Abstract
The isolation of genes influencing long-term memory is critical for an understanding of learning at the molecular level. Recently, chromosomal substitution rat strains, known as consomics, have been developed. Here we report the results of the first study on aversive learning and memory with these consomic rats. We compared the Fawn Hooded Hypertensive (FHH) and Brown Norway (BN) parent strains with a Brown Norway chromosome 1 substitution on the FHH background (FHH-1BN). Results indicated that while all strains had normal short-term memory, the FHH animals were impaired relative to BN in tests of long-term memory for a discrete auditory cue. This deficit was rescued by the introgression of the BN1 chromosome onto the FHH background. Furthermore, the FHH-1BN consomic showed an enhancement in long-term contextual fear memory relative to the FHH strain. These changes were not due to differences in pain sensitivity as both strains performed equally on two different pain tests. These results provide preliminary support that consomic rat strains can be a useful tool in identifying genes related to long-term fear memory formation.
Keywords: Consomics, Fear conditioning, Learning, Memory, QTL, Rats
Introduction
A vast amount of research has indicated that behavioral performance in complex learning tasks is influenced by genetic as well as environmental factors (Owen et al. 1997; Radcliffe et al. 2000). While methods for isolating quantitative trait loci (QTL) have contributed to our understanding of this genetic variation in both mice (e.g., Wehner et al. 1997) and rats (e.g., Bielavská et al. 2002; Fernández-Teruel et al. 2002), the exact function of genes isolated through this method remain unknown. Converging approaches are needed in order to determine this function, especially in the rat where due to its size, ease of manipulation, and breeding techniques, most of the behavioral studies on learning to date have been done (Aitman et al. 2008; Jacob 1999; Lazar et al. 2005). Consomic, or chromosomal substitution, rat strains provide such an approach (Kwetik-Black and Jacobs 2001).
This method uses breeding to introgress entire chromosomes from a donor strain to a recipient. With the introgression of this chromosome comes any QTL, either known or unknown, within this chromosomal region. Phenotyping of a complete consomic panel, which includes 20 autosomes plus the X and Y chromosomes, can map QTL to a single chromosome. Once narrowed, an F2 intercross with the inbred strain could produce a panel of congenic strains which could further narrow the QTL region. Thus, consomic rat strains provide a powerful means by which specific genes identified by QTL analysis could be isolated and their function, with regard to a specific phenotype could be identified giving it the potential to be an important tool in understanding mechanisms of memory formation and stability.
Pavlovian fear conditioning is a widely used paradigm for examining the systems and molecular neurobiological substrates of long-term memory formation and stability (Helmstetter et al. 2008; LeDoux 2000). This paradigm involves exposing animals to paired presentations of a neutral conditional stimulus (CS) with a noxious unconditional stimulus (UCS). Once this association has been established, upon later presentation the CS will elicit conditional responses. Learning in this paradigm is rapid and robust and lasts for an extended period after only a few pairings. This makes fear conditioning ideal for the study of long-term memory.
Results from pharmacological (e.g., Bailey et al. 1999; Parsons et al. 2006), gene expression (e.g., Ahn et al. 2008; Han et al. 2007, 2008), and transgenic manipulation studies (e.g., Pineda et al. 2004) have indicated a potential role for a number of genes in long-term memory formation and stability. Of particular interest are studies showing that the serum/glucocorticoid kinase 1 (Sgk1; Lee et al. 2007; von Hertzen and Giese 2005), glutamate receptor subunits 1 and 5 (Gravius et al. 2006; Zhou et al. 2007), and protein phosphatase 1 (PP1; Genoux et al. 2002; Miller and Sweatt 2007) contribute critically to the formation of long-term fear memories. In the rat, genes for all these proteins involved in memory formation have been identified on chromosome 1 (Rat Genome Database, http://rgd.mcw.edu). A genetic manipulation which could isolate these genes could provide complementary support for their potential role in long-term memory formation.
In the present study, we performed the first comparison of consomic rats in a complex learning paradigm. We compared the Fawn-Hooded Hypertensive (FHH) and Brown Norway (BN) inbred rat strains on a standard auditory and contextual fear conditioning paradigm. Since a number of potential learning-related genes are known to exist on the rat chromosome 1, we tested these strains with the Fawn-Hooded Hypertensive Brown Norway chromosome 1 (FHH-1BN) consomic strain. Though acquisition of fear conditioning is known to be slightly retarded in variations of the FHH strain, it is one of a few inbred rat models that have been shown to be capable of learning both contextual fear and two-way avoidance conditioning paradigms when normotensive (Calcagnetti and Schechter 1994; Overstreet et al. 1992). To account for the FHH’s possible retarded acquisition, we used a strong training protocol which results in near asymptotic freezing for the discrete auditory cue in the Long Evans strain which is generally used as an excellent model for LTM formation (Helmstetter and Fanselow 1987). Additionally, since the slower acquisition in the FHH strain is thought to be due to an increased analgesic response (Calcagnetti and Schechter 1994), all animals underwent several tests of analgesia to control for differences in long-term memory due to variations in pain sensitivity.
Materials and methods
Subjects
The Fawn-Hooded Hypertensive (FHH), Brown Norway (BN) and Fawn-Hooded Hypertensive Brown Norway chromosome 1 (FHH-1BN) strains were developed by PhysionGenix (Wauwatosa, WI, USA) and maintained by commercial vendors. For this experiment, 20 male FHH and 20 male FHH-1BN were obtained from Hilltop Labs (Scottsdale, PA, USA) and 15 male Brown Norway (BN) rats were obtained from Charles Rivers Laboratories (Kingston, NY, USA). All rats were received at ~4–6 weeks of age and housed individually in stainless steel cages with free access water and 0.4% NaCl rat chow (Harlan, Madison, WI, USA) throughout the experiment. Though some evidence exists suggesting that isolated housing can differentially affect sensitivity to aversive events in some rat strains (for e.g., Nunes Mamede Rosa et al. 2005), such effects have not been reported in the FHH and BN strains. Since the FHH strain is known to progressively develop hypertension across their lifespan (Kwetik-Black and Jacobs 2001), all animals were conditioned at 90 days (~6–8 weeks from arrival) which is an age at which untreated FHH rats are reported to still be normotensive when maintained on a 4.0% NaCl diet (Mattson et al. 2005). The colony was maintained on a 14:10 h light:dark cycle, with an average temperature of 69°F and average relative humidity of 52%. All procedures were approved by the University of Wisconsin-Milwaukee institutional animal care and use committee, and carried out in accordance with the NIH guidelines for using animals in experimental procedures.
Apparatus
Fear conditioning took place in four identical observation chambers (28 × 20.5 × 1 cm) constructed of Plexiglas and stainless steel (Context A). The floor of each chamber was composed of stainless steel rods spaced 1.5 cm apart through which foot shock could be delivered and each chamber was illuminated by a 7.5 W white light bulb. Ventilation fans provided a constant background noise of ~60 dB. Testing to the auditory cue took place in a separate set of chambers (Context B) with stainless steel rods covered with floors made of Plexiglas and fans that provided a background noise of ~58 dB. All chambers were housed in sound-attenuating boxes. Context A was cleaned with a 5% ammonium hydroxide solution before each set of animals, while Context B was cleaned with a 2% acetic acid solution.
Fear conditioning procedures
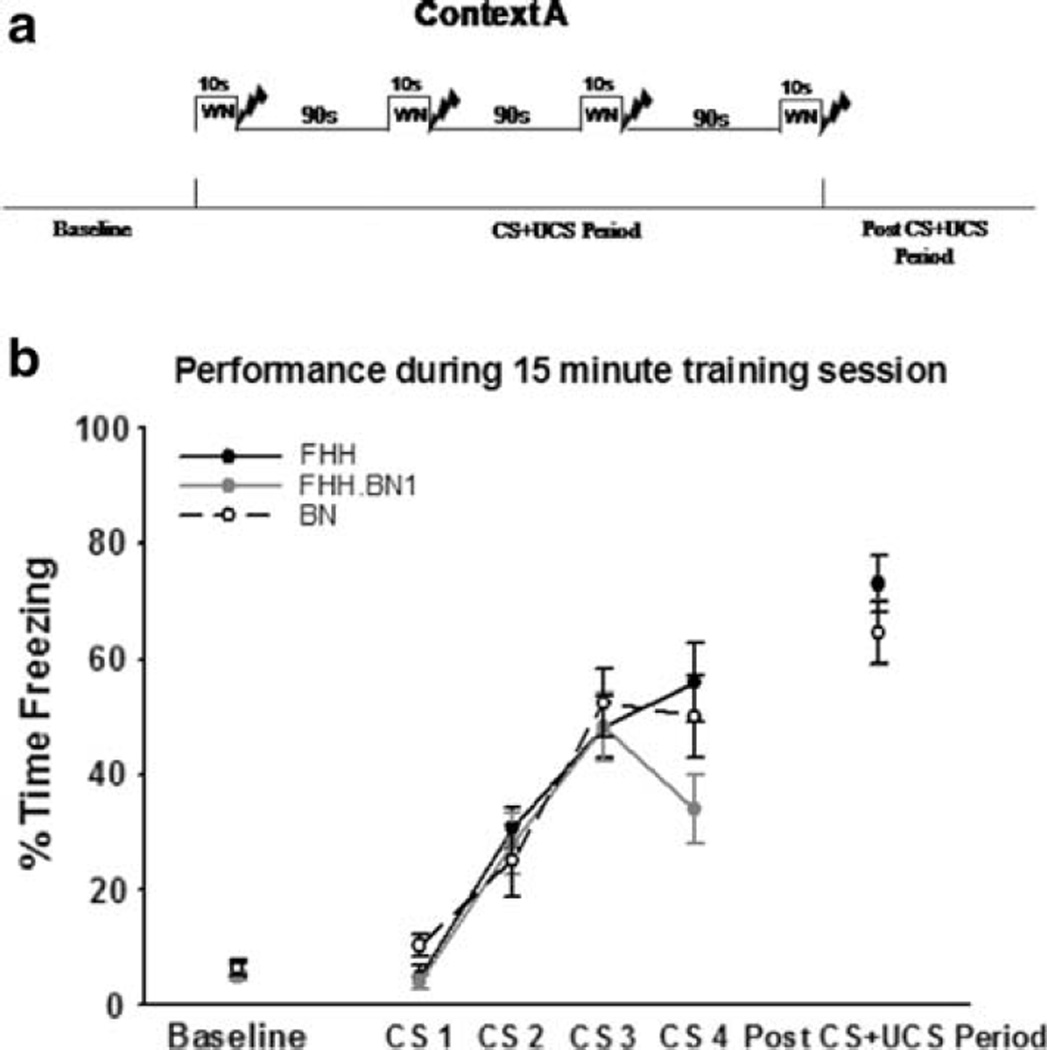
Fear conditioning was performed as described previously (Baruch et al. 2004). All animals were handled 5 min a day for three consecutive days followed by 3 days of habituation to the transport procedure. After the handling and habituation procedures were complete, animals were individually trained (1 per chamber) to a contextual plus auditory fear conditioning procedure (Fig. 1a). Training involved a 6 min baseline followed by four white noise (72 dB; 10 s)-shock (1 mA/1 s) pairings separated by a 90 s inter-trial interval. After a 4 min post-shock period, animals were removed from the training context (Context A).
Fig. 1.
BN1 substitution does not alter the acquisition of fear memory. a Procedure for contextual plus auditory fear conditioning. Animals were placed into Context A and received four white noise-shock (WN-SK) pairings after a 6 min baseline period. Following the final shock, there was a 4 min short-term memory test for the context CS. b All strains showed similar baselines (left), CS-UCS acquisition (middle), and short-term memory for the context CS (right)
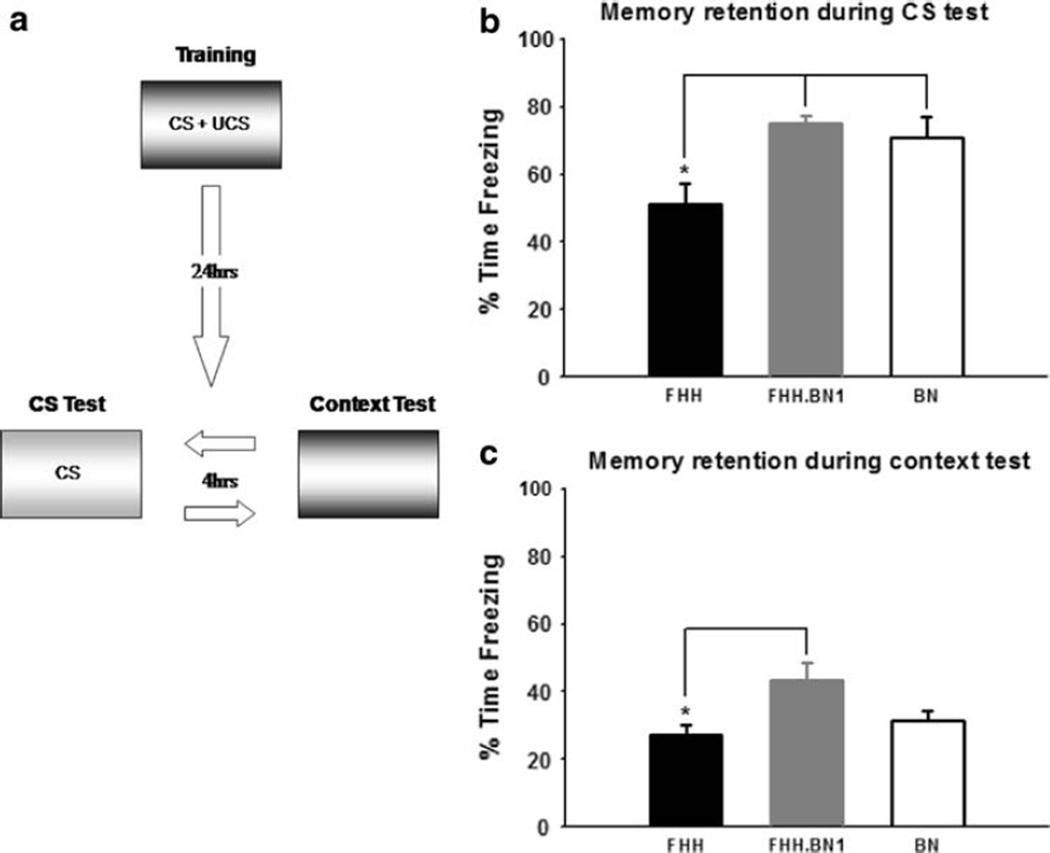
This training procedure results in two separate memories, one for the context in which training took place and another for the discrete (i.e., white noise) cue, and each can be tested through the animals’ display of conditional responses (i.e., freezing behavior). In order to distinguish between conditional responses for each cue, the animals were tested for context fear by exposure to Context A and to the white noise CS in the novel Context B 24 h after acquisition (Fig. 2a). The order in which the animals were tested was counterbalanced so that half of the rats from each group were tested first in Context A and the remaining were tested first in Context B and then returned to their home cages. About 4 h later, the rats previously tested in Context A were tested in Context B, and vice versa.
Fig. 2.
BN1 substitution rescues long-term auditory and contextual fear memory deficits in the FHH strain. a Procedure for the 2 days of testing. 24 h after training, animals were placed into Context B and given a 5 min, nonreinforced CS presentation after a 6 min baseline period and were placed into Context A for a 15 min test of the contextual CS. There was 4 h between each test and the order was counterbalanced for all strains. b The FHH strain had a long-term memory deficit for the auditory CS, relative to the BN strain. Introgression of the BN1 chromosome rescues this impairment. c The FHH strain showed marginal impairments in long-term memory for the contextual CS relative to the BN strain. Substitution of the BN1 chromosome onto the FHH background significantly enhances LTM for the contextual CS. * denotes P < 0.05
Context testing consisted of exposing rats to the training context for 15 min, with no discrete CS or shock UCS presentation. White noise tests consisted of exposure to the novel context for 6 min, followed by a 5-min white noise CS (72 dB) presentation in the absence of any shock UCS. The rats remained in the chambers for 4 min after the termination of the white noise CS and were then returned to their home cages. All freezing scores were obtained and calculated by Clever Systems software (FreezeScan 1.0, CleverSys. Inc., Reston, VA, USA) and analyzed by analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) post hoc tests. Subjects were determined to be outliers if they scored more than two standard deviations above or below the mean.
Hotplate
After completion of fear memory testing, all animals were assessed for reactivity to thermal pain. Animals were transported to a novel room where they were individually placed onto a Hotplate Analgesia Meter (Columbus Instruments, Columbus, OH). The base of the Hotplate was set at a constant temperature of 50°C. Animals remained on the plate until they licked one of their back feet (Paylor et al. 1998). Trials were terminated after 120 s if a rat failed to perform an appropriate response. All sessions were scored live by two experimenters and were also recorded for future analysis. The plate was cleaned with 10% ethanol between animals.
Shock reactivity analysis
Shock reactivity scores were obtained from each animal’s reaction to the four shocks given during the training session. For each shock, the time from when the shock terminated until the first instance of freezing behavior occurred was recorded. This period from shock termination until freezing behavior is exhibited is an “activity burst” and results as an unconditioned response to the shock UCS (Fanselow 1984). Behavior was scored offline using FreezeScan 1.0.
Results
Fear conditioning
All strains demonstrated similar freezing behavior throughout the entire training session (Fig. 1b). Baseline freezing levels indicated that there was no initial fear to the training context prior to the CS-UCS pairings. During the 5 min period in which the animals were presented with CS-UCS pairings, there were no differences between groups in overall freezing behavior (F(2,52) = 0.921, P = 0.405, data not shown). All strains acquired the CS-UCS association at a relatively equal rate, as indicated by freezing behavior obtained from each of the CS presentations. A repeated measures ANOVA was conducted with freezing to each CS presentation as the dependent measure and strain as the independent variable. Results indicated a main effect for CS trial (F(1,52) = 116.831, P < 0.001) but not for strain (F(2,52) = 1.527, P = 0.227) and there was not an interaction (F(2,52) = 2.006, P = 0.145). This suggests that all strains gradually acquired the CS-UCS association across the training session and that the rate of acquisition did not significantly differ between strains. The last 4 min of the training session can serve as an index of short-term memory (Bailey et al. 1999). During this time, there were no significant differences between groups for freezing behavior (F(2,52) = 0.862, P = 0.428) indicating that all strains reacted normally to the training experience.
On the following day all animals were given two long-term memory (LTM) tests. Although all groups did exhibit some freezing during the baseline period of the auditory CS test, there were no significant differences between groups (F(2,52) = 0.304, P = 0.739; data not shown) and this freezing behavior was significantly lower than freezing during the context test for all groups (all P’s < 0.05, data not shown) indicating that all strains could distinguish between the two contexts. There were large differences in overall freezing levels during the auditory CS presentation (Fig. 2b). An ANOVA revealed a main effect for strain (F(2,52) = 6.766, P < 0.01). Fisher LSD Post hoc tests revealed that the FHH animals froze significantly less than the BN animals, showing ~25% difference in overall time spent freezing. Interestingly, introgression of the BN chromosome 1 onto the FHH background, the FHH-1BN, rescued this deficit and resulted in freezing levels comparable to BN animals. This finding suggests that there are one or more learning-related genes located on the Brown Norway chromosome 1 which significantly influence long-term memory for a discrete cue when introgressed onto the FHH background.
All strains were also given a 15 min context test in the original training environment (Fig. 2a). During this test one FHH and one FHH-1BN animal were determined to be statistical outliers because they scored more than two standard deviations above the group mean and were excluded from context retention analysis. Similar to the auditory CS test, there were noticeable differences in overall freezing behavior between strains during the context test (Fig. 2c). An ANOVA was run for average percent freezing for the entire 15 min context test and results indicated a main effect for strain (F(2,50) = 3.540, P < 0.05). Fisher LSD Post hoc analyses revealed that while the FHH and BN strains did not significantly differ on memory retention for the context, introgression of the BN chromosome 1 onto the FHH background resulted in a significant increase in retention in comparison to the FHH strain. Combined with the results from the auditory CS test, this suggests that chromosome 1 may contain learning-related genes that improve the long-term retention of fear memory.
Nociceptive testing
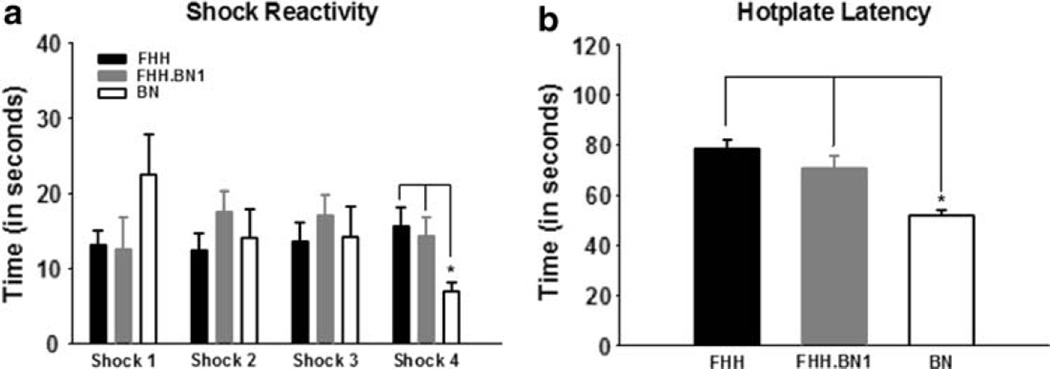
The differences in LTM for both the auditory and contextual cues could potentially be attributed to differences in sensory processing and not related to learning per se. For example, if the FHH-1BN consomic was differentially sensitive to the shock UCS, this could have contributed to what appears to be enhanced learning for both cues. In order to evaluate this, each animal’s reaction to shock, or “activity burst” (Fanselow 1991) was scored. The amount of time between shock termination and the first instance of freezing behavior was measured for each animal. On average, the FHH-1BN strain responded equally to the shock as did its FHH parent strain (Fig. 3a). A repeated measures ANOVA was run with time to unconditioned response to each shock as the dependent measure and strain as the independent variable. Results did not indicate a main effect for strain (F(2,52) = 0.207, P = 0.814) or shock number (F(1,52) = 1.858, P = 0.179), but did reveal an interaction (F(2,52) = 4.531, P < 0.05). Fisher LSD Post hoc analyses revealed that BN animals showed a significantly smaller reaction to shock than did both the FHH and FHH-1BN strains on shock 4. However, the FHH and FHH-1BN strains did not differ at any point in the series. This suggests that even though the BN strain may differ slightly than the FHH strain in response to the final UCS, the BN1 substitution did not modify this.
Fig. 3.
Introgression of BN1 chromosome does not alter pain tolerance or shock reactivity. a FHH and FHH-1BN strains had similar responses to the shock UCS given during training, while the BN strain was significantly more reactive to the UCS after shock 4. b The FHH strain showed a higher pain tolerance on the Hotplate than the BN strain and this was not altered by the BN1 substitution. * denotes P < 0.05
To further evaluate potential sensory/motor differences between the strains that might have an impact on the interpretation of the learning data, we also measured the rats’ reaction to a thermal nociceptive stimulus using the standard “hotplate” test. Animals were individually placed onto the copper hotplate, which was maintained at a constant temperature of 50°C. The session ended when the animal licked one of its back feet or 120 s had passed. An ANOVA indicated that there were significant differences in response latencies between the strains (F(2,52) = 11.048, P < 0.001; Fig. 3b). Fisher LSD Post hoc analyses revealed that the BN strain had a significantly higher sensitivity to the thermal pain than the FHH strain and introgression of the BN1 chromosome did not significantly alter the FHH strain’s sensitivity, as indicated by comparable response latencies. These results suggest that while the BN may be more sensitive to pain or more ready to respond than is the FHH strain, the chromosome 1 substitution does not normalize all of the phenotypic differences between these two strains. Combined with the shock reactivity results, this suggests that the learning enhancements noted in FHH-1BN animals, in comparison with the FHH strain, were not due to alterations in UCS processing.
Discussion
Our study highlights the potential utility of consomic rat strains as an approach to understand memory phenotypes. We show that while the FHH strain exhibits normal acquisition of contextual and auditory fear conditioning, it performs poorly on LTM tests for both cues. Interestingly, these deficits are rescued by the introgression of the BN chromosome 1. Additionally, while the FHH strain demonstrated slower responses to painful stimuli on two separate tests in comparison with the BN strain, the introgression of the BN1 chromosome did not alter these responses, suggesting that the FHH and FHH-1BN strains responded equally to painful stimuli. Importantly, this result indicates that the chromosome substitution did not normalize all phenotypic differences between the two strains. Collectively, these results are consistent with the hypothesis that a learning-related gene or genes may exist on chromosome 1 which significantly influences LTM formation.
While our results suggest that the FHH-1BN strain has enhanced LTM retention in comparison with the FHH strain, other interpretations may exist (e.g., see Mori and Makino 1994). For example, since our measure of memory was freezing behavior, it could be argued that the FHH-1BN strain is simply more inclined to freeze than the FHH parent strain. While a possibility, our results suggest that this is unlikely. In comparison with the FHH parent strain, FHH-1BN animals did not show enhanced freezing to the baseline of either the training session or the LTM test for the auditory CS. Additionally, FHH-1BN animals showed similar levels of freezing behavior when receiving shocks during training as did the FHH parent strain. Together, these results suggest that the increase in FHH-1BN freezing behavior during the retention tests was not due to an inclination to display the behavior more often under normal conditions.
Another interpretation could be that the FHH-1BN strain had enhanced acquisition of the fear memory relative to the FHH strain, which resulted in superior LTM. Again, our results suggest this is not the case. During the four CS presentations given in the training session, the FHH and FHH-1BN strains do not differ in their freezing behavior, suggesting that both strains acquired the CS-UCS association at an equivalent rate. Furthermore, these two strains did not differ during the final 4 min of the training session, which serves as a short-term memory test for the contextual CS, suggesting that these two strains did not differ on their strength of short-term memory. Conversely, when given a LTM test to the context CS, the FHH-1BN animals show enhanced memory in comparison with the FHH strain. Since there were no differences in contextual freezing during the short-term memory test, this suggests that both strains likely formed the memories equally.
Results from our test of thermal pain reactivity, the hotplate, revealed that the FHH strain had a significantly lower sensitivity for certain kinds of pain than did the BN strain. Considering these results, it could be suggested that the BN strain demonstrated superior LTM relative to the FHH strain because it reacts more to the shock UCS. As indicated during the LTM test for the discrete CS, BN animals showed significantly higher retention which could be attributed to their greater pain sensitivity. The recovery of LTM performance in the FHH-1BN consomic therefore could have been due to increased pain sensitivity as a result of the BN1 substitution. Our results indicate that this is unlikely. The FHH-1BN consomic did not show significantly increased pain sensitivity relative to the FHH parent strain, suggesting that the recovery in LTM was not likely due to changes in pain sensitivity.
Recently, several studies have indicated that a fine-mapped QTL for cued and context fear conditioning exists on chromosome 5 in rats (Fernández-Teruel et al. 2002; Johannesson et al. 2009). Interestingly, the gene for the protein kinase C zeta isoform (PKCf) exists on chromosome 5 (Rat Genome Database, http://rgd.mcw.edu). The atypical isoform of PKCζ is PKMζ, which has been implicated in the maintenance of long-term fear memories (e.g., Kwapis et al. 2009). Of interest would be whether the FHH strain with a BN5 substitution (FHH-5BN) would show recovery of LTM deficits for both the auditory and context cues similar to the FHH-1BN consomic. Additionally, preliminary evidence suggests that the FHH strain with a BN12 substitution (FHH-12BN) does not show any significant recovery of these deficits for both auditory or context cues (data not shown). This suggests that not all BN chromosome substitutions may rescue LTM deficits in the FHH strain, however, further studies are needed to confirm this finding. Future research should address these questions.
We provide the first evidence that a chromosome substitution can rescue a deficit in LTM formation in the rat. Future studies could be designed based on this finding. For example, the strength of using the consomic technology is that a genetic component has been identified on chromosome 1 and having the FHH-1BN rat strain already in hand speeds the mapping process to find the genes on this chromosome influencing fear memory formation. Future work could narrow the chromosome 1 region of interest with congenic lines constructed from an F2 intercross with the FHH-1BN and FHH rats, which we demonstrate here differ significantly in the fear memory formation. Once the region has been narrowed, gene expression analysis can be employed to compare congenic lines still possessing the phenotype of interest with the FHH strain. This strategy could potentially identify the gene(s) or pathway(s) of interest influencing the significant recovery of long-term memory. Once identified, production of transgenic rats that have inducible overexpression and knockouts could further indicate the importance of these genes in the formation of long-term fear memory in the rat. Ultimately, consomic rat strains provide a powerful means by which learning and memory can be genetically manipulated in the rat and provide a framework for future research looking to identify genes important in LTM formation.
Acknowledgments
We thank R. Parsons, G. Gafford of UW-Milwaukee and M. Wolter of PhysioGenix for technical assistance. This work was supported by National Institute of Mental Health grants MH069558 and MH060668 (FJH) and by National Center for Research Resources grant R44RR022671 (SHN).
Contributor Information
Timothy J. Jarome, Department of Psychology, University of Wisconsin, 2441 East Hartford Ave, Milwaukee, WI 53201, USA
Janine L. Kwapis, Department of Psychology, University of Wisconsin, 2441 East Hartford Ave, Milwaukee, WI 53201, USA
Steven H. Nye, PhysioGenix Inc., Wauwatosa, WI 53226, USA
Fred J. Helmstetter, Department of Psychology, University of Wisconsin, 2441 East Hartford Ave, Milwaukee, WI 53201, USA
References
- Ahn HJ, Hernandez CM, Levenson JM, Lubin FD, Liou HC, Sweatt JD. c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn Mem. 2008;15(7):539–549. doi: 10.1101/lm.866408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman TJ, Crister JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, et al. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40(5):516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on pavlovian fear conditioning. Behav Neurosci. 2004;118(5):1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Bielavská E, Kren V, Musilová A, Zídek V, Pravenec M. Genome scanning of the HXB/BXH sets of recombinant inbred strains of the rat for quantitative trait loci associated with conditioned taste aversion. Behav Genet. 2002;32(1):51–60. doi: 10.1023/a:1014407928865. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Deficits in shock-induced freezing and naltrexone enhancement of freezing in fawn hooded rats. Brain Res Bull. 1994;35(1):37–40. doi: 10.1016/0361-9230(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Opiate modulation of the active and inactive components of the postshock reaction: parallels between naloxone pretreatment and shock intensity. Behav Neurosci. 1984;98:269–277. doi: 10.1037//0735-7044.98.2.269. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Analgesia as a response to aversive pavlovian conditioned stimuli: cognitive and emotional mediators. In: Denny MR, editor. Fear, avoidance, and phobias. Hillsdale: Erbaum; 1991. pp. 61–86. [Google Scholar]
- Ferná ndez-Teruel A, Escorihuela RM, Gray JA, Aguilar R, Gil L, Giménez-Llort L, Tobeña A, Bhomra A, Nicod A, Mott R, Driscoll P, Dawson GR, Flint J. A quantitative trait locus influencing anxiety in the laboratory rat. Genome Res. 2002;12(4):618–626. doi: 10.1101/gr.203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schäfer D, Schmidt WJ, Danysz W. The role of group 1 metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats—a comparison. Neuropharmacology. 2006;51(7–8):1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Han J-H, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during fear memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han J-H, Yiu AP, Cole CJ, Hsiang H–L, Neve RL, Josselyn SA. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem. 2008;15:443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Strain differences in reversal of conditioned analgesia by opioid antagonists. Behav Neurosci. 1987;101(5):735–737. doi: 10.1037//0735-7044.101.5.735. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89(3):324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. Functional genomics and rat models. Genome Res. 1999;9:1013–1016. doi: 10.1101/gr.9.11.1013. [DOI] [PubMed] [Google Scholar]
- Johannesson M, Lopez-Aumatell R, Stridh P, et al. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res. 2009;19:150–158. doi: 10.1101/gr.081497.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Inhibition of protein kinase Mζ erases an established fear memory in the amygdale but not the hippocampus. Behav Neurosci. 2009;123(4):844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwetik-Black AE, Jacobs HJ. The use of designer rats in the genetic dissection of hypertension. Curr Hypertens Rep. 2001;3:12–18. doi: 10.1007/s11906-001-0072-0. [DOI] [PubMed] [Google Scholar]
- Lazar J, Moreno C, Jacob HJ, Kwitek AE. Impact of genomics on research in the rat. Genome Res. 2005;15:1717–1728. doi: 10.1101/gr.3744005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee CT, Ma YL, Lee EHY. Serum- and glucocorticoidinducible kinase 1 enhances contextual fear memory formation through downregulation of the expression of Hes5. J Neurochem. 2007;100:1531–1542. doi: 10.1111/j.1471-4159.2006.04284.x. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW., Jr Substitution of chromosome 1 ameliorates L-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol. 2005;288:F1015–F1022. doi: 10.1152/ajprenal.00374.2004. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Mori T, Makino J. Response types to shock and avoidance learning in inbred strains of mice. Shinrigaku Kenkyu. 1994;65(4):295–302. doi: 10.4992/jjpsy.65.295. [DOI] [PubMed] [Google Scholar]
- Nunes Mamede Rosa ML, Nobre MJ, Oliviera AR, Brandao ML. Isolation induced changes in ultrasonic vocalization, fear-potentiated startle and prepulse inhibition in rats. Neuropsychobiology. 2005;51(4):248–255. doi: 10.1159/000085820. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Janowski DS. Genetic models of depression and ethanol preference provide support for cholinergic and serotonergic involvement in depression and alcoholism. Biol Psychiatry. 1992;31(9):919–936. doi: 10.1016/0006-3223(92)90118-j. [DOI] [PubMed] [Google Scholar]
- Owen EH, Christensen SC, Paylor R, Wehner JM. Identification of quantitative trait loci involved in contextual and auditory-cued fear conditioning in BXD Recombinant inbred strains. Behav Neurosci. 1997;111(2):292–300. doi: 10.1037//0735-7044.111.2.292. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Arca7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Pineda VV, Athos JI, Wang H, Celver J, Ippolito D, Boulay G, Birnbaumer L, Storm DR. Removal of G(ialpha1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron. 2004;41:153–163. doi: 10.1016/s0896-6273(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Lowe MV, Wehner JM. Confirmation of contextual fear conditioning QTLs by short-term selection. Behav Genet. 2000;30(3):183–191. doi: 10.1023/a:1001910107167. [DOI] [PubMed] [Google Scholar]
- Rat Genome Database. http://rgd.mcw.edu. [Google Scholar]
- von Hertzen LSJ, Giese KP. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J Neurosci. 2005;25(8):1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Rosman ST, Christensen SC, Rasmussen DL, Fulker DW, Wiles M. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]
- Zhou D, Huang J, Wu X, Li L. Metabotropic glutamate subtype 5 receptors modulate fear-conditioning induced enhancement of prepulse inhibition in rats. Neuropharmacology. 2007;52(2):476–486. doi: 10.1016/j.neuropharm.2006.08.016. [DOI] [PubMed] [Google Scholar]