Abstract
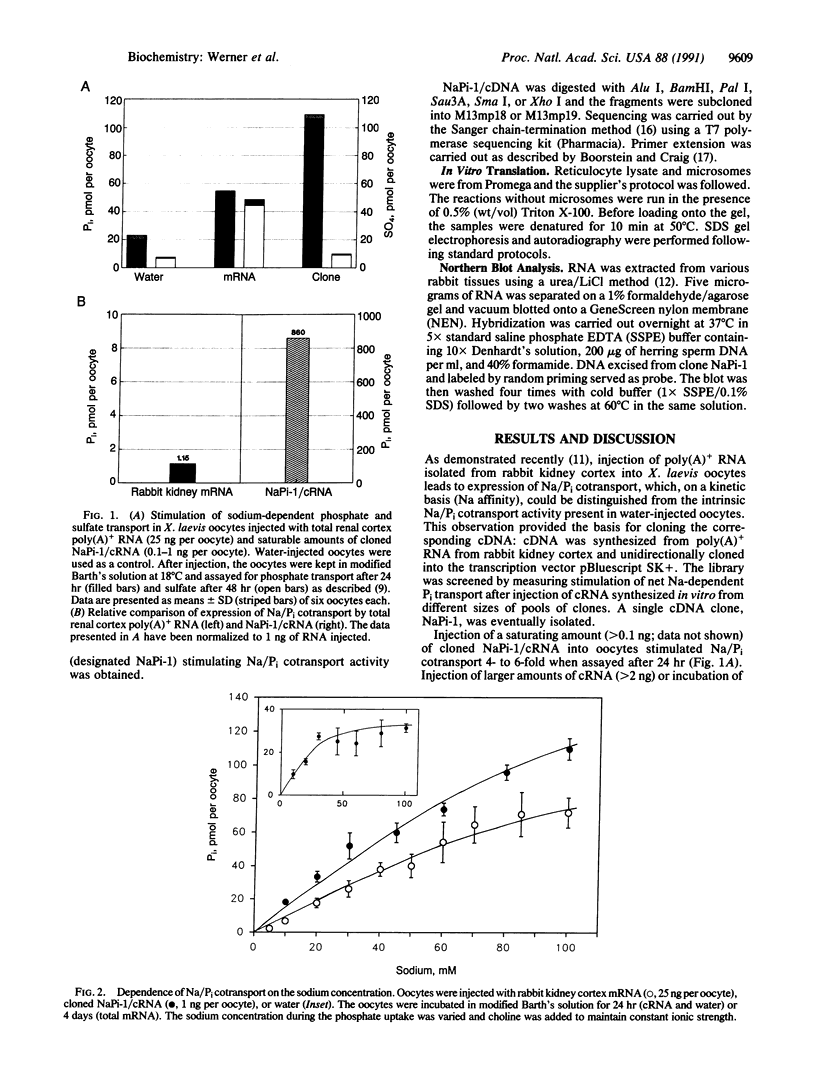
A cDNA library from rabbit kidney cortex was screened for expression of Na-dependent transport of phosphate (Pi) using Xenopus laevis oocytes as an expression system. A single clone was eventually isolated (designated NaPi-1) that stimulated expression of Na/Pi cotransport approximately 700-fold compared to total mRNA. The predicted sequence of the Na/Pi cotransporter consists of 465 amino acids (relative molecular mass, 51,797); hydropathy profile predictions suggest six (possibly eight) membrane-spanning segments. In vitro translation of NaPi-1/complementary RNA in the presence of pancreatic microsomes indicated NaPi-1 to be a glycosylated protein; four potential N-glycosylation sites are present in the amino acid sequence. Northern blot analysis demonstrated the presence of NaPi-1/mRNA in kidney cortex and liver; no hybridization signal was obtained with mRNA from other tissues (including small intestine). Kinetic analysis of Na/Pi cotransport expressed by NaPi-1/complementary RNA demonstrated characteristics (sodium interaction) similar to those observed in cortical apical membranes. The alignment of 5 amino acid residues (Gly342/Ala381-Xaa-Xaa-Xaa-Xaa-Leu386-Xaa-Xaa-Xaa-P ro390- Arg391) is consistent with a motif proposed for Na-dependent transport systems. We conclude that we have cloned a cDNA for a Na/Pi cotransport system present in rabbit kidney cortex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Biber J. Cellular aspects of proximal tubular phosphate reabsorption. Kidney Int. 1989 Sep;36(3):360–369. doi: 10.1038/ki.1989.204. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Primer extension analysis of RNA. Methods Enzymol. 1989;180:347–369. doi: 10.1016/0076-6879(89)80111-9. [DOI] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Ibnoul-Khatib H., Bergeron M., Beauregard G., Potier M. Radiation-inactivation studies on brush-border-membrane vesicles. General considerations, and application to the glucose and phosphate carriers. Biochem J. 1988 Jun 15;252(3):807–813. doi: 10.1042/bj2520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Sacktor B. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. J Biol Chem. 1981 Feb 25;256(4):1556–1564. [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990 Dec 15;265(35):21704–21708. [PubMed] [Google Scholar]
- Ferreira G. C., Pratt R. D., Pedersen P. L. Energy-linked anion transport. Cloning, sequencing, and characterization of a full length cDNA encoding the rat liver mitochondrial proton/phosphate symporter. J Biol Chem. 1989 Sep 15;264(26):15628–15633. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Turk E., Wright E. M. Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Vitamin D: metabolism and biological actions. Annu Rev Nutr. 1984;4:493–520. doi: 10.1146/annurev.nu.04.070184.002425. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mizgala C. L., Quamme G. A. Renal handling of phosphate. Physiol Rev. 1985 Apr;65(2):431–466. doi: 10.1152/physrev.1985.65.2.431. [DOI] [PubMed] [Google Scholar]
- Murer H., Werner A., Reshkin S., Wuarin F., Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol. 1991 May;260(5 Pt 1):C885–C899. doi: 10.1152/ajpcell.1991.260.5.C885. [DOI] [PubMed] [Google Scholar]
- Nakao T., Yamato I., Anraku Y. Nucleotide sequence of putP, the proline carrier gene of Escherichia coli K12. Mol Gen Genet. 1987 Jun;208(1-2):70–75. doi: 10.1007/BF00330424. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol. 1990 Sep;117(3):201–221. doi: 10.1007/BF01868451. [DOI] [PubMed] [Google Scholar]
- Surin B. P., Jans D. A., Fimmel A. L., Shaw D. C., Cox G. B., Rosenberg H. Structural gene for the phosphate-repressible phosphate-binding protein of Escherichia coli has its own promoter: complete nucleotide sequence of the phoS gene. J Bacteriol. 1984 Mar;157(3):772–778. doi: 10.1128/jb.157.3.772-778.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Biber J., Forgo J., Palacin M., Murer H. Expression of renal transport systems for inorganic phosphate and sulfate in Xenopus laevis oocytes. J Biol Chem. 1990 Jul 25;265(21):12331–12336. [PubMed] [Google Scholar]
- Wuarin F., Wu K., Murer H., Biber J. The Na+/Pi-cotransporter of OK cells: reaction and tentative identification with N-acetylimidazole. Biochim Biophys Acta. 1989 Jun 6;981(2):185–192. doi: 10.1016/0005-2736(89)90027-8. [DOI] [PubMed] [Google Scholar]
- al-Mahrouq H. A., Kempson S. A. Photoaffinity labeling of brush-border membrane proteins which bind phosphonoformic acid. J Biol Chem. 1991 Jan 25;266(3):1422–1427. [PubMed] [Google Scholar]