Abstract
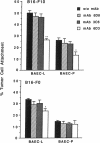
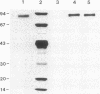
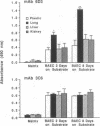
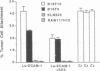
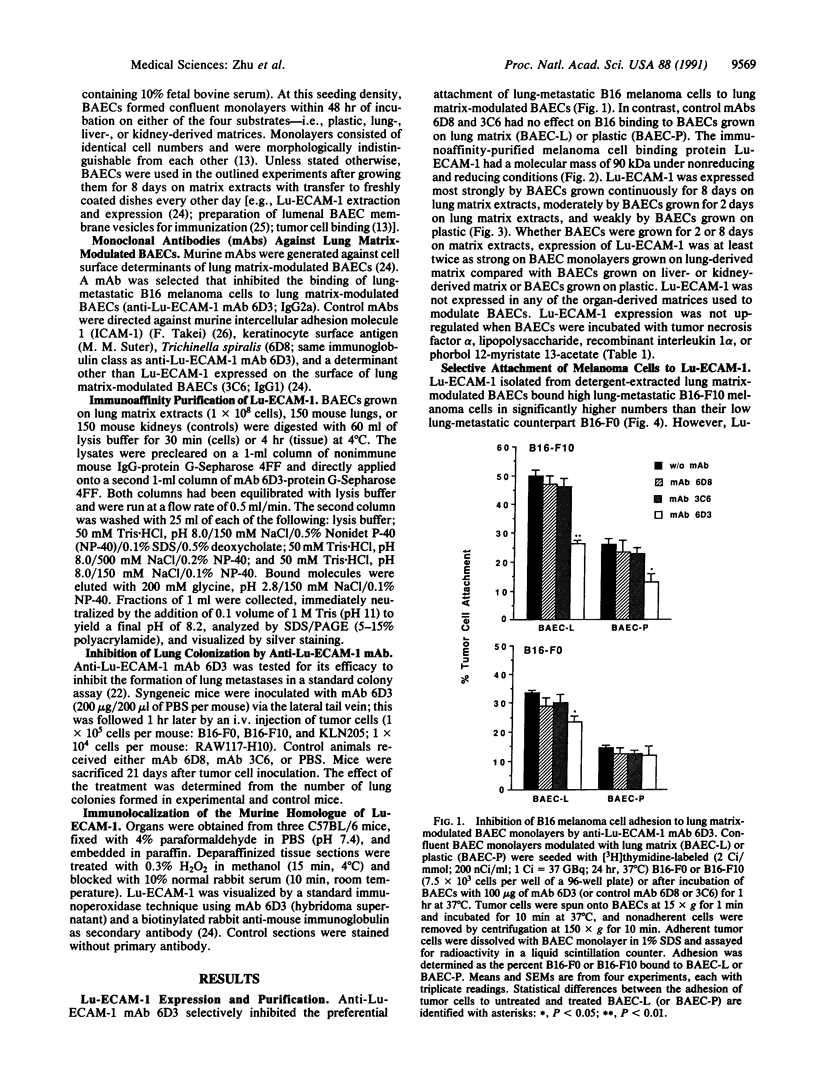
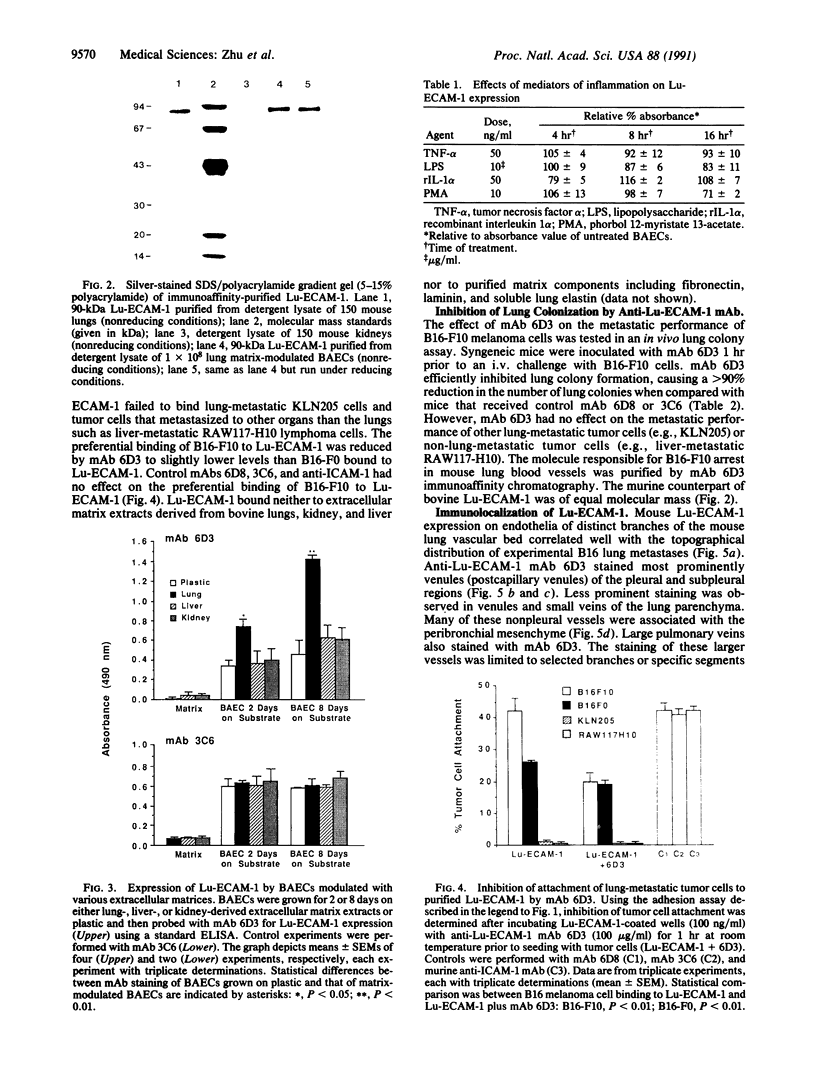
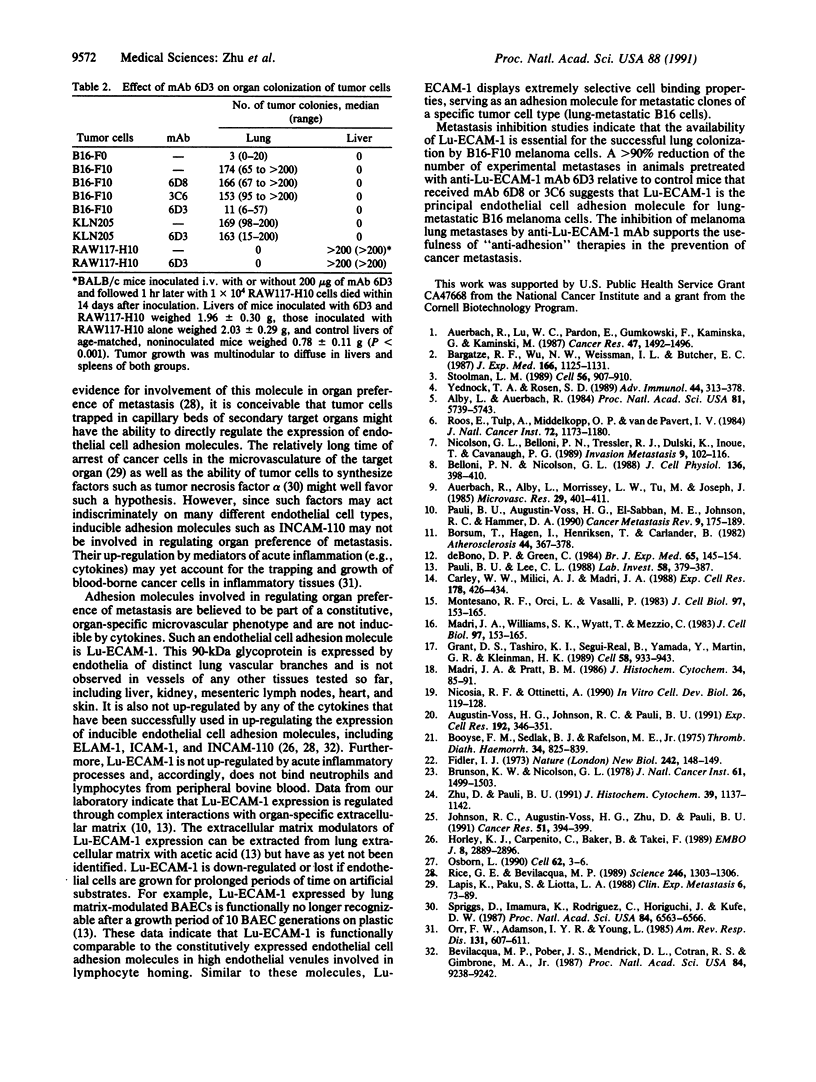
Organ-specific adhesion molecules expressed by vascular endothelial cells have been implicated in the arrest of blood-borne cancer cells in selective, secondary sites. A lung-specific endothelial cell adhesion molecule (Lu-ECAM-1) localized on endothelia of distinct branches of lung blood vessels has been purified by immunoaffinity chromatography from detergent extracts of lung matrix-modulated endothelial cells using monoclonal antibody (mAb) 6D3. It has a molecular mass of 90 kDa and promotes the selective attachment of lung-metastatic B16 melanoma cells. Corresponding with their metastatic performance, B16-F10 tumor cells selected for higher lung colonization bind to Lu-ECAM-1 in significantly higher numbers than their low lung metastatic counterpart B16-F0. Binding of B16-F0 and B16-F10 is reduced with mAb 6D3 to slightly lower levels than B16-F0 bound to Lu-ECAM-1. mAb 6D3 injected into C57BL/6 mice 1 hr prior to an i.v. challenge with B16-F10 causes a 90% reduction in the number of lung colonies compared with animals injected with control mAb (6D8 or 3C6). Lu-ECAM-1 neither binds nor effects metastasis of other lung-colonizing tumor cells (e.g., KLN205). Thus, site-specific metastasis of tumor cells is regulated by similar mechanisms as the homing of lymphocytes--namely, by the ability of blood-borne cancer cells to recognize and adhere to distinct endothelial cell adhesion molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alby L., Auerbach R. Differential adhesion of tumor cells to capillary endothelial cells in vitro. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5739–5743. doi: 10.1073/pnas.81.18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R., Alby L., Morrissey L. W., Tu M., Joseph J. Expression of organ-specific antigens on capillary endothelial cells. Microvasc Res. 1985 May;29(3):401–411. doi: 10.1016/0026-2862(85)90028-7. [DOI] [PubMed] [Google Scholar]
- Auerbach R., Lu W. C., Pardon E., Gumkowski F., Kaminska G., Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987 Mar 15;47(6):1492–1496. [PubMed] [Google Scholar]
- Augustin-Voss H. G., Johnson R. C., Pauli B. U. Modulation of endothelial cell surface glycoconjugate expression by organ-derived biomatrices. Exp Cell Res. 1991 Feb;192(2):346–351. doi: 10.1016/0014-4827(91)90051-u. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Wu N. W., Weissman I. L., Butcher E. C. High endothelial venule binding as a predictor of the dissemination of passaged murine lymphomas. J Exp Med. 1987 Oct 1;166(4):1125–1131. doi: 10.1084/jem.166.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni P. N., Nicolson G. L. Differential expression of cell surface glycoproteins on various organ-derived microvascular endothelia and endothelial cell cultures. J Cell Physiol. 1988 Sep;136(3):398–410. doi: 10.1002/jcp.1041360303. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Brunson K. W., Nicolson G. L. Selection and biologic properties of malignant variants of a murine lymphosarcoma. J Natl Cancer Inst. 1978 Dec;61(6):1499–1503. [PubMed] [Google Scholar]
- Børsum T., Hagen I., Henriksen T., Carlander B. Alterations in the protein composition and surface structure of human endothelial cells during growth in primary culture. Atherosclerosis. 1982 Sep;44(3):367–378. doi: 10.1016/0021-9150(82)90011-9. [DOI] [PubMed] [Google Scholar]
- Carley W. W., Milici A. J., Madri J. A. Extracellular matrix specificity for the differentiation of capillary endothelial cells. Exp Cell Res. 1988 Oct;178(2):426–434. doi: 10.1016/0014-4827(88)90411-9. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Horley K. J., Carpenito C., Baker B., Takei F. Molecular cloning of murine intercellular adhesion molecule (ICAM-1). EMBO J. 1989 Oct;8(10):2889–2896. doi: 10.1002/j.1460-2075.1989.tb08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Augustin-Voss H. G., Zhu D. Z., Pauli B. U. Endothelial cell membrane vesicles in the study of organ preference of metastasis. Cancer Res. 1991 Jan 1;51(1):394–399. [PubMed] [Google Scholar]
- Lapis K., Paku S., Liotta L. A. Endothelialization of embolized tumor cells during metastasis formation. Clin Exp Metastasis. 1988 Jan-Feb;6(1):73–89. doi: 10.1007/BF01580408. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem. 1986 Jan;34(1):85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Belloni P. N., Tressler R. J., Dulski K., Inoue T., Cavanaugh P. G. Adhesive, invasive, and growth properties of selected metastatic variants of a murine large-cell lymphoma. Invasion Metastasis. 1989;9(2):102–116. [PubMed] [Google Scholar]
- Nicosia R. F., Ottinetti A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol. 1990 Feb;26(2):119–128. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Adamson I. Y., Young L. Pulmonary inflammation generates chemotactic activity for tumor cells and promotes lung metastasis. Am Rev Respir Dis. 1985 Apr;131(4):607–611. doi: 10.1164/arrd.1985.131.4.607. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Augustin-Voss H. G., el-Sabban M. E., Johnson R. C., Hammer D. A. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990 Nov;9(3):175–189. doi: 10.1007/BF00046359. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Lee C. L. Organ preference of metastasis. The role of organ-specifically modulated endothelial cells. Lab Invest. 1988 Apr;58(4):379–387. [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Roos E., Tulp A., Middelkoop O. P., van de Pavert I. V. Interactions between lymphoid tumor cells and isolated liver endothelial cells. J Natl Cancer Inst. 1984 May;72(5):1173–1180. [PubMed] [Google Scholar]
- Spriggs D., Imamura K., Rodriguez C., Horiguchi J., Kufe D. W. Induction of tumor necrosis factor expression and resistance in a human breast tumor cell line. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6563–6566. doi: 10.1073/pnas.84.18.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Rosen S. D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]
- Zhu D. Z., Pauli B. U. Generation of monoclonal antibodies directed against organ-specific endothelial cell surface determinants. J Histochem Cytochem. 1991 Aug;39(8):1137–1142. doi: 10.1177/39.8.1856462. [DOI] [PubMed] [Google Scholar]
- de Bono D. P., Green C. The adhesion of different cell types to cultured vascular endothelium: effects of culture density and age. Br J Exp Pathol. 1984 Feb;65(1):145–154. [PMC free article] [PubMed] [Google Scholar]