Abstract
In Pavlovian fear conditioning, an aversive unconditional stimulus (UCS) is repeatedly paired with a neutral conditional stimulus (CS). As a consequence, the subject begins to show conditional responses (CRs) to the CS that indicate expectation and fear. There are currently two general models competing to explain the role of subjective awareness in fear conditioning. Proponents of the single-process model assert that a single propositional learning process mediates CR expression and UCS expectancy. Proponents of a dual-process model assert that these behavioral responses are expressions of two independent learning processes. We used backward masking to block perception of our visual CSs and measured the effect of this training on subsequent unmasked performance. In two separate experiments we show a dissociation between CR expression and UCS expectancy following differential delay conditioning with masked CSs. In Experiment I, we show that masked training facilitates CR expression when the same CSs are presented during a subsequent unmasked reacquisition task. In Experiment II we show that masked training retards learning when the CS+ is presented as part of a compound CS during a subsequent unmasked blocking task. Our results suggest that multiple memory systems operate in a parallel, independent manner to encode emotional memories.
Keywords: fear conditioning, humans, backward masking, awareness, skin conductance response
In Pavlovian fear conditioning, an aversive unconditional stimulus (UCS) is repeatedly paired with a neutral conditional stimulus (CS). As a consequence, the subject begins to show conditional responses (CRs) to the CS that indicate expectation and fear. In fear conditioning with humans, autonomic CRs can be measured via changes in skin conductance level (SCL). In addition, humans are also able to explicitly express knowledge of the programmed experimental contingencies. Changes in autonomic arousal and knowledge of the stimulus contingencies may represent two different expressions of conditional learning (Carter, O'Doherty, Seymour, Koch, & Dolan, 2006; Cheng, Knight, Smith, Stein, & Helmstetter, 2003). However, the degree to which these implicit and explicit forms of expression reflect independent learning mechanisms is controversial (Lovibond & Shanks, 2002). Currently there are two models competing to explain the role of awareness in classical conditioning. Proponents of the single-process model assert that both forms of expression are dependent upon a single propositional learning mechanism (Lovibond & Shanks, 2002). Proponents of a dual-process model assert that these behavioral responses are expressions of two independent learning processes (Manns, Clark, & Squire, 2002; Wiens & Öhman, 2002). If a single propositional learning process is to account for these two forms of expression, then there should be little or no dissociation between implicit and explicit responses to the CS. However if two parallel learning processes are taking place, then it may be possible to express learning implicitly without being able to express it explicitly (Lovibond & Shanks, 2002). The purpose of this study was to investigate implicit learning in the absence of explicit awareness by training subjects on a differential conditioning task while manipulating their perception of CS-UCS contingencies using backward masking.
Support for a dual process model of Pavlovian conditioning comes from a growing body of studies using delay eyeblink conditioning, where the CS coterminates with the UCS (Clark & Squire, 1998). In this paradigm a tone (CS) predicts the delivery of a puff of air to the eye (UCS), which leads to a reflexive eyeblink (UCR). After repeated pairings, the CS evokes an eyeblink CR prior to the UCS presentation (McCormick & Thompson, 1984). Researchers have repeatedly shown that CR production with this paradigm is independent of awareness of the CS-UCS contingencies (Manns, Clark, & Squire, 2001; Smith, Clark, Manns, & Squire, 2005).
Consistent with the eyeblink literature, several researchers have examined the role of awareness in fear conditioning and found support for the dual-process model (Bechara et al., 1995; Esteves, Parra, Dimberg, & Öhman, 1994; Knight, Nguyen, & Bandettini, 2003, 2006; Öhman & Soares, 1998). For example, Bechara et al. (1995) tested three patients in a standard differential delay fear conditioning task. The authors paired presentations of neutral geometric shapes with a mild electric stimulation. The first patient had bilateral amygdala damage, the second patient had bilateral hippocampal damage, and the third patient had bilateral hippocampal and amygdala damage. The individual with amygdala damage was unable to express conditional SCRs, but was able to acquire declarative knowledge of the stimulus contingencies. The individual with hippocampal damage expressed conditional SCRs, but was unable to acquire declarative knowledge about the stimulus contingencies. Finally, the individual with hippocampal and amygdala damage was unable to express conditional SCRs or acquire declarative knowledge of the stimulus contingencies (Bechara et al., 1995). Results suggest that the amygdala is key in the production of the CR, while the hippocampus is important for acquisition or expression of awareness of the CS-UCS contingencies.
These findings are not limited to patients with lesions. In two experiments using auditory cues, Knight and colleagues demonstrated a clear dissociation between autonomic CRs and knowledge of stimulus contingencies in healthy adults during delay but not trace fear conditioning (Knight et al., 2003, 2006). They presented neutral tones at perithreshold volumes, using an adaptive thresholding technique. In these differential conditioning experiments, one CS was paired with the UCS (CS+), while the other was explicitly unpaired (CS−). Participants indicated on a trial by trial basis whether or not they perceived the tone. The researchers also measured skin conductance responses (SCRs) and UCS expectancy ratings (Knight et al., 2003, 2006). In the first experiment, they used a simple differential delay conditioning task. In this experiment, they observed differential CRs during both perceived and unperceived trials. However, they observed differential UCS expectancy only during the perceived trials (Knight et al., 2003). In the second experiment, they extended this paradigm to trace conditioning. They found that introducing a trace interval between the offset of the CS+ and the onset of the UCS eliminated differential responding during unperceived trials but not perceived trials (Knight et al., 2006). These results suggest that awareness is not necessary for expression of fear during delay conditioning, but is necessary for expression during trace conditioning.
Knight et al. (2003, 2006) manipulated awareness by changing the volume of tone CSs; however, this approach does not work for visual stimuli. Öhman and colleagues manipulated awareness of visual stimuli by using backward masking (Esteves et al., 1994; Öhman & Soares, 1998). In this paradigm, a target picture is presented briefly (<50 ms) and is followed by the presentation of a masking picture, in the same area of the visual field (Enns & Di Lollo, 2000). The presentation of this masking stimulus interrupts the cortical processing of the target stimulus (Noguchi & Kakigi, 2005; Rolls, Tovee, & Panzeri, 1999), and renders the target invisible (Kim & Blake, 2005).
In these masking studies, Öhman and colleagues paired masked CSs with an aversive UCS in a differential conditioning paradigm and measured the effect of such training on CR expression during a subsequent unmasked extinction session (Esteves et al., 1994; Öhman & Soares, 1998). In addition, these researchers used both evolutionary fear-relevant and neutral stimuli (angry vs. happy faces and spiders/snakes vs. flowers/mushrooms, respectively). In both cases, when subjects were unaware of the CS-UCS contingencies they showed differential CRs to only the fear-relevant (prepared) stimuli during the extinction session. Because subjects trained with masked neutral stimuli failed to show evidence of conditioning, Öhman hypothesized that conditioning with masked CSs is mediated by dedicated neural circuits that were evolved to rapidly and automatically detect fear-relevant stimuli (Öhman, 2005; Öhman, Carlsson, Lundqvist, & Ingvar, 2007).
However, it is also possible that the lack of conditioning observed with masked neutral stimuli is due to the fact that the CS and the UCS were separated by a temporal gap of at least 500 ms. Consistent with this hypothesis, more recent studies using trace conditioning with neutral CSs suggest that awareness is needed to maintain the CS representation during gaps between CS offset and UCS onset (Knight et al., 2006; Manns, Clark, & Squire, 2000). Therefore, it may be possible to show conditioning with masked neutral CSs if there is no temporal gap between the CS and the UCS. The goal of this study was to test this hypothesis by presenting masked geometric shapes in a differential delay conditioning paradigm and measure the effects of this masked training on performance during subsequent unmasked tasks.
Experiment I
The purpose of this experiment was to test the predictions of the single and dual process models, as well as Öhman's preparedness hypothesis in a differential delay conditioning paradigm. In Phase I of this experiment, participants underwent differential delay conditioning with masked CSs. We used simple geometric shapes as CSs and a checkerboard pattern as a mask. CSs were presented for 15 ms. A painful 500 ms electrical stimulation served as the UCS and was presented immediately upon CS offset. To ensure that our masking manipulation truly blocked participants' ability to consciously perceive the CSs, we gave them 700 ms on each trial to determine which of the two stimuli was presented.
After a short break (~5 min), groups underwent differential conditioning and extinction with unmasked, 8-s CSs. One group saw the same CSs, with the same CS-UCS contingencies; the other group saw completely new CSs and learned new CS-UCS contingencies. We measured contingency awareness and CR expression during Phase II. If individuals implicitly learn about the CS-UCS contingencies during Phase I, then they should learn the task more readily during Phase II, but only if they are presented with the same CSs.
According to the single process model, CR expression is dependent upon the ability to perceive the CS and thus associate it with the UCS. Therefore, if subjects fail to perceive the stimuli during Phase I, they should show equivalent CRs during Phase II whether they see the same CSs or different CSs. Likewise, according to Öhman's preparedness hypothesis, conditioning with masked CSs is dependent upon the preparedness of the CSs used; therefore, if subjects see neutral CSs during Phase I, then they should show equivalent CRs during Phase II whether they see the same CSs or different CSs. In contrast, according to the dual process model, CR expression is not dependent upon stimulus features or the ability to perceive the CSs; therefore, subjects shown the same CSs during both phases should show an enhanced differential CR during Phase II.
Method
Participants
Twenty-two undergraduate students (Age: M = 19.83, SD = 2.53; 13 female) at the University of Wisconsin-Milwaukee participated in this experiment for extra credit in their psychology classes. One individual was excluded from the study because of computer failure during data collection. Another individual was excluded from the study based on his performance during Phase I (see results). All participants gave informed consent, and the protocol was approved by the Institutional Review Board for human subject research at the University of Wisconsin-Milwaukee.
Stimuli
Stimuli were presented using the software package Presentation (Neurobehavioral Systems, Inc., Albany, CA). They were presented on a Dell laptop (model: Inspiron 9300, Dell Inc., Red Rock, TX) with a 17” monitor with a 60-Hz refresh rate. The stimuli consisted of simple geometric shapes, and they were masked by a checkerboard pattern, which was presented immediately after their offset. The stimuli in this study consisted of gray (RGB: 128, 128, 128) shapes (circle, oval, square, diamond) against a black background, and were masked by a gray and black checkerboard pattern. All shapes with the exception of the oval had a height and width of 100 pixels. The oval had a height of 100 pixels and a width of 83 pixels. The checkerboard pattern was 13 × 13 and had a height and width of 125 pixels.
Electrical stimulation
Electrical stimulation was administered via an AC (60 Hz) source (Lafayette Instruments, Model 82400, Lafayette, IN) through two aluminum surface electrodes (2 cm diameter) treated with electrode cream (Med-Tek Corp., Joliet, IL), and cued by the software. The electrodes were placed on the skin over the subject's right tibial nerve over the right medial malleolus. The stimulation was calibrated prior to the experiment, according to each person's pain tolerance. Each individual rated the shock on a scale from zero (no sensation) to 10 (painful but tolerable). We then gradually increased the shock intensity until the individual rated the sensation as a 10. Presentations during the experiment were administered at the subjective level 10 for 500 ms.
Skin conductance responses
SCL was recorded via two surface cup electrodes (silver/silver chloride, 8 mm diameter, Biopac model EL258-RT, Goleta, CA) filled with electrolyte gel (Signa Gel, Parker Laboratories Fairfield, NJ) attached to the bottom of the participants left foot, approximately 2 cm apart, and sampled at 80 Hz throughout the experiment. For each trial, we sampled SCL during the 7.5-s CS period prior to the shock and the preceding 2-s baseline period. Raw values for each trial were normalized to that trials average baseline SCL and expressed as a percent change from that baseline value. SCR time course data were obtained by averaging the percent change values across participants at each time point during the sample period. Statistical tests were computed at each time point.
Monte Carlo simulations
Monte Carlo simulations were conducted to correct for multiple comparisons carried out on SCRs, in a manner similar to cluster thresholding of fMRI data (Forman et al., 1995). This procedure is based on the assumption that the SCL at any given time point is correlated with the SCL at surrounding time points. Therefore true significant differences should be sequentially located; whereas spurious differences should be randomly distributed throughout the time course.
We generated 200,000 strings of random p values that were the same length as the SCR samples, using a random number generator. We then counted the number of sequential significant p values (Tp = time point p value), using a time point α (Tα) of 0.05, and determined the likelihood that a given stretch of sequential significant p values (λp = length of observed stretch; λα = length of threshold) could have arisen due to chance alone. We found that less than 0.026% of the simulations yielded λps greater than or equal to 5 time points in length, which is equivalent to 62.5 ms in duration (See Supplemental Table 1). Therefore to correct for multiple comparisons, we thresholded our data using a combination of individual time point p value (Tα = 0.05) and length of sequential significant p values (λα = 62.5 ms).
UCS expectancy
Throughout Phase II of the experiment, participants continuously rated their expectancy of receiving the electrical stimulation. To do so, they controlled a visual analog scale on the computer screen using the laptop touchpad. The analog scale was anchored with 0 and 100. They were instructed to move the cursor to 0 if they were absolutely sure that they would not receive an electrical stimulation, to move the cursor to 100 if they were absolutely sure that they would receive a stimulation, and to keep the cursor near 50 if they felt like there was an equal probability of receiving or not receiving a stimulation. Responses were recorded throughout the phase and sampled at 40 Hz. For each trial, we sampled the last 4 s of the CS period.
Procedure
During Phase I, participants underwent differential delay conditioning with masked stimuli (See Figure 1a). Each individual viewed presentations of two of the four stimuli. Before the experiment began, participants were given one memorization trial with each of the two stimuli. During these trials the participant was instructed to memorize the stimulus and press the corresponding mouse key to move on. During the experiment, subjects were instructed to identify the stimulus presented on each trial by pressing the correct mouse key. They were instructed to press the left mouse key if they saw stimulus 1 and the right mouse key if they saw stimulus 2. The stimuli and button assignments were counterbalanced across participants with respect to the UCS. Subjects were given 700 ms from CS onset to respond, after which the trial was scored as incorrect. Performance on this task was used to assess their ability to identify the CS presentation and therefore associate the CSs with the UCS. Above chance performance on this task resulted in exclusion from the study. Above chance performance was defined as greater than 14 out of 20 correct identifications χ2(1, N = 20) = 3.2, p = .074).
Figure 1.
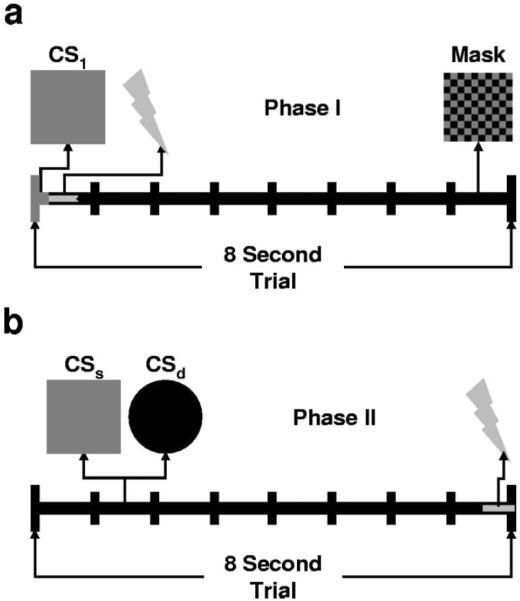
We manipulated awareness in a fear conditioning task, and assessed the effects of conditioning without awareness on subsequent learning. (a) During Phase I, we presented 20 differential conditioning trials. We presented 15-ms CSs that were masked by a 7.985-s masking stimulus to block CS1 perception. We presented an immediate shock UCS on CS1+ trials. (b) During Phase II, we split participants into two groups that underwent differential acquisition and extinction with 8-s unmasked CSs. We exposed one group to the same CSs (CSS) and another to different CSs (CSD). For both groups, the UCS coterminated with the CS+.
We conducted pilot studies to determine the masking parameters that most effectively blocked subjects' perception of the masked stimuli. Each trial began with a 1-s presentation of a fixation cross, which appeared randomly in the center of one of four quadrants on the screen. Subjects were instructed to focus on the fixation point even though its location would not predict the location of the stimulus. Upon fixation offset, the stimulus and mask were presented in one of the three remaining quadrants. The stimulus was presented first for 15 ms and was immediately followed by a 7.985-s presentation of the mask. One stimulus was paired with the shock (CS1+) while the other stimulus was presented alone (CS1−). The 500-ms shock began at the onset of the mask presentation. There were 10 trials of each CS type. CSs were presented in quasi-random order so that the same CS was not presented more than two times consecutively. Trial one CS type was also counterbalanced. The shock was presented on 8 of the 10 CS1+ presentations. Participants were not told that there would be a relationship between the CSs and the shock. In fact, the purpose of the mask was to minimize the likelihood that they would become aware of the CS-UCS contingencies. The intertrial interval varied randomly between 16 and 24 s. Upon completion of Phase I of the experiment, participants were given a short break (~5 min) before the start of the Phase II of the experiment.
During Phase II, participants underwent differential conditioning and extinction with unmasked CSs (See Figure 1b). They were randomly assigned to one of two groups. The first group viewed presentations of the same CSs that were presented during Phase I, with the same CS-UCS contingencies (Group SAME; n = 10). The second group viewed presentations of novel CSs, and learned novel CS-UCS contingencies (Group DIFFERENT; n = 10). Trials during Phase 2 began with the presentation of a 1-s fixation cross, which was immediately followed by an 8-s presentation of the CS. For Group SAME, the CS1+ was paired with a shock (S+) and the CS1− was presented alone (S−). For Group DIFFERENT, the stimuli were novel; one stimulus was paired with the shock (D+) and the other was presented alone (D−). During conditioning, the UCS coterminated with the stimulus on all CS2+ trials. During extinction the UCS was not presented. Each CS was presented three times during both conditioning and extinction. Conditioning and extinction were carried out in a single experimental phase, and participants were not signaled at the onset of extinction. CSs were presented in quasi-random order so that the same CS was not presented more than two times consecutively. As in Phase I, trial one CS type was counterbalanced. UCS expectancy and SCRs were recorded.
As a manipulation check, participants were given a postexperimental questionnaire designed to assess their knowledge of the Phase I CS-UCS contingencies. It included questions, such as: “In the first phase of the experiment, were you able to predict when you would receive an electrical stimulation?” and “Was there a relationship between the shapes and the electrical stimulation in the first phase of the experiment?”
Two parallel analyses were carried out on Phase II UCS expectancy and SCR data. We were interested in the effect of the training with masked CSs during Phase I on subsequent learning with unmasked CSs during Phase II. Therefore, we analyzed data across trials within the conditioning and extinction sessions. We were also interested in the effect of Phase I CS-UCS pairings on fear expression, independent of Phase II CS-UCS pairings; therefore, we further subdivided groups based on Phase II trial one CS type (S+ n = 5; S− n = 5; D+ n = 6; D− n = 4), and compared across groups. This allowed us to measure UCS expectancy and SCR expression to the CSs before participants are exposed to the Phase II CS-UCS associations.
Results
Phase I perception
We assessed perception of Phase I CSs at an individual level and a group level. Individual performance was assessed using a χ2 test. Group performance was assessed using a one-sample t test with a test value of 10, which reflects chance performance.
One individual correctly identified 15 of the 20 stimulus presentations during Phase I, and was therefore excluded from all analyses. However, the group as a whole correctly identified an average of 9.4 (SD = 2.7) stimulus presentations. Group performance did not significantly differ from the test value (t(19) = −/−.993, p = 0.333), suggesting that the group as a whole could not identify the stimulus presentations during Phase I.
Consistent with these data, only one person felt that he could predict when he would receive the electrical stimulation during Phase I. This individual also felt that there was a relationship between the shapes and the stimulation during this phase, but incorrectly identified the CS1− as the shape that was paired with the shock.
Phase II behavior prior to CS2–UCS contingencies
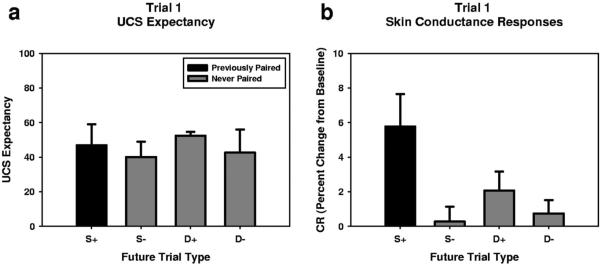
In order to assess behavior before exposure to the CS2-UCS contingencies, we conducted a planned comparisons analysis of variance (ANOVA) on UCS expectancy and SCR expression. For SCR expression, we conducted the ANOVA at each time point during the CS period. We were interested specifically in the effect of CS1-UCS pairing on subsequent behavior, so our first comparison was between those who were presented with the previously reinforced stimulus (S+) and those who were presented with all other stimuli (S−, D+, D−; See Figure 2). Next we wanted to assess the effect of previous exposure on subsequent behavior, so our second comparison was between those who were presented with the previously presented, but unreinforced stimulus (S−) and those who were presented with novel stimuli (D+, D−). Our final comparison was between the two remaining groups (D+ vs. D−).
Figure 2.
Groups show different patterns of UCS expectancy and SCR expression. (a) Participants shown the previously reinforced stimulus (n = 5) show similar levels of UCS expectancy as those shown the previously unreinforced stimulus (n = 5) and those shown novel stimuli (D+, n = 6; D−, n = 4) during the first trial of Phase II. Bars represent M + SEM. (b) In contrast, participants shown the previously reinforced stimulus (n = 5) show larger magnitude SCRs than those shown the previously unreinforced stimulus (n = 5) or those shown novel stimuli (D+, n = 6; D−, n = 4) during the first trial of Phase II. Graph represents the peak SCR value during the CS period. Bars represent M + SEM. For SCR time course data see Supplemental Figure 1 and Supplemental Table 2.
When conducting the ANOVA on UCS expectancy, we failed to show any significant effects (Fs < 1), which suggests that training with masked CSs did not affect UCS expectancy to CSs prior exposure to the CS2-UCS pairings.
In contrast to the UCS expectancy data, those presented with the previously reinforced stimulus (S+) show significantly larger SCRs than those presented with all other stimuli (S−, D+, D−; See Figure 2; See Supplemental Figure 1 and Supplemental Table 2 for time course data), during the second half of the CS period (Onset = 6.23 s; Offset = Trial End). However, those presented with the previously presented but unreinforced stimulus (S−) showed similar magnitude SCRs to those presented with novel stimuli (D+, D−). Similarly, the groups presented with the novel stimuli showed similar magnitude SCRs. Taken together, these results suggest that training with masked CSs affects SCR production in a stimulus specific manner, dependent upon CS1-UCS pairings.
Phase II UCS expectancy
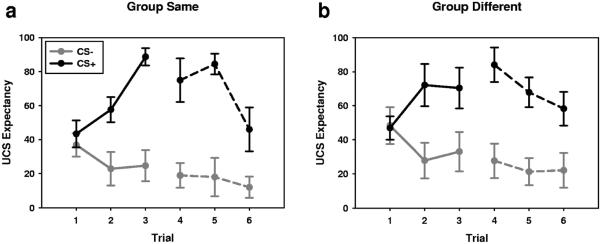
To assess UCS expectancy during conditioning and extinction, we conducted two repeated-measures ANOVAs with the following factors: group (SAME vs. DIFFERENT), CS (CS2+ vs. CS2−), and trial. See Figure 3 for UCS expectancy data.
Figure 3.
Groups show similar patterns of UCS expectancy during Phase II. (a, b) Average UCS expectancy across all trials of Phase II. Group SAME (a; n = 10), showed a similar pattern of UCS expectancy as Group DIFFERENT (b; n = 10). Lines represent M ± SEM.
During conditioning, both groups readily learn the CS-UCS contingencies, as evidenced by a significant trial by CS interaction, F(2, 72) = 7.16, p = .001 and a significant main effect for CS, F(1, 36) = 30.94, p < .00049. However, UCS expectancy did not seem to vary as a function of group. We failed to find a significant main effect for group, F(1, 36) = 0.548, p = .464 or any significant group interactions (Fs < 1). These results are consistent with the Phase I perception data, and suggest that participants did not consciously learn the CS-UCS contingencies during that phase.
During extinction, both groups appear to be extinguishing the CS-UCS contingency, as evidenced by a trial by CS trend. However, this trend did not reach significance, F(2, 72) = 1.95, p = .15. More important, UCS expectancy did not seem to vary as a function of group. Again, we failed to find a significant main effect for group, F(1, 36) = 0.474, p = .496, or any significant group interactions (Fs < 2).
Phase II SCRs
To assess SCR expression during conditioning and extinction, we conducted two repeated-measures ANOVAs with the following factors: group (SAME vs. DIFFERENT), CS (CS2+ vs. CS2−), and trial at each time point during the CS period.
During conditioning, both groups readily learn the CS-UCS contingencies, as evidenced by a significant trial by CS interaction, which occurred during the onset of the second interval response, as well as a significant main effect for CS which began during the onset of the second interval response and continued until trial end (See Supplemental Table 3).
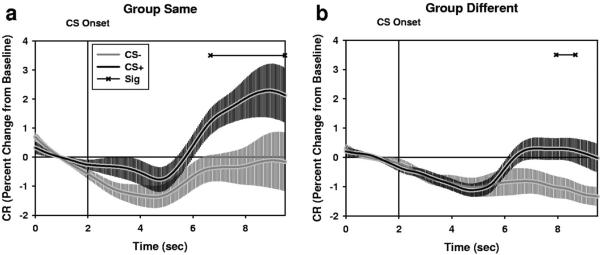
Although we did not observe the expected Group by CS interaction, there still seems to be some evidence for group differences in SCR expression during acquisition. For instance, we observed a significant Group by trial interaction during the onset of the of the second interval response. In addition, there seem to be qualitative differences in SCR expression across groups. We conducted paired-sample t tests for both groups during conditioning at each time point during the CS period. CS2+ responses were compared to CS2− responses on a participant by trial basis. During conditioning, Group SAME showed a differential SCR (See Figure 4 and Supplemental Table 3) starting at 4.66 s into the trial and lasting the duration of the trial. Group DIFFERENT also showed a differential SCR, however the CS+ is significantly different from the CS− during only a small portion of the CS period (Onset = 5.94 s; Offset = 6.66 s). These results seem to suggest that Group SAME is showing savings in the production of conditional SCRs during acquisition. However, one must use caution when coming to this conclusion because it is based on a descriptive comparison of the SCR time courses of the two groups, not on an inferential comparison.
Figure 4.
Participants in Group SAME seem to show savings in the production of Phase II differential SCRs, compared to Group DIFFERENT. Group SAME (a; n = 10) shows a differential SCR that is that is longer in duration than that of Group DIFFERENT (b; n = 10). Horizontal lines at top of graph indicate p values <0.05. Plots represent M ± SEM.
During extinction, groups do not differ in SCR expression, both groups seem to extinguish at similar rates. We observe significant CS by trial interaction during the first half of the trial, but no main effect for CS or Group (See Supplemental Figure 2 and Supplemental Table 3).
Discussion
In support of the dual process model, subjects presented with the previously reinforced stimulus on the first trial showed a significantly larger SCR than those presented with previously unreinforced or novel stimuli, before being exposed to the CS2-UCS contingencies. We found it to be interesting that these effects are not seen on the measure of UCS expectancy. Furthermore, the duration of the CR for Group SAME during the Phase II conditioning session was over three times longer than that of Group DIFFERENT. In contrast, Phase II UCS expectancy did not seem to vary as a function of Phase I training.
Taken together, these results suggest a dissociation between CR expression and UCS expectancy during Phase II. This pattern of behavior is specific to the stimuli used during Phase I, and independent of stimulus perception during Phase I. In contrast to the Phase II acquisition data, we observed no group differences during the Phase II extinction session, which could be due to contamination by the Phase II conditioning session.
Originally we predicted that participants would implicitly learn the CS-UCS contingencies during Phase I, and that this training would lead to differences in the rate of acquisition for the different groups. Because we did not observe the predicted Group by CS interaction, one might be lead to conclude that subjects were not able to implicitly learn the Phase I CS-UCS contingencies. However, that conclusion is inconsistent with the Trial 1 data, and there may be several other possible explanations for why we may have not observed the CS by Group interaction during Phase II acquisition. For instance, with so few trials we may not have had the statistical power to detect group differences in acquisition rates. Alternatively, our masking manipulation may have been too effective or our reacquisition task may have been too simple.
Another limitation to our study is that we used a forced choice stimulus identification task to assess CS perception during Phase I. In order to complete the task, subjects were presented with two memorization trials (one for each CS) prior to the experiment. This preexposure to the CSs was necessary for the forced choice task because it allowed the subject to associate a given stimulus with the corresponding response. However, it also introduces a potential confounding variable into our experiment, because the preexposure was different for the different groups in the experiment. Both groups receive preexposure to the Phase I CSs, but only Group SAME saw those same CSs during Phase II. Therefore, differences seen in Phase II could be due to this preexposure, rather than the Phase I CS-UCS contingencies. To address this issue, we performed a second experiment in which this necessary stimulus preexposure was consistent across groups. In addition, we increased the number of acquisition trials to increase our statistical power.
Experiment II
Like Experiment I, the purpose of this experiment is to test the hypothesis that differential delay conditioning with masked CSs affects subsequent CR expression, but not subsequent UCS expectancy. However unlike Experiment I, all participants were exposed to novel stimuli in Phase II, thus eliminating the potential confound of CS preexposure introduced by the memorization trials. In this experiment, Phase I CSs were used as part of a compound stimulus during Phase II in a forward blocking paradigm.
In blocking, a stimulus (CSA) is initially paired with the UCS, and is subsequently presented as part of a compound stimulus (CSAB), which is then also paired with the UCS. When tested to CSB alone, subjects that have previously undergone training with CSA show a diminished CR to CSB. In other words, learning the CSA-UCS association prior to learning the CSAB-UCS association blocks the subject's ability to learn the CSB-UCS association (Kamin, 1968, 1969a, 1969b).
The current experiment included two training sessions. As in Experiment I, during Phase I training, participants underwent differential delay conditioning with masked CSs. Also as in Experiment I, during Phase II, they underwent differential delay conditioning with unmasked CSs. Because we did not observe group differences during extinction in Experiment I, we did not include an extinction session in Phase II of this experiment. By using six trials of acquisition instead of three, we were able to increase the statistical power of our testing procedure. Unlike Experiment I, all CSs during Phase II were novel. The new CSs were presented as part of a compound stimulus, with either the previous CS+ (CS1+) or the previous CS− (CS1−), which was presented on all CS2+ and CS2− trials. Subjects were randomly assigned to groups, and groups differed based on which CS served as the blocking stimulus (CS1+=Group BLOCK, CS1− = Group CONTROL).
If the CS1+ is presented as part of a compound stimulus with CS2+, it should block the CS2-UCS association. According to the dual process model, blocking perception of the CSs in Phase I with masking should affect the acquisition of explicit but not implicit CS1-UCS association. Therefore according to the dual process model, Phase II explicit performance should not be affected, but Phase II implicit performance should be affected. According to this logic, Group BLOCK should show a diminished differential CR when compared to Group CONTROL. However, because participants are unable to perceive the CSs during Phase I, Phase II UCS expectancy should not vary across groups.
Method
Participants
Twenty-six undergraduate students (Age: M = 22.23, SD = 3.84; 13 female) at the University of Wisconsin-Milwaukee participated in this experiment for extra credit in their psychology classes. Five individuals were excluded from the study based on performance during Phase I. Four were classified as aware of stimulus contingencies (see results), one failed to make responses during Phase I, therefore stimulus perception could not be assessed. All participants gave informed consent, and the protocol was approved by the Institutional Review Board for human subject research at the University of Wisconsin-Milwaukee.
Apparatus
Apparatus used in this experiment were the same as those used in Experiment I.
Procedure
Phase I procedure in this experiment was the same as in Experiment I (See Figure 5a). Briefly, participants underwent differential delay conditioning with masked CSs. We measured stimulus perception using the same forced-choice task is in Experiment I. Individuals that were able to consciously identify stimulus presentations at an above chance level were excluded from further analyses.
Figure 5.
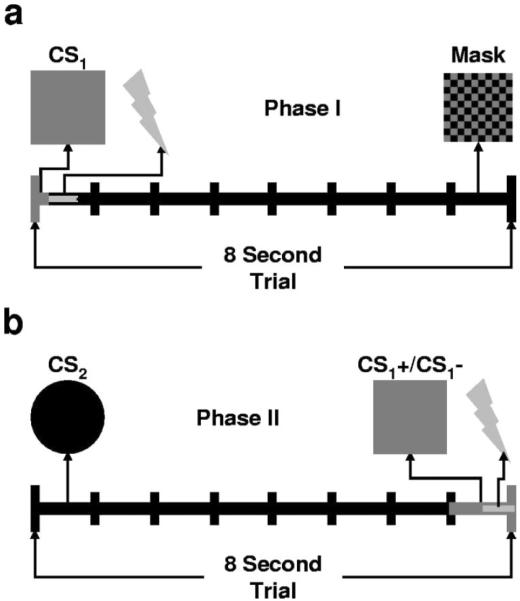
As in Experiment I, we manipulated awareness in a fear conditioning task and assessed the effects of conditioning without awareness on subsequent learning. (a) During Phase I, we presented 20 differential conditioning trials. We presented 15-ms CSs that were masked by a 7.985-s masking stimulus to block CS1 perception. We presented an immediate shock UCS on CS1+ trials. (b) During Phase II, we split participants into two groups that underwent differential acquisition with 7-s unmasked novel CSs. These CSs were paired with one of the Phase I stimuli. Group BLOCK CSs were paired with CS1+. Group CONTROL CSs were paired with CS1−. The blocking stimulus was presented during the last second of all trials. The UCS coterminated with the blocking stimulus on all CS+ trials.
In phase II, participants underwent differential conditioning with unmasked novel CSs that were paired with a blocking stimulus (See Figure 5b). The blocking stimulus was either the previous CS+ or the previous CS−. Individuals were randomly assigned to one of two groups. The first group (BLOCK; n = 11) had CSs paired with the previous CS+. The second group (CONTROL; n = 10) had CSs paired with the previous CS−. Trials during Phase II began with the presentation of a 1-s fixation cross, which was immediately followed by a seven second presentation of the CS, then by a 1-s presentation of the blocking stimulus. By pairing the CSs with the blocking stimulus temporally as opposed to spatially, we were able to measure both SCR production and UCS expectancy to the CSs without adding an additional testing phase. One compound stimulus was paired with the shock (CS2+) and the other was presented alone (CS2−). The UCS coterminated with the blocking stimulus on all CS2+ trials. Each CS was presented six times during Phase II. The blocking stimulus was presented on all CS2+ and CS2− trials and coterminated with the UCS on CS2+ trials. CSs were presented in quasi-random order so that the same CS was not presented more than two times consecutively. As in Phase I, trial one CS type was counterbalanced. UCS expectancy and SCRs were recorded.
As a manipulation check, participants were given the same postexperimental questionnaire used in Experiment I. As before, we were interested in the effect of the Phase I training on subsequent performance during Phase II. Therefore, we assessed UCS expectancy and SCR production across all Phase II trials.
Results
Phase I perception
We assessed stimulus perception at an individual level and a group level, as in Experiment I. Four individuals correctly identified 14 or more (14/20, 14/20, 17/20, 18/20) stimulus presentations during Phase I, and were therefore excluded from all analyses. However, the group as a whole correctly identified an average of only 9.38 (SD = 2.01) stimulus presentations. Group performance did not significantly differ from the test value, t(20)=−1.41, p = 0.174, suggesting that the group as a whole could not identify the stimulus presentations during Phase I.
Consistent with the perception data, only three (one of which was classified as “aware”) subjects felt that they could predict when they would receive the electrical stimulation during Phase I. However, none of these individuals associated the stimulation with a particular stimulus.
Phase II UCS expectancy
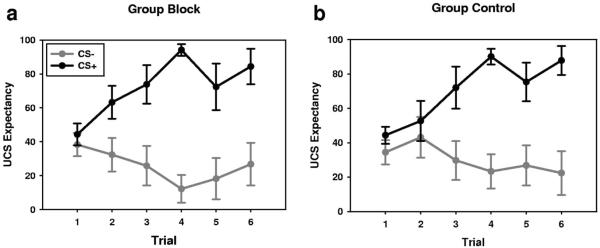
To assess UCS expectancy during Phase II we conducted a repeated measures ANOVA with the following factors: group (BLOCK vs. CONTROL), CS (CS2+ vs. CS2−), and trial. All trials from Phase II were included. See Figure 6 for UCS expectancy data.
Figure 6.
As in Experiment I, Groups show similar patterns of UCS expectancy during Phase II. Group BLOCK (a; n = 11), showed a similar pattern of UCS expectancy as Group CONTROL (b; n = 10). Lines represent M ± SEM.
As in Experiment I, both groups readily learn that the CS-UCS contingencies, as evidenced by a significant trial by CS interaction, F(5, 190) = 7.912, p < .00049 and a significant main effect for CS, F(1, 38) = 50.29, p < .00049. However, UCS expectancy did not seem to vary as a function of group. We failed to find a significant main effect for group, F(1, 38) = 0.052, p = .821 or any significant group interactions (Fs < 1). These results are consistent with the Phase I perception data and with Experiment I, and suggest that participants did not consciously learn the CS-UCS contingencies during Phase I training.
Phase II SCRs
To assess conditional SCR expression during Phase II, we conducted repeated measures ANOVAs with the following factors: group (BLOCK vs. CONTROL), CS (CS2+ vs. CS2−), and trial at each time point during the CS period. All trials from Phase II were included (See Figure 7 and Supplemental Table 4).
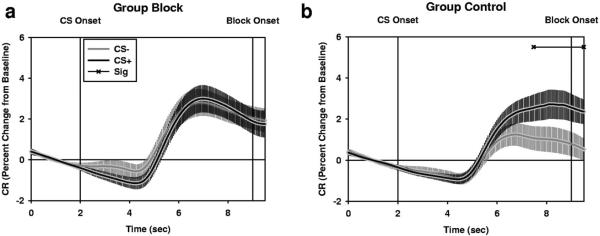
Figure 7.
Training during Phase I affects SCR expression during Phase II when the previous CS+ is used as a blocking stimulus. (a) Group BLOCK (n = 11) does not show differential SCR production. In contrast, Group CONTROL (n = 10) shows robust differential SCR production. Horizontal lines at top of graph indicate p values <0.05. Plots represent M ± SEM.
We see evidence of learning during Phase II. We see significant main effects for CS and Trial, and a significant CS by Trial interaction during the second interval period. However, this learning does not seem to be consistent across groups. We see a significant Group by CS interaction during the second interval period as well.
To characterize the Group by CS interaction, we conducted paired sample t tests for both groups at each time point during the CS period. CS2+ responses were compared to CS2− responses on a participant by trial basis. Pairing the previous CS− with the CSs during Phase II did not disrupt differential conditioning, because Group CONTROL shows a significantly larger SCR to the CS+ than to the CS− during the second interval period. However, pairing the previous CS+ with the CSs during Phase II blocked differential conditioning, because Group BLOCK does not show differences in SCR magnitude during Phase II.
Discussion
In support of the dual process model, we again find that differential delay conditioning with masked CSs affects subsequent CR expression, independent of the participants' ability to consciously identify the masked CSs. When the Phase I CS+ is used as a blocking stimulus in a subsequent unmasked conditioning phase, differential CR expression is blocked. This is not the case when the previous CS− is used as the blocking stimulus. Because stimuli were counterbalanced and preexposure to the CSs was equivalent across groups, these differences cannot be accounted for by differences in stimulus characteristics or preexposure.
As in Experiment I, these results suggest that subjects are implicitly learning about the CS-UCS contingencies during Phase I. Also as in Experiment I, subjects were unable to consciously identify the CSs during Phase I, and the choice of blocking stimulus did not affect UCS expectancy during Phase II. This dissociation between conscious perception of the CSs and subsequent CR expression is inconsistent with both the single process model and the preparedness hypothesis, but consistent with the dual process model and the results from Experiment I.
The results of this experiment suggest that pairing the new CSs with the previously reinforced blocking stimulus in Phase II leads to diminished differential CR expression. There are two possible explanations for these results. First, it could be that subjects' ability to learn the new CS-UCS relationship is diminished because the blocking stimulus is competing with the new CS+ for association with the UCS. Second, it could be that subjects' ability to discriminate between the new CSs is diminished because they are learning second-order conditioning during the Phase II training. That is, subjects may be learning that the new CSs predict the blocking stimulus, which previously predicted the shock. Therefore, they learn to associate the new CS+ and the new CS− with the UCS via its association with the blocking stimulus. Although both possibilities lead to the same conclusion (that subjects were implicitly learning about the CSs during Phase I), future studies should be designed to dissociate the effects of blocking and second-order conditioning in this paradigm.
General Discussion
Here we demonstrate evidence of implicit Pavlovian conditioning in the absence of explicit CS perception using a delay fear conditioning paradigm. In both experiments, we conditioned subjects with masked presentations of simple geometric shapes, and measured the effect of this training on performance in a subsequent unmasked conditioning phase. We show that individuals implicitly learn CS-UCS contingencies even when masking of the CSs prevents explicit identification of the stimulus, and that this learning affects CR expression, but not UCS expectancy during the subsequent conditioning tasks.
In Experiment I, participants underwent unmasked conditioning and extinction with either the same or different stimuli. On the first trial, individuals showed larger CRs if exposed to the previously reinforced stimulus, even though they showed similar levels of UCS expectancy as the other groups. During conditioning, groups seemed to show qualitative differences in CR expression, but not UCS expectancy. Subjects shown the same CSs had a longer duration differential CR than those shown different CSs. However, these results are merely descriptive and should be interpreted with caution.
In Experiment II, we addressed many of the issues of the first experiment. We added trials to increase our statistical power and used a sequential blocking paradigm with novel stimuli to control for preexposure effects. We showed that CSs presented during masked training can block CR expression to a novel CS-UCS contingency when used as part of a compound stimulus in a subsequent unmasked training task. Furthermore, this blocking effect occurs only if the blocking stimulus has been previously paired with the UCS, and it is unaccompanied by a similar UCS expectancy effect. In addition, with both experiments we show that delay conditioning with masked CSs is not dependent upon the preparedness of the CSs used. These results are consistent with the dual process model, but inconsistent with the single process model and the preparedness hypothesis.
Our results are consistent with a number of studies showing that awareness is not necessary for CR production in delay conditioning. Knight et al. (2003, 2006) observed differential SCR expression without awareness using perithreshold audio tones paired with a white noise. Similarly, an individual with hippocampal lesions showed differential SCRs, but not awareness of CS-UCS contingencies (Bechara et al., 1995). Jovancovic and colleagues observed fear potentiated startle in unaware subjects, using lights paired with an aversive airblast to the larynx (Jovanovic et al., 2006). In addition to the fear conditioning literature, researchers have repeatedly found that delay eyeblink conditioning is independent of awareness as well (Clark, Manns, & Squire, 2002; Manns et al., 2002; Smith et al., 2005).
Our results are however different from those of Esteves et al. (1994) and Öhman and Soares (1998), who used trace fear conditioning. They observed differential SCR production with unaware participants only with prepared stimuli. In light of more recent findings with trace conditioning, it is not surprising that they failed to observe differential SCRs with masked neutral CSs. As noted earlier, several groups have failed to find CR expression without awareness during trace conditioning (Knight et al., 2006; Manns et al., 2000). Trace conditioning differs from delay conditioning in that it requires the subject to actively maintain a sensory representation of the CS during the trace period. Consequently, this active maintenance appears to be dependent upon awareness of CS, as well as the participation of additional neural substrates. In a comparison of the neural substrates mediating trace and delay conditioning, our lab has shown that trace conditioning is associated with increased activity in the hippocampus and middle frontal gyrus over delay conditioning (Knight, Cheng, Smith, Stein, & Helmstetter, 2004). This is similar to work suggesting that hippocampal activity is also important for trace eyeblink conditioning (Clark et al., 2002; Clark & Squire, 1998) as well as work suggesting that the hippocampus and parahippocampal gyrus play a role in contingency awareness (Bechara et al., 1995; Carter et al., 2006).
The trace conditioning/awareness hypothesis may explain why we show evidence of masked conditioning with neutral CSs but Öhman and colleagues do not (Esteves et al., 1994; Öhman & Soares, 1998). However in both of their previous studies (Esteves et al., 1994; Öhman & Soares, 1998) they were able to show differential CR expression in the absence of awareness using prepared stimuli, which is inconsistent with the trace conditioning/awareness hypothesis. Therefore, the hypothesis that awareness is necessary for trace conditioning needs to be adapted to account for their finding.
Several lines of evidence suggest that trace conditioning requires additional neural processing to maintain the sensory representation of the CSs during the trace period (Clark et al., 2002; Clark & Squire, 1998; Knight et al., 2004; Knight et al., 2006; Manns et al., 2000). In most cases this means that awareness of the CS-UCS contingencies is necessary for CR production, but with prepared visual stimuli there may be brain regions capable of maintaining this representation below the threshold for conscious identification (Liddell et al., 2005; Morris, de Gelder, Weiskrantz, & Dolan, 2001; Morris, Öhman, & Dolan, 1999). LeDoux (2000) has suggested that there is a thalamic pathway to the amygdala that serves to automatically detect prepared visual stimuli, this model has been supported by researchers using masking in normal participants (Liddell et al., 2005; Morris et al., 1999) as well as observations of brain activity in patients with cortical blindness (de Gelder & Hadjikhani, 2006; Morris et al., 2001). According to this model, the superior colliculus sends inputs to the pulvinar nucleus of the thalamus, which then send inputs to the amygdala (LeDoux, 2000; Öhman, 2005; Öhman et al., 2007). However, rather than simply detecting the stimuli prior to awareness, we hypothesize that activity in these regions may be capable of actively storing a sensory representation of the prepared stimulus, thus enabling the CS-UCS associations to be acquired even if there is a trace interval between the CS and the UCS. Consistent with this hypothesis, pulvinar lesions block the effects of threatening images on reaction time when such images are used as distracters (Ward, Danziger, & Bamford, 2005).
The current results differ from previous findings in that we observed differential conditioning using masked neutral pictures as CSs, whereas others have not (Esteves et al., 1994; Öhman & Soares, 1998). We believe that this is because we used delay conditioning and they used trace conditioning. However there are other possible explanations for the differing results.
One such explanation is that we used simple geometric shapes as our neutral stimuli, whereas the previous researchers (Esteves et al., 1994; Öhman & Soares, 1998) used complex pictures as their CSs (neutral faces and flowers/mushrooms, respectively). The amygdala has been shown be especially sensitive to low spatial frequency information (Vuilleumier, Armony, Driver, & Dolan, 2003), which may suggest that simple low spatial frequency pictures may be more easily associated with a UCS during masked training. However this is unlikely the case, because Vuilleumier et al. (2003) used pictures that had been passed through spatial frequency filters, and the amygdala responded to both the low-frequency fear pictures and the unfiltered fear pictures, but not the high-frequency fear pictures. These results suggest that it is not presence of high spatial frequency information that interferes with amygdala response to fear pictures; rather it is the absence of low spatial frequency that causes the interference.
Another possible reason why our results differed from previous findings is that our method of analyzing SCR may be a more sensitive index of CRs. Methods of analyzing SCR that use the peak response are based on a single data point for each trial. These peak values occur at different points on each trial. That is, the peak value of one trial may not necessarily occur at the same time point as the peak value of another trial. Such differences can be due to differences in the slope of the baseline relative to the magnitude of the response. Systematic differences in this relationship across trial types may lead to a decreased sensitivity. Using our current method, we make direct comparisons at each time point, which accounts for differences in the baseline/response magnitude relationship. Furthermore, with this method we were able to observe differences in response topography in addition to response magnitude. For example, in the Experiment I Phase II conditioning session, groups show a similar magnitude differential response; however, the differential response for Group SAME is roughly three times as long as that of Group DIFFFERENT. An analysis based on the peak response would have missed this difference in topography.
One limitation to our study is that the interval between the onset of the CS and the onset of the UCS was so brief during the masking phase; we were unable to directly observe behavior during this phase. During the masking phase of Experiment I, there were two unreinforced CS+ presentations. Although responses were recorded during those presentations, comparisons were difficult to interpret because of contamination from the masking stimulus, which was presented on both CS+ and CS− trials.
We believe that our results may differ from those of Öhman and colleagues (Esteves et al., 1994; Öhman & Soares, 1998) because we used delay conditioning and they used trace conditioning. However, we did not directly compare trace and delay conditioning with masked neutral CSs. Also, based on this discrepancy we hypothesize that there may be additional neural substrates that are capable of maintaining an active representation of prepared stimuli, even when perception of these stimuli is blocked by backward masking. Unfortunately, our data do not address this directly. These hypotheses could be tested by manipulating conditioning type (trace vs. delay) and stimulus category (prepared vs. neutral) in a single parametric masked conditioning experiment. If our hypotheses are correct then groups presented with prepared stimuli should show evidence of conditioning whether or not they are trained with delay or trace conditioning, but groups presented with neutral stimuli should show evidence of conditioning only if they are trained with delay conditioning.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH060668).
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/a0019927.supp
References
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. doi:10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. doi:10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. doi:10.1037/0735–7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends in Cognitive Sciences. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. doi:10.1016/S1364-6613(02)02041–7. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. doi:10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Hadjikhani N. Non-conscious recognition of emotional body language. Neuroreport. 2006;17:583–586. doi: 10.1097/00001756-200604240-00006. PMID: 16603916. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V. What's new in visual masking? Trends in Cognitive Sciences. 2000;4:345–352. doi: 10.1016/s1364-6613(00)01520-5. doi:10.1016/S1364-6613(00)01520–5. [DOI] [PubMed] [Google Scholar]
- Esteves F, Parra C, Dimberg U, Öhman A. Nonconscious associative learning: Pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. doi:10.1111/j.1469–8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. doi:10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. doi:10.1037/0735–7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ. “Attention-like” processes in classical conditioning. In: Jones MR, editor. Miami Symposium on the Prediction of Behavior, 1967: Aversive stimulation. University of Miami Press; Coral Gables, FL: 1968. pp. 9–31. [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. Appleton-Century-Crofts; New York: 1969a. pp. 279–296. [Google Scholar]
- Kamin LJ. Selective association and conditioning. In: Mackintosh NJ, Honig WK, editors. Fundamental issues in associative learning; proceedings of a symposium held at Dalhousie University, Halifax, June 1968. Dalhousie University Press; Halifax, UK: 1969b. pp. 42–64. [Google Scholar]
- Kim CY, Blake R. Psychophysical magic: Rendering the visible `invisible.'. Trends in Cognitive Sciences. 2005;9:381–388. doi: 10.1016/j.tics.2005.06.012. doi:10.1016/j.tics.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. doi:10.1523/JNEUROSCI.0433–03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences, USA of the United States of America. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. doi:10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:157–162. doi: 10.3758/cabn.6.2.157. doi:10.3758/CABN.6.2.157. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. doi:10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Williams LM. A direct brainstem-amygdala-cortical `alarm' system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. doi:10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. doi:10.1037//0097–7403.28.1.3. [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Parallel acquisition of awareness and trace eyeblink classical conditioning. Learning & Memory. 2000;7:267–272. doi: 10.1101/lm.33400. doi:10.1101/lm.33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Single-cue delay eyeblink conditioning is unrelated to awareness. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:192–198. doi: 10.3758/cabn.1.2.192. doi:10.3758/CABN.1.2.192. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Standard delay eyeblink classical conditioning is independent of awareness. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:32–37. doi:10.1037//0097–7403.28.1.32. [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. doi:10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Morris JS, de Gelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–1252. doi: 10.1093/brain/124.6.1241. doi:10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences, USA of the United States of America. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. PMID: 9990084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y, Kakigi R. Neural mechanisms of visual backward masking revealed by high temporal resolution imaging of human brain. Neuroimage. 2005;27:178–187. doi: 10.1016/j.neuroimage.2005.03.032. doi:10.1016/j.neuroimage.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. doi:10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Öhman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiology & Behavior. 2007;92:180–185. doi: 10.1016/j.physbeh.2007.05.057. doi:10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of Experimental Psychology: General. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. PMID: 9503652. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ, Panzeri S. The neurophysiology of backward visual masking: Information analysis. Journal of Cognitive Neuroscience. 1999;11:300–311. doi: 10.1162/089892999563409. doi:10.1162/089892999563409. [DOI] [PubMed] [Google Scholar]
- Smith CN, Clark RE, Manns JR, Squire LR. Acquisition of differential delay eyeblink classical conditioning is independent of awareness. Behavioral Neuroscience. 2005;119:78–86. doi: 10.1037/0735-7044.119.1.78. doi:10.1037/0735–7044.119.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6:624–631. doi: 10.1038/nn1057. doi:10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Bamford S. Response to visual threat following damage to the pulvinar. Current Biology. 2005;15:571–573. doi: 10.1016/j.cub.2005.01.056. doi:10.1016/j.cub.2005.01.056. [DOI] [PubMed] [Google Scholar]
- Wiens S, Öhman A. Unawareness is more than a chance event: Comment on Lovibond and Shanks (2002) Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:27–31. doi:10.1037//0097–7403.28.1.27. [PubMed] [Google Scholar]