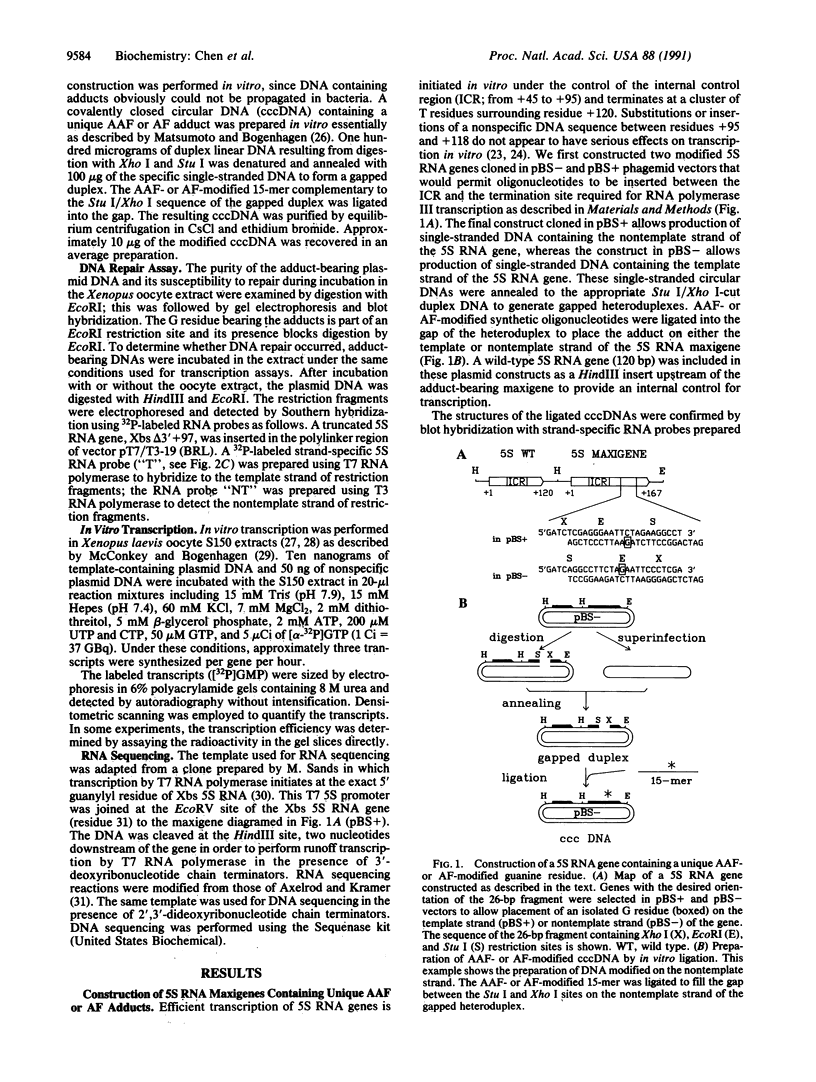
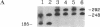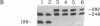Abstract
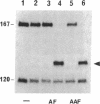
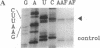
Unique N-acetyl-2-aminofluorene (AAF) or 2-aminofluorene (AF) adducts were introduced into the Xenopus borealis somatic 5S RNA gene between the intragenic control region and the transcription termination site. The effects of these bulky adducts on transcription were studied in a cell-free extract derived from Xenopus laevis oocytes. AAF and AF adducts inhibit transcription only when they are on the template strand, whereas transcription passes through these adducts when they are placed on the nontemplate strand. In the presence of the AAF or AF adduct on the template strand, transcription usually terminates one nucleotide before the altered guanine residue. Premature termination at these bulky adducts does not block reinitiation of transcription, since several transcripts are produced per gene per hour on these damaged templates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armier J., Mezzina M., Leng M., Fuchs R. P., Sarasin A. N-acetoxy-N-2-acetylaminofluorene-induced damage on SV40 DNA: inhibition of DNA replication and visualization of DNA lesions. Carcinogenesis. 1988 May;9(5):789–795. doi: 10.1093/carcin/9.5.789. [DOI] [PubMed] [Google Scholar]
- Axelrod V. D., Kramer F. R. Transcription from bacteriophage T7 and SP6 RNA polymerase promoters in the presence of 3'-deoxyribonucleoside 5'-triphosphate chain terminators. Biochemistry. 1985 Oct 8;24(21):5716–5723. doi: 10.1021/bi00342a005. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Broyde S., Hingerty B. Conformation of 2-aminofluorene-modified DNA. Biopolymers. 1983 Nov;22(11):2423–2441. doi: 10.1002/bip.360221109. [DOI] [PubMed] [Google Scholar]
- Burnouf D., Koehl P., Fuchs R. P. Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4147–4151. doi: 10.1073/pnas.86.11.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Johnson D. L., Reid T. M., Lee M. S., Romano L. J., King C. M. Mutagenesis by single site-specific arylamine-DNA adducts. Induction of mutations at multiple sites. J Biol Chem. 1989 Nov 25;264(33):20120–20130. [PubMed] [Google Scholar]
- Hansson J., Munn M., Rupp W. D., Kahn R., Wood R. D. Localization of DNA repair synthesis by human cell extracts to a short region at the site of a lesion. J Biol Chem. 1989 Dec 25;264(36):21788–21792. [PubMed] [Google Scholar]
- Heflich R. H., Djurić Z., Zhuo Z., Fullerton N. F., Casciano D. A., Beland F. A. Metabolism of 2-acetylaminofluorene in the Chinese hamster ovary cell mutation assay. Environ Mol Mutagen. 1988;11(2):167–181. doi: 10.1002/em.2850110203. [DOI] [PubMed] [Google Scholar]
- Kaplan L. A., Weinstein I. B. Preferential inhibition of the synthesis of 45S ribosomal rna precursor by N-acetoxyacetylaminofluorene in rat liver epithelial cultures. Chem Biol Interact. 1976 Jan;12(1):99–108. doi: 10.1016/0009-2797(76)90071-5. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site in DNA in a Xenopus laevis oocyte extract. Mol Cell Biol. 1989 Sep;9(9):3750–3757. doi: 10.1128/mcb.9.9.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site involves concerted reactions of DNA synthesis followed by excision and ligation. Mol Cell Biol. 1991 Sep;11(9):4441–4447. doi: 10.1128/mcb.11.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey G. A., Bogenhagen D. F. TFIIIA binds with equal affinity to somatic and major oocyte 5S RNA genes. Genes Dev. 1988 Feb;2(2):205–214. doi: 10.1101/gad.2.2.205. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Johnson D. L., Reid T. M., King C. M., Romano L. J. Evidence for in vitro translesion DNA synthesis past a site-specific aminofluorene adduct. J Biol Chem. 1987 Oct 25;262(30):14648–14654. [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- Millette R. L., Fink L. M. The effect of modification of T7 DNA by the carcinogen N-1-acetylaminofluorene: termination of transcription in vitro. Biochemistry. 1975 Apr 8;14(7):1426–1432. doi: 10.1021/bi00678a012. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya M., Takeshita M., Johnson F., Peden K., Will S., Grollman A. P. Targeted mutations induced by a single acetylaminofluorene DNA adduct in mammalian cells and bacteria. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1586–1589. doi: 10.1073/pnas.85.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. H., Grunberger D., Cantor C. R., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. IV. Circular dichroism and proton magnetic resonance studies of oligonucleotides modified with N-2-acetylaminofluorene. J Mol Biol. 1971 Dec 14;62(2):331–346. doi: 10.1016/0022-2836(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Norman D., Abuaf P., Hingerty B. E., Live D., Grunberger D., Broyde S., Patel D. J. NMR and computational characterization of the N-(deoxyguanosin-8-yl)aminofluorene adduct [(AF)G] opposite adenosine in DNA: (AF)G[syn].A[anti] pair formation and its pH dependence. Biochemistry. 1989 Sep 5;28(18):7462–7476. doi: 10.1021/bi00444a046. [DOI] [PubMed] [Google Scholar]
- Orfanoudakis G., Gilson G., Wolff C. M., Ebel J. P., Befort N., Remy P. Repair of acetyl-aminofluorene modified pBR322 DNA in Xenopus laevis oocytes and eggs; effect of diadenosine tetraphosphate. Biochimie. 1990 Apr;72(4):271–278. doi: 10.1016/0300-9084(90)90083-s. [DOI] [PubMed] [Google Scholar]
- Sands M. S., Bogenhagen D. F. The carboxyterminal zinc fingers of TFIIIA interact with the tip of helix V of 5S RNA in the 7S ribonucleoprotein particle. Nucleic Acids Res. 1991 Apr 25;19(8):1791–1796. doi: 10.1093/nar/19.8.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Fuchs R. P. Acetylaminofluorene bound to different guanines of the sequence -GGCGCC- is excised with different efficiencies by the UvrABC excision nuclease in a pattern not correlated to the potency of mutation induction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):191–194. doi: 10.1073/pnas.87.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J Biol Chem. 1990 Dec 5;265(34):21330–21336. [PubMed] [Google Scholar]
- Shibutani S., Gentles R., Johnson F., Grollman A. P. Isolation and characterization of oligodeoxynucleotides containing dG-N2-AAF and oxidation products of dG-C8-AF. Carcinogenesis. 1991 May;12(5):813–818. doi: 10.1093/carcin/12.5.813. [DOI] [PubMed] [Google Scholar]
- Strauss B. S., Wang J. Role of DNA polymerase 3'----5' exonuclease activity in the bypass of aminofluorene lesions in DNA. Carcinogenesis. 1990 Dec;11(12):2103–2109. doi: 10.1093/carcin/11.12.2103. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Tang M. S., Bohr V. A., Zhang X. S., Pierce J., Hanawalt P. C. Quantification of aminofluorene adduct formation and repair in defined DNA sequences in mammalian cells using the UVRABC nuclease. J Biol Chem. 1989 Aug 25;264(24):14455–14462. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Jordan E., Brown D. D. A bacteriophage RNA polymerase transcribes through a Xenopus 5S RNA gene transcription complex without disrupting it. Cell. 1986 Feb 14;44(3):381–389. doi: 10.1016/0092-8674(86)90459-9. [DOI] [PubMed] [Google Scholar]
- Xing Y. Y., Worcel A. A 3' exonuclease activity degrades the pseudogene 5S RNA transcript and processes the major oocyte 5S RNA transcript in Xenopus oocytes. Genes Dev. 1989 Jul;3(7):1008–1018. doi: 10.1101/gad.3.7.1008. [DOI] [PubMed] [Google Scholar]