | No binding | – | – | – | – |
| Imidazole | No binding | – | – | – | – |
| 1,2,4‐triazole | No binding | – | – | – | – |
| l‐glycine | No binding | – | – | – | – |
| l‐arginine | 3 ± 1 μM | 1 ± 0.04 | 0.53 ± 0.02 | 0.027 | Slow |
| Homo‐l‐arginine | 2 ± 0 μM | 1 ± 0 | 2.52 ± 0.01 | 0.034 | + |
| Nα‐acetyl‐l‐arginine | No binding | – | – | – | + |
| l‐arginine amide | No binding | – | – | – | + |
| N G‐methyl‐l‐arginine | 1.3 ± 0.8 μM | 0.9 ± 0.2 | −3.2 ± 0.8 | 0.016 | + |
| N G, N G‐ dimethyl‐l‐arginine | No‐binding | – | − | − | + |
| N G, N G′‐ dimethyl‐l‐arginine (asymmetric) | 6 ± 1 μM | 0.3 ± 0.05 | 6.54 ± 1 | 0.045 | + |
| l‐lysine | 19.5 ± 6 μM | 1 ± 0 | 2.3 ± 0.06 | 0.029 | Fast |
| Nα‐acetyl l‐lysine | No binding | – | – | – | + |
| Nɛ‐acetyl‐l‐lysine | No binding | – | – | – | + |
| l‐lysine amide | No binding | – | – | – | + |
| Nɛ, Nɛ‐dimethyl‐l‐lysine | No binding | – | – | – | + |
| Nɛ, Nɛ, Nɛ‐trimethyllysine | No binding | – | – | – | + |
| l‐ornithine | 72 ± 4 μM | 1.3 ± 0.4 | 1.7 ± 0.1 | 0.020 | Fast |
| l‐2, 4‐diaminobutyric acid | No binding | – | – | – | + |
| 2,6‐diaminopimelic acid | No binding | – | – | – | – |
The ITC data are the average of at least three experiments with standard deviation. For the NMR data “+” means changes were observed in the HSQC spectra implying ligand binding, while “−” means no changes could be detected and the ligand did not bind.
aNo binding means that binding was tested but was too weak to be observed by ITC.
Ligand binding: ITC experiments
We used ITC to quantitatively determine the binding of HisJ toward l‐His, l‐Lys, l‐Arg, l‐Orn, d‐His and various amino acid analogs, His‐metabolites and post‐translationally modified amino acid derivatives (Supporting Information Fig. S1). As the data presented in Table 1 indicates, HisJ binds with l‐His with nM affinity [Fig. 3(A)] as was reported by us previously.12 The crystal structure data for HisJ bound to L‐His show several non‐covalent interactions between the COO− (S72 N, R77 guanidino, T121 N) and (S70 O, S72 —OH with T121 —OH via a water molecule) groups on L‐His and HisJ, indicating the possibility of important ligand recognition mechanism.16, 17, 19 Hence we also studied the binding of l‐histidine amide and N‐acetyl l‐histidine to probe the importance of the negatively charged carboxylate group and the positively charged amino terminal group.
Figure 3.
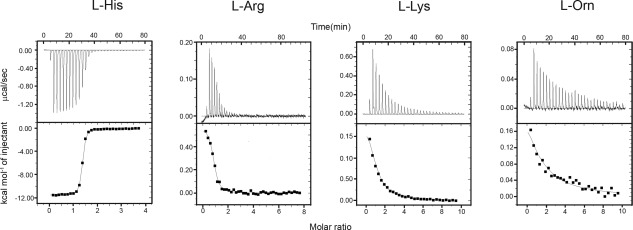
Representative binding data as measured by ITC HisJ binding with (a) l‐His, (b) l‐Arg, (c) l‐Lys, and (d) l‐Orn. The smooth lines in the lower plots represent the best fit to the data using one‐site binding model whereas the dots represent actual injection. All experiments were carried out by in 20 mM HEPES, 100 mM NaCl, pH 7.0 at 30°C.
The ring nitrogens (Nδ1 and Nɛ2) of l‐histidine also participate in the binding event by forming noncovalent interactions with various amino acid side chains of HisJ. In this work we also studied the binding of two naturally occurring amino acids 1‐methyl‐His and 3‐methyl‐His having different steric properties compared to l‐His in the vicinity of those two nitrogens. 1‐methyl‐His is a marker of meat consumption32 and 3‐methyl‐His is considered an important biomarker for patients that suffer from cachexia, a muscle wasting disease.33 As the data in Table 1 show l‐histidine amide does not bind to HisJ, whereas N‐acetyl l‐histidine can still bind with HisJ, however with reduced affinity [Table 1, Supporting Information Fig. S7(A)]. Similarly, 1‐methyl‐l‐histidine and 3‐methyl‐l‐histidine also bound to HisJ with much reduced affinities (µM) compared to the affinity of l‐His with the HisJ protein (Table 1). This weakened interaction of 3‐methyl‐l‐histidine compared to l‐His with HisJ is also reflected by the crystallographic data where additional hydrogen bonding was observed between position‐3 of the ring nitrogen of histidine and S69 of HisJ.17 It is also apparent that position‐3 of the ring nitrogen must play a different role than the —NH at position‐1 as 3‐methyl‐l‐histidine binds slightly stronger than 1‐methyl histidine (Table 1). Taken together, our ITC data indicate that although the COO−, and ring nitrogen groups of l‐histidine form important noncovalent interactions with HisJ amino acid residues as shown by X‐ray crystallography,16, 17, 19 the COO− group plays a more crucial role for ligand recognition and high affinity binding to HisJ. While the free carboxylate is important, interactions with the side chain are clearly also significant contributors, as l‐Gly does not bind. Likewise imidazole representing part of the His side chain does not bind by itself either. Overall, histidine and all histidine analogs that display interactions in the ITC experiments, show a negative enthalpy of binding which in turn indicates favorable exothermic reactions [Supporting Information Fig. S7(A)]. We also titrated in a solution of D‐histidine into a HisJ solution and observed no interaction, indicating that the binding is stereospecific as well (Table 1).
We further investigated the ability of HisJ to recognize the l‐histidine backbone for high affinity binding by titrating in a histidine analog which does not exist naturally, such as β‐(1, 2, 4‐triazol‐3‐yl) dl‐alanine as well as the naturally occurring dipeptide carnosine (for structures see Supporting Information Fig. S1). In addition to differences in chemical features, successful incorporation of the histidine analog, β‐(1, 2, 4‐triazol‐3‐yl) dl‐alanine, into histidine‐auxotrophic E. coli 34 led us to investigate the possible role of HisJ as a potential transporter. Because of the potential beneficial effects of carnosine supplementation on several diseases such as diabetes, neurodegenerative diseases, diseases of the sense organs, aging and cancers,35 we also selected this physiologically important dipeptide as a possible candidate for HisJ binding. However, both of these histidine analogs did not show any interaction with HisJ in the ITC experiments (Table 1).
Finally, we evaluated the binding of naturally occurring breakdown products derived from histidine, such as histamine and cis‐ or trans‐urocanic acids (Supporting Information Fig. S3). Histamine, is a biogenic amine that is well known for its regulatory role in inflammation, gastric acid secretion, and neurotransmission.36 On the other hand, as a major absorber of ultraviolet radiation, urocanic acid, a breakdown product of His, is also well known for its role in immunosuppression, skin cancer development and skin barrier function.37 Table 1 shows that histamine and both forms of urocanic acids fail to bind with HisJ. These data further confirm our observations for an important role of COO− and —NH2 groups in ligand recognition and binding with HisJ because histamine does not contain the terminal carboxyl group and urocanic acid lacks the —NH2 group.
Table 1 also provides the ITC binding data obtained for HisJ with l‐Arg, l‐Lys, and l‐Orn and Figure 3 shows the representative ITC plots recorded for those titrations. As can be seen in Table 1, both l‐Arg and l‐Lys bind to HisJ with K d = 3 and 19.5 μM, respectively, whereas l‐Orn shows the weakest interaction with HisJ at ∼72 μM (Fig. 3). These data not only confirm that l‐Arg, l‐Lys, and l‐Orn by comparison form low affinity ligands for HisJ, but also reveal the previously reported trend of binding affinities between the low affinity ligands.9, 14, 15 In addition to the fact that l‐Arg, l‐Lys, and l‐Orn have a lower affinity for HisJ compared to l‐His, the ITC experiments also show that these ligands interact with HisJ in an endothermic fashion, whereas the high affinity ligand l‐His binds to HisJ in an exothermic reaction (Fig. 3). The differences seen for the thermodynamic parameters measured for His and its analogs as opposed to the other basic amino acids, could be related to the partial protonation of the His sidechain at our experimental pH. Therefore, the possible effects of His sidechain protonation were evaluated by performing additional ITC experiments as a function of pH (5.8–7.8) for l‐His, N‐acetyl His and l‐Arg (data not shown). However these data did not support this idea, as the enthalpy for His binding remained negative throughout. Possibly a change in the binding pattern with additional water molecules being displaced from the binding site of HisJ, when the longer Lys and Arg sidechains bind, may be responsible for this difference in the thermodynamic parameters. Such a displacement of bound water molecules has in fact been seen in the crystal structure of the related LAOBP protein.18
The crystal structure of HisJ bound to l‐His shows that the and COO− groups of L‐His are positioned deep within the binding cleft between the N‐ and the C‐lobes of the protein.19 Hence we wanted to explore the importance of the α‐ and ɛ‐ groups of l‐Arg and l‐lysine for binding to the HisJ protein. When Nα‐acetyl‐l‐Arg and Nα‐acetyl‐l‐Lys were titrated into a solution of HisJ, they did not show any binding (Table 1), in contrast to the observation for N‐acetyl l‐histidine, which still bound to HisJ with μM affinity [Supporting Information Fig. S7(A)]. Interestingly, modified versions of the ɛ‐ group of l‐Lys, with di‐ and tri‐ methyl groups were also unable to interact with HisJ. Not unexpectedly, the Nɛ‐acetyl‐l‐lysine, which does not retain its positive charge on the side chain, does not bind either. Therefore, we conclude that the unmodified ɛ‐ ‐ group of lysine is essential to establish an interaction with the HisJ protein. Naturally occurring variants of methylated and acylated Arg (and Lys) play an important role in the epigenetic code of histone proteins.38
Intriguingly, homo‐L‐Arg, having an additional —CH2 group in the side chain compared to the naturally occurring L‐Arg, shows comparable affinity (Table 1). Similarly, NG‐methyl‐L‐Arg shows a very similar binding affinity, whereas N G, N G′‐dimethyl l‐Arg (asymmetric) has a lower affinity [Supporting Information Fig. S7(B)]. Taken together, the slightly stronger binding of the longer side chain with HisJ, is likely caused by additional hydrophobic interactions, taking into account that there are numerous hydrophobic residues in the HisJ binding pocket.17, 19 Our ITC data also support this idea, as most of the binding events were driven by unfavorable enthalpy and favorable entropy except for N G‐methyl‐l‐Arg [Supporting Information Fig. S7(B)]. Therefore, hydrophobic forces might play a major role. Nonetheless, this type of interaction was completely absent during titration with N G, N G‐dimethyl l‐arginine (symmetric). Possibly, the presence of the additional —CH3 group at both terminal positions makes the side chain bulkier and it prevents proper hydrogen bonding required to fit in the HisJ binding pocket. Nonetheless, an interaction was detected in the NMR screen, suggesting that weak binding of the compound may still occur.
We also titrated in L‐2,4‐diaminobutyric acid and 2,6‐diaminopimelic acid solutions into HisJ to investigate the influence of chain length and terminal group on high and low affinity binding (Table 1). Diaminobutyric acid is shorter than L‐Orn and it shows essentially no binding. Likewise 2,6‐diaminopimelic acid, which is a constituent of peptidoglycan in Gram‐negative bacteria, did not bind.
Thermal stability of apo‐ and liganded‐HisJ
The thermal stability of apo‐HisJ and HisJ protein in the presence of L‐His, L‐Arg, L‐Lys, and L‐Orn has been previously reported.39 In our study we have reevaluated the thermal stability of apo‐HisJ and HisJ in the presence of the aforementioned amino acids under our experimental conditions (20 mM HEPES, 100 mM NaCl at pH 7.0). In addition, we also studied the protein thermal stability in the presence of various other related compounds.
Our DSC experiments show that apo‐HisJ has a T m at 55.9°C [Table 2, Fig. 4(A)]. This T m for apo‐HisJ is very close to the Tm previously published that was obtained for the protein in 5 mM Tris/HCl buffer, pH 8.3. Our DSC experiments were done at a different pH value than a previous report, where the authors observed independent unfolding of the two domains of the protein.39 However, at pH 7.0, the ratio of ΔH/ΔH vH is close to 1 suggesting that apo‐HisJ and ligand‐bound HisJ both unfold as a single cooperative unit similar to the results obtained with the related maltose binding protein and the arabinose‐binding protein.40, 41 Values of ΔH/ΔH vH close to unity for two domain type I and type II periplasmic proteins, reflect the fact that there are substantial interactions between the two domains which causes them to denature in a single two‐state transition.40, 41
Table 2.
DSC Data Obtained for HisJ and Various Ligands at pH 7.0
| Ligands | Melting temperature | ΔH/ΔH vH |
|---|---|---|
| Apo‐HisJ | 55.9 ± 0.04a | 0.88 |
| l‐histidine | 61.7 ± 0.01 | 1.20 |
| N‐acetyl‐l‐histidine | 57.0 ± 0.04 | 1.03 |
| 1‐methyl‐l‐histidine | 56.6 ± 0.02 | 1.04 |
| 3‐methyl‐l‐histidine | 58.5 ± 0.04 | 1.06 |
| β‐(1, 2, 4‐triazol‐3‐yl) dl‐alanine | 56.7 ± 0.02 | 1.11 |
| Histamine | 56.4 ± 0.20 | 0.67 |
| l‐arginine | 60.0 ± 0.05 | 1.21 |
| Homo‐l‐arginine | 60.0 ± 0.07 | 1.20 |
| N G ‐methyl‐l‐arginine | 59.9 ± 0.05 | 1.31 |
| N G, N G′‐ dimethyl‐l‐arginine | 57.2 ± 0.02 | 1.11 |
| l‐lysine | 57.1 ± 0.02 | 1.07 |
| l‐ornithine | 56.1 ± 0.02 | 1.05 |
The error in the curve fitting is indicated.
Figure 4.
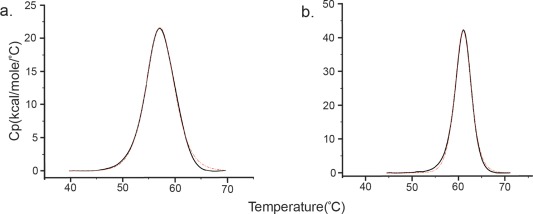
Representative DSC data obtained for (a) apo‐HisJ and for (b) HisJ in the presence of a fivefold concentration of l‐histidine. Experiments were carried out in 20 mM HEPES, 100 mM NaCl at pH 7.0. The red curve is a fit of the heat of unfolding data for HisJ to a simple non‐2‐state unfolding model.
Our DSC experiments also showed that the HisJ protein in the presence of five times excess of β‐(1, 2, 4‐triazol‐3‐yl) dl‐alanine and histamine showed a T m value very close to the thermal denaturation temperature of apo‐HisJ, pointing towards a very weak interaction for these ligands with HisJ. These data further confirm our observations from the NMR screening and ITC experiments (see Table 1), as we find that only those compounds that demonstrated binding in ITC experiments give rise to an increased T m in the DSC experiments [Fig. 4(B)]. Moreover, as anticipated, the more tightly binding ligands have the largest effect on the T m of HisJ.
Discussion
In this work we have explored the specificity of the binding pocket of E. coli HisJ, a protein that can interact with the ligands l‐histidine, l‐arginine, l‐lysine, and l‐ornithine with affinities ranging from nM to weak μM, respectively. We also have compared the data with available structural data for the related PBP LAOBP.12, 16, 17, 18, 19 From the chemical shift changes observed for characteristic residues in our NMR titration data, we conclude that HisJ forms a similar global fold, with all four ligands bound. Similar observations have also been reported to the folding events observed during the binding of the same four ligands to the LAOBP protein. This is of interest because a previous report has indicated that only the closed form of HisJ can lead to efficient ATP hydrolysis when the liganded protein interacts with HisQMP2, and hence give rise to substrate translocation.42 A common liganded form for both HisJ and LAOBP will make the signal transduction that leads to ATP hydrolysis and subsequent transport more efficient.
1H,15N NMR HSQC titration experiments could identify differences in the side chain orientation of the same amino acid residues in the binding cleft on HisJ, that interact with l‐Arg, l‐Lys, and l‐Orn in the LAOBP crystal structure.43 According to our NMR titration data, all these residues are involved in strong binding for l‐His and l‐Arg whereas due to the differences of dynamics of the exchange rate of the same residues, l‐Lys and l‐Orn appeared as weaker binders of HisJ. This forms a possible molecular discrimination mechanism between high and low affinity ligands for HisJ.
Additionally, our ITC data confirm the ligand binding abilities towards all four ligands reported earlier.12, 14, 15 Moreover, we also show that HisJ interacts selectively with l‐His over d‐histidine and is capable of binding to some biologically relevant l‐His analogs, such as 1‐methyl‐l‐His and 3‐methyl‐l‐His. We did not observe any binding when either l‐glycine, imidazole, or triazole solutions were titrated into HisJ to probe the impact of either backbone or sidechain alone, which is an indication of higher selectivity and specificity of HisJ toward different ligands in terms of molecular recognition. Using ITC experiments we further established that the presence of the free α‐COO− group on l‐histidine is crucial for high affinity binding as no interactions were observed for histamine binding. This is consistent with the fact that l‐histidine amide only binds very weakly to HisJ.
Although we did not observe any interaction with either of the N‐acetyl‐Arg/Lys, or l‐His/Arg/Lys amide compounds in the ITC experiments, in the initial NMR screening analyses, all these ligands were shown to participate in complex formation with HisJ. We also observed a similar trend of complex formation in all the methylated‐l‐Lys analogs in NMR screening along with β‐(1,2,4‐triazol‐3‐yl) dl‐alanine, carnosine and L‐2,4‐diaminobutyric acids. We hypothesize that this type of interaction is too weak (K d > 10−4 M) to detect by ITC, whereas NMR spectroscopy is highly sensitive technique for detecting weak protein‐protein/ligand interactions, where the Kd can be in the mM range.44 Somehow HisJ could bind the aforementioned ligands but due to rapid dissociation rates of these interactions, there was no significant heat generated in the ITC titrations. The binding affinities we observed in the ITC are also reflected in DSC where HisJ with the strongly associated ligands appears to unfold at higher melting temperature. It seems likely that these calorimetric observations are related to the fact that only the binding of some ligands can generate the closed form of the protein.
From all the biophysical data obtained in this investigation, we can conclude that the α‐COO− group plays a pivotal role for higher selectivity of ligands by HisJ. Overall our results form a preliminary step for designing a robust sensitive reagentless biosensor, that could detect either l‐His, or 3‐methyl l‐His. Developing of a biosensor with good sensitivity and selectivity can have a profound impact on health and biosafety.45 Fluorescent adducts of several other PBP's are currently under investigation as protein based biosensors for the detection of glucose27 and other metabolites46, 47, 48 in the hope that they can promote detection of disease and be used to follow the efficacy of treatments. Novel approaches for introducing fluorescent moieties in these protein biosensors are also being explored, and together such studies sets the stage for future work towards the development of a biosensor that is based on HisJ.
Furthermore, our current studies on HisJ, as a representative of type I and type II PBPs, will help us to understand how this protein family displays a remarkable degree of specificity and high affinity towards one amino acid but not others. Further ligand‐screening investigations of the interactions with other proteins from the PBP family will also help shed light on the evolutionary relationship of these proteins in terms of their selectivity for different amino acids.
Materials and methods
All chemicals and materials used in this study are of the highest purity grade and most chemicals were procured from Sigma–Aldrich. l‐arginine amide and l‐lysine amide were purchased from Santa Cruz Biotechnology.
Cloning and purification of E. coli HisJ
Cloning and purification of HisJ was achieved following a method published by us previously.12 In short, the mature HisJ protein (residues 1–238), was amplified from the E. coli K12 genome with 5′ NdeI and 3′ XhoI restriction sites and cloned into the pET15 vector. The pET15 vector has a hexa‐histidine tag and a TEV (tobacco etch virus) protease cleavage site located near the N terminus of the expressed protein. Cleavage of the expressed protein with TEV protease removes the His‐tag and results in the addition of three residues (G‐G‐M) to the N‐terminus of mature HisJ.12
The HisJ protein was over‐expressed by growing E. coli BL21(DE3) (Novagen) cells containing the vector of interest in 1 L of Luria‐Bertani (LB) media (for unlabeled protein) or 1 L of D2O M9 media containing 1g L−1 of 15NH4Cl (for 2D‐15N HisJ) in the presence of 100 μg mL−1 ampicillin.49 Cells were grown until the OD600 reached 0.8 at 37°C and then were induced with 0.8 mM isopropyl‐d−1‐thiogalactopyranoside for 3 h. Cells were harvested by centrifugation and resuspended in 20 mM Tris, 100 mM NaCl, 20 mM imidazole pH 8.0 buffer and lysed using French Pressure Cell. The cell lysate was then centrifuged at 18,000g for 45 min at 4°C and the supernatant was applied to a Ni‐affinity column (Sigma‐Aldrich). The bound protein was washed with 200 mL 20 mM Tris, 100 mM NaCl, 20 mM imidazole at pH 8.0 and HisJ was eluted subsequently using 20 mM Tris, 100 mM NaCl, 250 mM imidazole pH 8.0 buffer. The purified protein was then buffer exchanged with 20 mM Tris, 100 mM NaCl, 500 mM EDTA buffer (pH 8.0), and 3 mM reduced l‐glutathione and 0.3 mM oxidized (−)‐glutathione was added to this solution and the fusion protein was subjected to TEV digestion for 5 h at 30°C. This solution was then centrifuged and applied once again to the Ni‐affinity column where the cleaved N‐terminal histidine tag bound to the column and the tag‐free HisJ was eluted in the flow through. The protein was then dialyzed (three times) with 5 mM Tris pH 8.0 buffer and ion‐exchanged using DEAE Sepharose Resource Q column (GE Health care) and eluted with a steady gradient of 0.1 M NaCl, 5 mM Tris pH 8.0 to obtain pure HisJ protein. The purity of the protein was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) (98%) and the concentration of the protein was determined using the extinction coefficient at 280 nm obtained theoretically from the ExPASy Prot‐Param program.
NMR spectroscopy
All NMR experiments were performed at 298 K on a Bruker Avance 700 MHz NMR spectrometer equipped with a triple‐resonance inverse cryoprobe with a single axis z‐gradient. NMR samples were prepared in 50 mM Na‐phosphate pH 7.0 according to the method published by Chu et al.12 Titration experiments were carried out by adding either l‐His, l‐Arg, l‐Lys, or l‐Orn in ∼ 0.3 mM 2D,15 N‐isotope labeled HisJ protein samples. Samples were prepared with 10% D2O and DSS (4,4‐dimethyl‐4‐silapentane‐1‐sulfonic acid) was used as an internal standard. Two‐dimensional transverse relaxation‐optimized spectroscopy (TROSY) 1H,15N‐HSQC experiments were acquired for each titration experiment. Previously published NMR chemical shift assignments of HN and N for holo‐HisJ12 were used to analyze chemical shift perturbations according to the following equation.50
For NMR “fingerprint” binding experiments with the various amino analogs and derivatives a 0.3 mM 1H,15N‐ HisJ protein was used and a 20 times excess of the compounds was added. Because NMR spectra are sensitive both to strong (nM and μM) and weak (mM) binding events, this procedure functioned as a screening method. All spectra were processed and analyzed by NMRPipe51 and NMRView52 software.
Isothermal titration calorimetry
ITC experiments were carried out on a Microcal VP‐ITC (Malvern) instrument. For ITC experiments the protein was dialyzed with NH4HCO3 (three times) and subsequently lyophilized and the solid obtained was stored at 4°C. The desired amount of lyophilized HisJ was weighed out and dissolved in 20 mM HEPES, 100 mM NaCl at pH 7.0 and experiments were carried out at 30°C. The protein concentration was calculated based on the extinction coefficient at 280 nm. The heats of dilution were determined in separate experiments and were negligible compared to the actual protein ligand titrations. The ITC raw data were fit to a one‐site binding model (MicroCal Origin version 7 software) to determine the stoichiometry (N) and association constant (K a) which were then converted to K d values using the relation K d = 1/K a.
Differential scanning calorimetry
All DSC experiments described in this article were carried out on a VP‐DSC microcalorimeter (Malvern). The desired amount of the lyophilised protein was dissolved in 20 mM HEPES, 100 mM NaCl at pH 7.0. The protein concentration was calculated based on the absorption measured at 280 nm and was maintained at ∼30 μM. In all experiments done in the presence of various ligands the final [HisJ]:[Ligand] was kept at 1:5. Detailed experimental procedure is mentioned in supplementary materials and methods. In each experiment the protein samples were loaded into the DSC instrument immediately after two consecutive buffer‐only heating cycles, accounting for the thermal memory compensation of the DSC calorimeter and baseline correction, respectively. The protein samples were heated from 10 to 80°C at a scan rate of 60°C h−1 with a filter period of 16 ps and the pressure was kept around 28 psi to keep the sample stable at higher temperatures. A fourth scan was done after cooling the sample after the third one to check for reversibility of the denaturation process. The non‐2‐state model was used to determine the van't Hoff heat change (ΔH v) that is the heat change per unfolding unit. Therefore the ratio of ΔH/ΔH v was used to measure the number of cooperative units per mole.
Supporting information
Supporting Information
Acknowledgments
The authors thank Dr. Byron Chu, Dr. Leo Nguyen, and Dr. Hiroaki Ishida for valuable suggestions made in the early stages of this work.
References
- 1. Holland I, Cole S, Kuchler K, Higgins C (2003) ABC proteins: From bacteria to man. Amsterdam: Elsevier. [Google Scholar]
- 2. Davidson AL, Dassa E, Orelle C, Chen J (2008) Structure, function, and evolution of bacterial ATP‐binding cassette systems. Microbiol Mol Biol Rev 72:317–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eitinger T, Rodionov DA, Grote M, Schneider E (2011) Canonical and ECF‐type ATP‐binding cassette importers in prokaryotes: diversity in modular organization and cellular functions. FEMS Microbiol Rev 35:3–67. [DOI] [PubMed] [Google Scholar]
- 4. Quiocho FA, Ledvina PS (1996) Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol 20:17–25. [DOI] [PubMed] [Google Scholar]
- 5. Krewulak KD, Vogel HJ (2008) Structural biology of bacterial iron uptake. Biochim Biophys Acta 1778:1781–1804. [DOI] [PubMed] [Google Scholar]
- 6. Berntsson RP‐A, Smits SHJ, Schmitt L, Slotboom D‐J, Poolman B (2010) A structural classification of substrate‐binding proteins. FEBS Lett 584:2606–2617. [DOI] [PubMed] [Google Scholar]
- 7. Ho WW, Li H, Eakanunkul S, Tong Y, Wilks A, Guo M, Poulos TL (2007) Holo‐ and apo‐bound structures of bacterial periplasmic heme‐binding proteins. J Biol Chem 282:35796–35802. [DOI] [PubMed] [Google Scholar]
- 8. Chu BCH, Vogel HJ (2011) A structural and functional analysis of type III periplasmic and substrate binding proteins: their role in bacterial siderophore and heme transport. Biol Chem 392:39–52. [DOI] [PubMed] [Google Scholar]
- 9. Ames GFL, Lever JE (1972) The histidine‐binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem 247:4309–4316. [PubMed] [Google Scholar]
- 10. Doige CA, luzzi Ames GF (1993) ATP‐dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Annu Rev Microbiol 47:291–319. [DOI] [PubMed] [Google Scholar]
- 11. Lever JE (1972) Quantitative assay of the binding of small molecules to protein: comparison of dialysis and membrane filter assays. Anal Biochem 50:73–83. [DOI] [PubMed] [Google Scholar]
- 12. Chu BCH, Dewolf T, Vogel HJ (2013) Role of the two structural domains from the periplasmic Escherichia coli histidine‐binding protein HisJ. J Biol Chem 288:31409–31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins CF, Ames GFL (1981) Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci USA 78:6038–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nikaido K, Ames GFL (1992) Purification and characterization of the periplasmic lysine‐, arginine‐, ornithine‐binding protein (LAO) from Salmonella typhimurium . J Biol Chem 267:20706–20712. [PubMed] [Google Scholar]
- 15. Pulido NO, Silva DA, Tellez LA, Perez‐Hernandez G, Garcia‐Hernandez E, Sosa‐Peinado A, Fernndez‐Velasco DA (2015) On the molecular basis of the high affinity binding of basic amino acids to LAOBP, a periplasmic binding protein from Salmonella typhimurium . J Mol Recognit 28:108–116. [DOI] [PubMed] [Google Scholar]
- 16. Oh BH, Kang C, De Bondt H, Kim S, Nikaido K, Joshi AK, Ames GFL (1994) The bacterial periplasmic histidine‐binding protein. J Biol Chem 269:4135–4143. [PubMed] [Google Scholar]
- 17. Yao N, Trakhanov S, Quiocho FA (1994) Refined 1.89‐A structure of the histidine‐binding protein complexed with histidine and its relationship with many other active transport/chemosensory proteins. Biochemistry 33:4769–4779. [DOI] [PubMed] [Google Scholar]
- 18. Oh BH, Ames GFL, Kim SH (1994) Structural basis for multiple ligand specificity of the periplasmic lysine‐, arginine‐, ornithine‐binding protein. J Biol Chem 269:26323–26330. [PubMed] [Google Scholar]
- 19. Wolf A, Shaw EW, Oh BH, De Bondt H, Joshi AK, Ames GFL (1995) Structure/function analysis of the periplasmic histidine‐binding protein: mutations decreasing ligand binding alter the properties of the conformational change and of the closed form. J Biol Chem 270:16097–16106. [DOI] [PubMed] [Google Scholar]
- 20. Wolf A, Shaw EW, Nikaido K, Ames GFL (1994) The histidine‐binding protein undergoes conformational changes in the absence of ligand as analyzed with conformation‐specific monoclonal antibodies. J Biol Chem 269:23051–23058. [PubMed] [Google Scholar]
- 21. Walmsley AR, Shaw JG, Kelly DJ (1992) Perturbation of the equilibrium between open and closed conformations of the periplasmic C4‐dicarboxylate binding protein from Rhodobacter capsulatus. Biochemistry 31:11175–11181. [DOI] [PubMed] [Google Scholar]
- 22. Floccos MM, Mowbray SL (1994) The 1.9 Å X‐ray structure of a closed unliganded form of the periplasmic glucose/galactose receptor from. Biochemistry 8931–8936. [PubMed] [Google Scholar]
- 23. Chu BCH, Chan DI, Dewolf T, Periole X, Vogel HJ (2014) Molecular dynamics simulations reveal that apo‐HisJ can sample a closed conformation. Proteins 82:386–398. [DOI] [PubMed] [Google Scholar]
- 24. Ames GFL, Liu CE, Joshi AK, Nikaido K (1996) Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J Biol Chem 271:14264–14270. [DOI] [PubMed] [Google Scholar]
- 25. Dwyer MA, Hellinga HW (2004) Periplasmic binding proteins: a versatile superfamily for protein engineering. Curr Opin Struct Biol 14:495–504. [DOI] [PubMed] [Google Scholar]
- 26. de Lorimier RM, Smith JJ, Dwyer MA, Looger LL, Sali KM, Paavola CD, Rizk SS, Sadigov S, Conrad DW, Loew L, HW Helinga. (2002) Construction of a fluorescent biosensor family. Protein Sci 11:2655–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeffery CJ (2011) Engineering periplasmic ligand binding proteins as glucose nanosensors. Nano Rev 2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrigno PK (2016) Non‐antibody protein‐based biosensors. Essays Biochem 60:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang AYC, Mancera RL (2008) Molecular dynamics simulations of ligand‐induced backbone conformational changes in the binding site of the periplasmic lysine‐, arginine‐, ornithine‐binding protein. J Comput Aided Mol Des 22:799–814. [DOI] [PubMed] [Google Scholar]
- 30. Shuker SB, Hajduk PJ, Meadows RP, Fesik SW (1996) Discovering high‐affinity ligands for proteins: SAR by NMR. Science 274:1531–1534. [DOI] [PubMed] [Google Scholar]
- 31. Vaynberg J, Qin J (2006) Weak protein‐protein interactions as probed by NMR spectroscopy. Trends Biotechnol 24:22–27. [DOI] [PubMed] [Google Scholar]
- 32. Myint T, Fraser GE, Lindsted KD, Knutsen SF, Hubbard RW, Bennett HW (2000) Urinary 1‐methylhistidine is a marker of meat consumption in Black and in White California Seventh‐day Adventists. Am J Epidemiol 152:752–755. [DOI] [PubMed] [Google Scholar]
- 33. Ballard FJ, Tomas FM (1983) 3‐Methylhistidine as a measure of skeletal muscle protein breakdown in human subjects: the case for its continued use. Clin Sci 65:209–215. [DOI] [PubMed] [Google Scholar]
- 34. Ikeda Y, Kawahara S, Taki M, Kuno A, Hasegawa T, Taira K (2003) Synthesis of a novel histidine analogue and its efficient incorporation into a protein in vivo. Protein Eng 16:699–706. [DOI] [PubMed] [Google Scholar]
- 35. Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93:1803–1845. [DOI] [PubMed] [Google Scholar]
- 36. Peters LJ, Kovacic JP (2009) Histamine: metabolism, physiology, and pathophysiology with applications in veterinary medicine. J Vet Emerg Crit Care 19:311–328. [DOI] [PubMed] [Google Scholar]
- 37. Gibbs NK, J Tye, Norval M (2008) Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochem Photobiol Sci 7:655–667. [DOI] [PubMed] [Google Scholar]
- 38. Beaver JE, Waters ML (2016) Molecular recognition of Lys and Arg methylation. ACS Chem Biol 11:643–653. [DOI] [PubMed] [Google Scholar]
- 39. Kreimer DI, Malak H, Lakowicz JR, Trakhanov S, Villar E, Shnyrov VL (2000) Thermodynamics and dynamics of histidine‐binding protein, the water‐soluble receptor of histidine permease implications for the transport of and low affinity ligands. Eur J Biochem 267:4242–4252. [DOI] [PubMed] [Google Scholar]
- 40. Novokhatny V, Ingham K (1997) Thermodynamics of maltose binding protein unfolding. Protein Sci 6:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukada H, Sturtevant JM, Quiocho FA (1983) Thermodynamics of the binding of l‐arabinose and of d‐galactose to the l‐arabinose‐binding protein of Escherichia coli . J Biol Chem 258:13193–13198. [PubMed] [Google Scholar]
- 42. Liu CE, Liu PQ, Wolf A, Lin E, Ames GFL (1999) Both lobes of the soluble receptor of the periplasmic histidine permease, an ABC transporter (traffic ATPase), interact with the membrane‐ bound complex. J Biol Chem 274:739–747. [DOI] [PubMed] [Google Scholar]
- 43. Wolf A, Lee KC, Kirsch JF, Ames GF (1994) Ligand‐dependent conformational plasticity of the periplasmic histidine‐binding protein HisJ. Involvement in transport specificity. J Biol Chem 269:21243–21250. [DOI] [PubMed] [Google Scholar]
- 44. Lian LY (2013) NMR studies of weak protein‐protein interactions. Prog Nucl Magn Reson Spectrosc 71:59–72. [DOI] [PubMed] [Google Scholar]
- 45. Njagi JI, Kagwanja SM (2011) The interface in biosensing: improving selectivity and sensitivity In: Interfaces and Interphases in Analytical Chemistry. Vol. 1062 ACS Symposium Series. American Chemical Society, pp 11–225. [Google Scholar]
- 46. Nadler DC, Morgan S, Flamholz A, Kortright KE, Savage DF (2016) Rapid construction of metabolite biosensors using domain‐insertion profiling. Nat Commun 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamura T, Hamachi I (2014) Recent progress in design of protein‐based fluorescent biosensors and their cellular applications. ACS Chem Biol 9:2708–2717. [DOI] [PubMed] [Google Scholar]
- 48. Solscheid C, Kunzelmann S, Davis CT, Hunter JL, Nofer A, Webb MR (2015) Development of a reagentless biosensor for inorganic phosphate, applicable over a wide concentration range. Biochemistry 54:5054–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tugarinov V, Kanelis V, Kay LE (2006) Isotope labeling strategies for the study of high‐molecular‐weight proteins by solution NMR spectroscopy. Nat Protoc 1:749–754. [DOI] [PubMed] [Google Scholar]
- 50. Yamniuk AP, Ishida H, Vogel HJ (2006) The interaction between calcium‐ and integrin‐binding protein 1 and the αIIb integrin cytoplasmic domain involves a novel C‐terminal displacement mechanism. J Biol Chem 281:26455–26464. [DOI] [PubMed] [Google Scholar]
- 51. Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. [DOI] [PubMed] [Google Scholar]
- 52. Johnson BA, Blevins RA (1994) NMR view: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


