Abstract
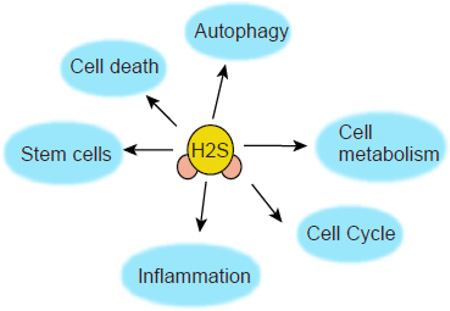
Hydrogen sulfide (H2S) a novel gasotransmitter is endogenously synthesized by multiple enzymes that are differentially expressed in the peripheral tissues and central nervous systems. H2S regulates a wide range of physiological processes ranging from cardiovascular, neuronal, immune, respiratory, gastrointestinal, liver, and endocrine systems by influencing cellular signaling pathways and sulfhydration of target proteins. This review focuses on the recent progress made in H2S signaling that affects mechanistic and functional aspects of several biological processes such as autophagy, inflammation, proliferation and differentiation of stem cell, cell survival/death, and cellular metabolism under both physiological and pathological conditions. Moreover we highlighted the crosstalk between nitric oxide (NO) and H2S in several bilogical contexts.
Graphical Abstract
Introduction
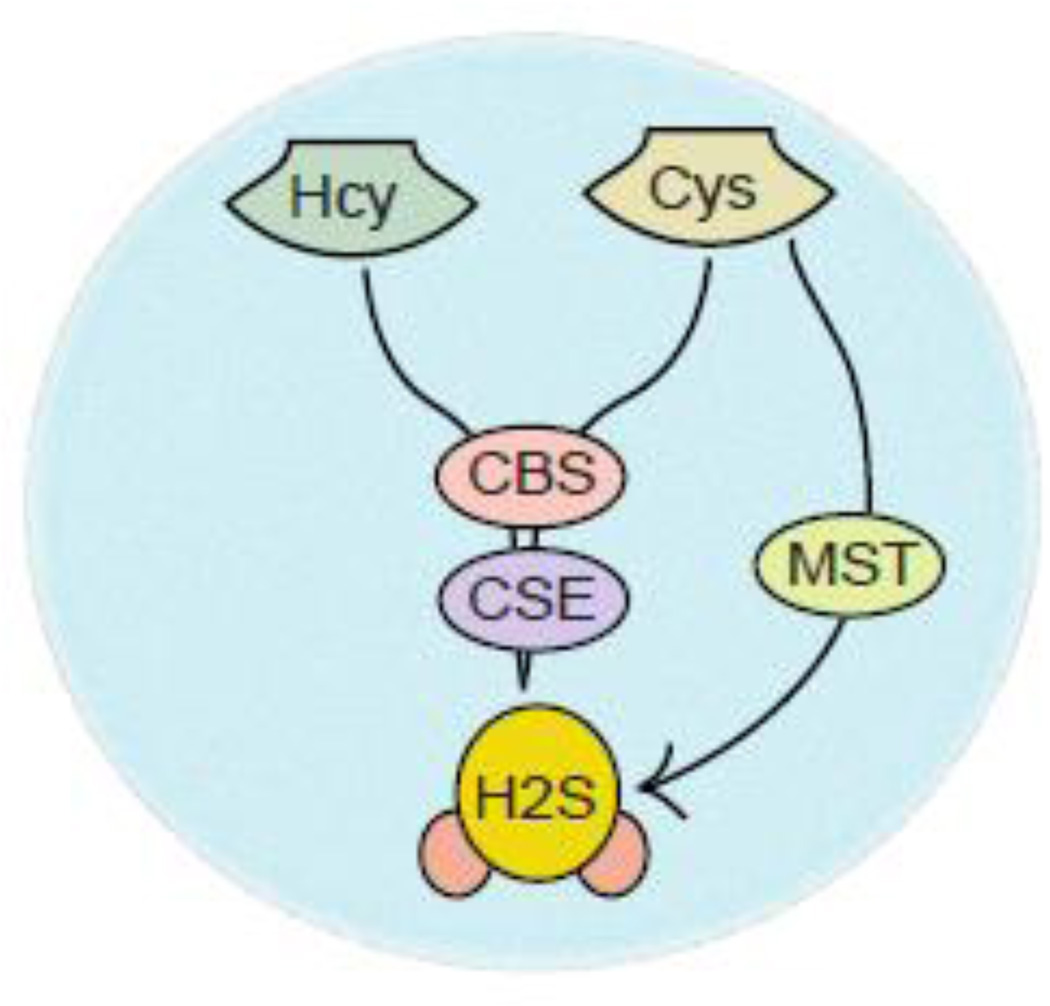
Unlike neurotransmitters or hormones, gasotransmitter such as hydrogen sulfide (H2S) can be primarily synthesized by an enzymatic activity of cystathionine gamma-lyase (CSE) or cystathionine beta synthase (CBS). The CSE essentially predominates in peripheral tissues to generate H2S in the liver, kidney, and heart, however, in the brain, CBS is mostly responsible for synthesizing H2S [1–4]. Recently, a third enzyme known as 3-mercaptopyruvate sulfurtransferase (MST) has been identified to synthesize H2S [2, 3] (Figure 1), but the evidence for its involvement in the physiological biosynthesis of H2S in mammals is weak compared to other enzymes [5, 6]. In general, CBS generates H2S using Cys and homocysteine as its substrate [7]; however, the regulation of the determining the substrate for CBS are unclear. Other than, producing H2S, CBS is one of the major regulator of homocysteine levels in mammalian. A compelling number of evidence indicates that impairment of CBS activity leads to markedly increase homocysteine levels that can affect cardiovascular function in vivo [7–10]. On the other hand, a reduction in the level of H2S due to an inactivation of CBS contributes to the vascular contribution in patients [11–14]. This was further supported by the fact that mice lacking CBS (CBS KO) cause death by 4 weeks of age along with growth retardation and developmental defects [15]. CBS generally exists in two states; the ferrous form of CBS is inactive by binding with carbon monoxide in the presence of NADPH or methionine synthase reductase, however, the ferric state of CBS is active both in vitro and in vivo [16–18]. On the other hand, CBS can be allosterically activated by S-adenosylmethionine (SAM) which can bind to the carboxy-terminal domain of CBS and produce H2S [19, 20]. Similar to CBS, the other enzyme CSE uses homocysteine and Cys as a substrate to produce H2S along with pyruvate and ammonia as a byproduct. The disulfide form of Cys, cystine can also serve as a substrate for rat CSE and produce H2S [8, 13, 14].
Figure 1.
Intracellular syntheis of H2S by CBS, CSE and MST. L-cysteine serves as a substrate for all three enzymes to produce H2S in cells; however, homocysteine can be used as a substrate to synthesize H2S by the enzymatic activity of CSE and CBS.
Obviously, the question arises how the level of H2S is regulated inside the cells. H2S can be metabolized by oxygen dependent catabolic processes in the mitochondria. The bound form of H2S, sulfane sulfur can generate H2S in the presence of a reducing agent but the role of sulfane sulfur in the signaling pathway is not well studied [21, 22]. H2S can also interact with haem proteins, such as hemoglobin, and cytochrome c oxidase, which may serve as a store for this gasotransmitter. In addition, H2S can be methylated in the cytosol by thiol S-methyltransferase to generate methanethiol, which can be further methylated to the less toxic compound dimethyl sulfide [23, 24].
H2S signaling via protein sulfhydration
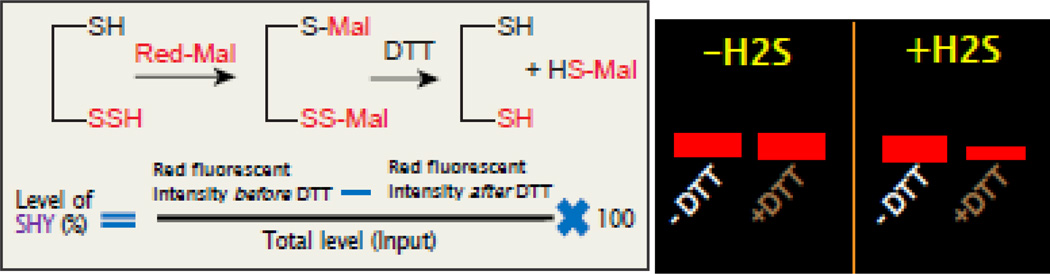
H2S signals inside cells through post-translational modification of proteins, known as sulfhydration [25, 26]. In this modification, H2S modifies a free Cys residue (–SH) of a target protein to −SSH. The modified biotin switch assay [25] was initially identified to detect sulfhydration of protein. But due to its limitation about specificity, another method of detection of sulfhydrated proteins have been identified which is known as Maleimide assay [27, 28]. In this procedure, a fluorescent conjugated maleimide interacts with both –SH and –SSH residues of a protein, but in the presence of a strong reducing agent such as DTT, the S–S bond is cleaved off and as a result, the fluorescent signal of sulfhydrated species is lost. The details of the procedure are highlighted in the diagram present in Figure 2. The sulfhydration of proteins determined by the acid dissociation constant (pKa) of Cys residues that can be governed by the local protein environment and protein architecture. Briefly, the thiolate anions (S−), produced from Cys residues with low pKa, functions as a strong nucleophile which is oxidized by hydrogen peroxide to form either sulfenic acid (SOH), sulfinic acid (SO2H) or sulfonic acid (SO3H) forms. The sulfenic form can react with HS− or H2S to form a persulfide bond [29–31]. Sulfhydration of proteins can affect its function, localization inside cells, stability in cells and resistance to oxidative stress [3]. We talked about the importance of H2S signaling and sulfhydration of several proteins in the following biological processes mostly autophagy, cellular metabolism, stem cells, inflammation, cell cycle and cell death under physiological and pathological conditions.
Figure 2.
The principle of red maleimide assay to detect sulfhydration of proteins. In this method, red malimide interacts with both –SH and -SSH groups and forms –S-Mal and –S-S-Mal respectively. Upon treatment with DTT, the –S-Mal will remain unaltered, however, –S-S-Mal will be cleaved off to form –SH and –S-Mal as the products. Thus the sulfhydrated protein will lose the signal of red fluorescence of red maleimide. A cartoon image (right side) of a gel showing that the red intensity of a target protein remains unaltered in the presence or absence of DTT in samples untreated with H2S. On the other hand, the red fluorescent intensity of protein will be lost after DTT treatment in samples treated with H2S. The residual red fluorescent intensity before and after DTT tretament divided by total protein will provide the percentage of sulfhydration of protein.
Autophagy and H2S signaling
Autophagy is a cellular protective and quality control process in cells and is an intracellular catabolic process in which the cytoplasmic constituents, such as aggregated proteins and dysfunctional organelles, are surrounded by a double membrane, termed the autophagosome, and are transported to lysosomes for degradation and recycling [32, 33]. In response to a variety of extracellular and intracellular stresses including nutrients deprivation, hormonal therapy, pathogenic infection, misfolded proteins and damaged organelles it serves as a salvage mechanism for recycling cytoplasmic materials and preserving energy via lysosome-driven degradations [34, 35]. The execution of autophagy involves a set of evolutionarily conserved gene products, known as Atg proteins. Among these usually correlates well with the accumulation of autophagosomes [36]. It has been suggested that autophagy malfunction can induce the pathogenesis of diverse human diseases and we will discuss how H2S influences autophagy in several disease conditions.
Pathological perspectives
Ischemia
Autophagy-related proteins and autophagy complex levels in the ischemic hemisphere results in neurological deficits and an increase in cerebral infarct volume in middle cerebral artery occlusion (MCAO) mice [37, 38]. It was shown that administration of H2S ameliorated infarct size and preserved LV function during development of myocardial infarction in mice, which suggest that H2S is cytoprotective and angioprotective during the evolution of myocardial infarction [39] along with an improvement in neurological deficits in a rapamycin-dependent manner [40]. Moreover, this effect is associated with its influence in down-regulating autophagosome accumulation [41]. Autophagy also plays a significant role in myocardial ischemia-reperfusion (IR) injury. H2S plays a myocardial protective role against IR injury by regulating autophagy via mTOR activation [42]. However, excessive autophagy aggravates myocardial ischemia/reperfusion (IR) injury [43]. It was shown that H2S can inhibit autophagy in neonatal rat cardiomyocytes exposed to H/R and exert a cardioprotective effect at least partly by regulating PI3K/SGK1/GSK3β signaling pathway [44]. Other groups have shown that H2S protected against myocardial I/R injury by activating AMPK and restoring I/R-impaired autophagic flux [45]. H2S also protects the spinal cord and induces autophagy via miR-30c micrRNA in a rat model of spinal cord hernia-reperfusion injury [46]. However, the mechanism is not well elucidated. Interestingly, it was shown that treatment with an antioxidant, Tetrahydrocurcumin (THC) ameliorates autophagy during ischemia/reperfusion injury by reducing homocysteinylation of cyto-c in cystathionine-beta-synthase heterozygote knockout (CBS+/−) mice (via activation of by MMP-9. This study suggests a potential therapeutic role of dietary THC in cerebral ischemia [47].
Ischemic post-conditioning (PC) lost cardioprotection in the aged hearts and cardiomyocytes. NaHS (an H2S donor) significantly restored cardioprotection of PC through decreasing myocardial damage, infarct size, and apoptosis, improving cardiac function, increasing cell viability and autophagy in the aged hearts and cardiomyocytes [48]. In addition, in the aged cardiomyocytes, NaHS up-regulated AMPK/mTOR pathway, and the effect of NaHS on PC was similar to the overexpression of Atg 5, which suggests that exogenous H2S restores cardioprotection from PC by up-regulation of autophagy via activation of AMPK/mTOR pathway in the aged hearts and cardiomyocytes [49]. Myocardial I/R induced autophagosome accumulation is evidenced by the increased ratios of LC3-II/LC3-I, upregulation of beclin-1 and P62 and reduction in LAMP-2 [45, 50]. Another H2S donor, ADT reduced these autophagic changes induced by I/R, indicating that H2S restored I/R-impaired autophagic flux [45]. Inhibition of AMPK blocked the effects of H2S on restoring I/R-impaired autophagy flux. Thus AMPK activation and subsequent restoration of I/R impaired autophagic flux are unrecognized mechanisms underlying cardioprotective effects conferred by H2S donors [45, 51]. During acute pancreatitis (AP) impairment of autophagy and accumulation of autophagic vacuoles and premature activation of trypsinogen are common phenotypes. It was shown that treatment with NaHS exacerbated both processes and attributed to over-activation of autophagy rather than hampered autophagosome-lysosome fusion in an AMPK and mTOR-dependent manner [52].
Myocardial fibrosis
Excessive autophagy induced by extravagant oxidative stress is the main reason for diabetes-induced vascular endothelial cells dysfunction. Exogenous H2S protect arterial endothelial cells by suppressing excessive autophagy induced by oxidative stress through the Nrf2-ROSAMPK signaling pathway [53]. Myocardial fibrosis is the predominant pathological characteristic of diabetic myocardial damage. Treatment with NaHS, myocardial fibrosis was ameliorated, myocardial autophagy was decreased and the PI3K/AKT1 pathway suppression was reversed [54]. Moreover, the protective effect of H2S against diabetes-induced myocardial fibrosis is associated with the attenuation of autophagy via the upregulation of the PI3K/AKT1 signaling pathway [54].
Metabolic disorders
H2S is effective in protection against neurodegeneration and neurovascular dysfunction [55]. Hyperglycemia (HG) reduces AMPK activation leading to impaired autophagy and matrix accumulation. H2S treatment improves HG-induced renovascular remodeling, however, its mechanism remains unclear [56]. HG decreased the expression of H2S regulating enzymes CBS and CSE, and autophagy markers Atg5, Atg7, Atg3 and LC3B/A ratio [56] and H2S plays a key role in renovasculopathy during HHcy and is mediated through Akt/FoxO3 pathways [57]. HG increased galectin-3 and periostin, markers of matrix accumulation. H2S mitigates adverse remodeling in HG by induction of autophagy and regulation of matrix metabolism through LKB1/STRAD/MO25 dependent pathway [56]. Diabetes and particularly high blood glucose levels are implicated in neurodegeneration. One of the hallmarks of neurodegeneration is protein aggregation. H2S treatment increased protein synthesis and aggregation in the diabetic ZDF rat brain and provided a novel strategy against protein aggregation in the diabetic brain [58].
Autophagy plays an important role in liver triglyceride (TG) metabolism. Inhibition of autophagy could reduce the clearance of TG in the liver. H2S is a potent stimulator of autophagic flux. Recent studies showed H2S is protective against hypertriglyceridemia (HTG) and nonalcoholic fatty liver disease (NAFLD), while the mechanism remains to be explored. H2S treatment reduces serum TG level and ameliorates NAFLD by activating liver autophagy via the AMPK-mTOR pathway [59]. Apoptosis and autophagy play important roles in concanavalin A (Con A)-induced acute hepatitis. Aspartate aminotransferase, alanine aminotransferase, and pathological damage were significantly ameliorated by NaHS pretreatment. NaHS pretreatment significantly reduced the levels of interleukin-6 and tumor necrosis factor-α (TNFα) compared with those of the Con A group [60]. The expression of Bcl-2, Bax, Beclin-1, and LC3-2, which play important roles in the apoptosis and autophagy pathways, were also clearly affected by NaHS. H2S attenuates Con A-induced acute hepatitis by inhibiting apoptosis and autophagy, in part, through activation of the PtdIns3K-AKT1 signaling pathway [60]. Other groups have shown that H2S attenuates hepatic I/R injury, at least in part, by regulating apoptosis through inhibiting JNK1 signaling [61]. The autophagy agonist rapamycin potentiated this hepatoprotective effect by reversing the inhibition of autophagy by H2S.
Metabolite accumulation in lysosomal storage disorders (LSDs) results in impaired cell function and multi-systemic disease [62]. Although substrate reduction and lysosomal overload-decreasing therapies can ameliorate disease progression, the significance of lysosomal overload-independent mechanisms in the development of cellular dysfunction is unknown for most LSDs. It was shown that impaired chaperone-mediated autophagy (CMA) in cystinosis, an LSD caused by defects in the cystine transporter cystinosis (CTNS) and characterized by cysteine lysosomal accumulation [62].
TBI
Traumatic Brain Injury (TBI) is one of the major reason for morbidity and mortality and it affects more than 1.7 million Americans each year. 3-mercaptopyruvate sulfurtransferase (3-MST) was a novel hydrogen sulfide (H2S)-synthesizing enzyme that may be involved in cyanide degradation and in thiosulfate biosynthesis. 3-MST was mainly located in living neurons and TBI leads to an augmentation in the level of 3-MST. Importantly, immunohistochemistry analysis revealed that injury-induced expression of 3-MST was partly co-labeled by LC3 [63]. However, there was no co-localization of 3-MST with propidium iodide (cell death marker) and LC3 positive cells were partly co-localized with propidium iodide. These data suggested that H2S may be implicated in the autophagy of neuron and involved in the pathophysiology of the brain after TBI [63, 64]. Other groups have shown that H2S pretreatment had reduced brain edema, improved motor performance and ameliorated performance in Morris water maze test after TBI [64]. Immunoblotting results showed that H2S pretreatment reversed TBI-induced cleavage of caspase-3 and decline of Bcl-2, suppressed LC3-II, Beclin-1, and Vps34 activation and maintained the p62 level in injured cortex and hippocampus post-TBI [64]. The results suggest a protective effect and therapeutic potential of H2S in the treatment of brain injury and the protective effect against TBI may be associated with regulating apoptosis and autophagy.
Cellular Metabolism and H2S signaling
Physiological perspectives
Cellular metabolism mostly depends on cellular energy production and it is known that H2S reduce cell energy production by inhibiting oxidative phosphorylation, ATP biosynthesis and cytochrome C oxidase in mitochondria [65, 66]. On the other hand, in hyperglycemia H2S protects the vascular endothelium by preserving mitochondrial function to improve endothelial metabolic state and maintain endothelial function [67]. H2S protects against vascular remodeling from endothelial damage by normalizing the levels of redox stress, MMPs, and TIMPs [68]. It was shown that CBS−/− mice exhibited thicker neointima and a higher percentage of luminal narrowing in vein grafts which is associated with vascular remodeling in vein graph in mice [69]. Moreover, Other groups have shown that in the liver of CBS−/− mice, mitochondrial oxidative stress is reduced along with a decrease in the level of a serine/threonine kinase, DYRK1A to counteract pro-apoptotic signals [70], [71].
H2S also affects cell proliferation or apoptosis by altering cell fate during the course of the cell cycle [72]. As a part of the mechanism, it was shown that H2S affects DNA synthesis, decreases Rb phosphorylation, and increases cell cycle arrest via augmenting p21Cip1level [72]. H2S is a reducing agent and possesses antioxidant effects to offer cytoprotection via reducing the cytotoxic effects of hydrogen peroxide and oxidized low-density lipoprotein (oxLDL) on cultured human umbilical vein endothelial cells (HUVECs). H2S attenuates high-fat diet–induced cardiac dysfunction via suppression of ER stress which suggest that H2S controls cellular metabolic processes via different processes [73]. Elevated retinal homocysteine in CBS+/− mice alters expression of genes involved in endoplasmic reticular stress, N-methyl-d-aspartate (NMDA) receptor activation, cell cycle, and apoptosis [74].
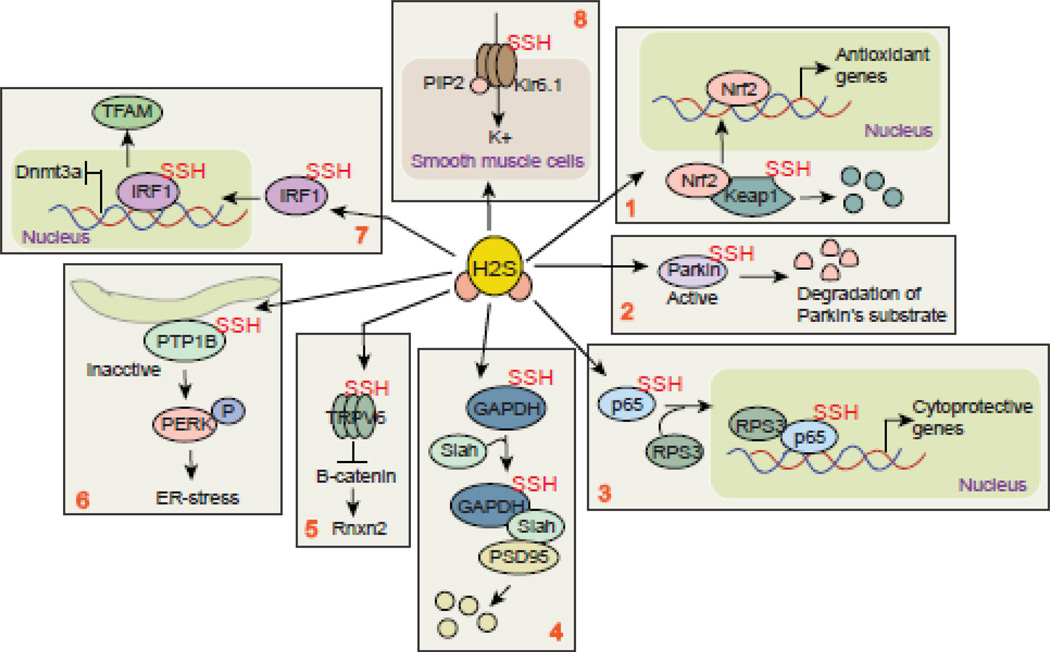
To identify the cellular targets of H2S, it was shown that H2S sulfhydrates KATP channel and sulfhydration of KATP channel prevents the association of KATP with ATP and promotes binding of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) [75] (Figure 3, box 8). This event results in the opening of the channel and vasodilation of smooth muscle cells. On the other hand, it was shown that in CBS+/− mice, where intracellular H2S was reduced significantly, there is no significant changes in muscle morphology except a reduction in large muscle fiber number in CBS+/− mice [76].Thus the balance of selecting target determine the overall effects of H2S. However, greater insights into the mechanistic details are needed to understand the precise role of H2S on biological effects and diseases related to defective H2S metabolism.
Figure 3.
Sulfhydration of proteins in general. Box 1: Sulfhydration of Keap1 leads Nrf2 to translocate to the nucleus and synthesize antioxidant genes. Box 2: sulfhydration of a E3 ligase, Parkin protein leads to degradation of its substrates and provides neuroprotection. Box 3: sulfhydration of p65 (NFkB) stimulates its transcriptional activation after its interaction with RPS3 protein. Box 4: sulfhydration of GAPDH leads to degradation of PSD95 after its interaction with a E3 ligase Siah protein. This event leads to memmory imapirment following an induction of a proinflammatory cytokine, IL-1β. Box 5: sulfhydration of a channel protein TRPV6 regulates maintenace of stem cell function via modulating β-catenin inside cells. Box 6: Sulfhydration of PTP1B induces PERK phopshorylation and triggers ER stress which has been implicated in several neurodegenerative diseases. Box 7: Sulfhydration of IRF1 serves as a transcriptional suppressor and facilititates the transcriptional activation of TFAM which is impaortant for mitochondrial DNA compaction.
Pathological perspectives
Imbalance in cellular bioenergetics is responsible for tumor growth, invasion, and metastasis and Tumor cells generate ATP mainly through mitochondrial oxidative phosphorylation and increased aerobic glycolysis. Recent studies have shown that H2S treatment influences mitochondrial phosphodiesterases and subsequently affect mitochondrial function and electron transfer [77]. Interestingly, CBS also present in mitochondria and depletion of CBS results in attenuation in mitochondrial oxygen consumption with a concomitant increase in the generation of reactive oxygen species in ovarian cancer cells [78]. Similarly, knockdown of CBS results in a decrease in the basal oxygen consumption rate, ATP production in colon cancer cells suggesting that H2S supports tumor growth and proliferation [78]. Mitochondrial transcription factor A (TFAM) encodes a key mitochondrial transcription factor containing two high mobility group motifs. The encoded protein also functions in mitochondrial DNA replication and repair. Recently, it was shown that CSE KO (CSE −/−) has lower mtDNA content and copy number due to decreased expression of TFAM [79]. Under physiological conditions, H2S sulfhydrates the transcriptional repressor interferon regulatory factor 1 (IRF-1) (Figure 3, box 7) and promotes its binding to the promoter of DNA methyltransferase 3a (Dnmt-3a) to inhibit its expression. The inhibition of Dnmt-3a results in lowering the level of DNA methylation and facilitates transcription of TFAM [80]. Metabolic complications may also lead to mitochondrial remodeling by interfering with fusion and fission, therefore, leading to mitochondrial mitophagy and skeletal muscle myopathy. Mitochondrial protection by H2S may mitigate deterioration of muscle function during metabolic syndromes [81].
Angiogenesis
Angiogenesis is a biological process where new blood vessels are formed from the existing one and it involves proliferation and migration of endothelial cells. Angiogenesis plays a pivotal role in the growth, invasion, and metastasis of solid tumors, which could supply oxygen and nutrients and dispose of metabolic wastes. Several factors including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and angiogenin are known to play an important role in angiogenesis. A compelling number of studies have shown that H2S can increase VEGF expression and stimulates angiogenesis in several tissues. H2S also promote tumor growth by promoting angiogenesis and vasorelaxation [82]. Depletion of CBS can reverse H2S-mediated angiogenesis in colon cancer [83]. Taken together, these results indicate that CBS plays an important role in promoting angiogenesis and tumor growth. Thus, CBS could be a promising molecular target for cancer therapy.
Recently it was demonstrated that lung alveolarization can be reduced to the extent of 50% in CBS−/− and CSE−/− mouse pups. Exogenous administration of H2S with GYY4137 promoted endothelial tube formation. These data confirm a key role for the H2S-generating enzymes CBS and CSE in pulmonary vascular development and homeostasis and in lung alveolarization [84]. The blood-brain barrier (BBB) integrity can be maintained by the cross-talk of endothelial cells, junction proteins, and neurogliovascular network and a recent study has shown that miR29b regulates BBB dysfunction by regulating BBB permeability through DNMT3b. In CBS+/− mice miR29b microRNA levels were high as compared with wild-type (WT) mice along with an increase in global hypermethylation and DNA methyltransferase-1 and -3a Thus treatment with a DNA methylation inhibitor, 5'-aza can improve BBB permeability by decreasing the expression of miR29b [85] and normalized blood pressure and reversed renal injury similar to The CBS+/− mice [86]. A mouse model of carotid artery air-dry endothelium denudation and re-endothelialization was established and used to evaluate post-injury endothelial repair in mice with the gene deletion of cystathionine-beta-synthase (CBS). Post-injury re-endothelialization was impaired in severe CBS−/− mice [87].
Stem cell and H2S signaling
Physiological perspectives
Tissue homeostasis can be maintained by the orchestration of several cell types such as osteoblasts, chondrocytes, myocytes and adipocytes. Mesenchymal stem cells (MSC) can serve as progenitor cells which have the capacity for self-renewal and multilineage differentiation to generate these cells. It was shown that endogenous H2S regulates the fate of MSC through several possible mechanisms. Endogenous H2S controls proliferation and differentiation of neural stem cells through activation of Erk1/2 and augments the proliferation and survival of human-induced pluripotent stem cell–derived MSCs by activation of the PI3K/Akt pathway [88]. A recent study showed that MSCs produce H2S and that endogenous H2S plays a crucial role in the maintenance of MSC function to ensure bone homeostasis [89]. Depletion of H2S in CBS KO mice, osteogenic differentiation, and proliferation in bone marrow MSCs were impaired [90, 91]. As a part of the mechanism, it was shown that reduction in sulfhydration of Ca2+ transient receptor potential (TRP) channels (TRPV3, TRPV6, and TRPM4) TRP causes aberrant intracellular Ca2+ influx (Figure 3, box 5). As a result, Wnt/β-catenin signaling is impaired and causes attenuation of osteogenic differentiation of BMMSCs [91]. NaHS dose-dependently decreased human osteoclast differentiation at concentrations which did not induce toxicity. The inhibition of human osteoclast differentiation was associated with a down-regulation in RANKL-dependent intracellular ROS levels in human pre-osteoclasts cells [92]. Furthermore, NaHS up-regulated NRF2 protein expression, its nuclear translocation, and the transcription of the two key downstream antioxidant genes Peroxiredoxin-1 and NAD(P)H dehydrogenase quinone 1, suggesting that NRF2 activation may inhibit human osteoclast differentiation by activating a sustained antioxidant response in osteoclast progenitors [92]. NaHS also downregulated the RANKL/OPG mRNA ratio in human mesenchymal stem cells, the key osteoclast-supporting cells [92]. These results suggest that NaHS shows a potential therapeutical role in erosive diseases of bone by regulating both direct and indirect mechanisms controlling the differentiation of circulating osteoclasts precursors. Recently, it has been shown that S-sulfhydration of Kelch-like ECH-associated protein 1 (KEAP1) is a negative regulator of the activity of nuclear factor erythroid 2-related factor 2 (NRF2). H2S-induced S-sulfhydration of KEAP1 at Cys151 (Figure 3, box1) facilitates the dissociation of KEAP1 from NRF2, thereby resulting in an enhancement of NRF2-mediated antioxidant responses [93].
H2S treatment augments MSC proliferation and survival and attenuates hypoxia-, oxidants, or serum deprivation–induced apoptosis. Activation of several signaling pathways including Erk1/2 and GSK-3β and an increase in an anti-apoptotic gene such as Bcl-2 are important for hypoxia-induced MSC apoptosis. Under ischemic conditions, H2S has a protective effect on mesenchymal stem cells and mature adipocytes, and this effect is mediated by the elevation of anti-apoptotic gene expression such as Bcl2 [94]. Thus modulation of H2S metabolism may serve as a therapeutic approach to promote the viability of transplanted MSCs and facilitate MSC-based regeneration. MSCs have the potential to facilitate cardiac repair following acute myocardial infarction. However, MSC therapy is limited by apoptosis of the stem cells following transplantation. It was demonstrated that hypoxia and serum deprivation is able to significantly induce apoptosis in MSCs. CSE overexpression, which enhances the endogenous H2S level, protects MSCs from H/SD-induced apoptosis via attenuation of the mitochondrial injury pathway, inhibition of endoplasmic reticulum stress and activation of the PI3K/Akt signaling pathway [95]. These findings suggest that modulation of, the CSE/H2S system may be a therapeutic approach with which can promote the viability of transplanted MSCs.
Other groups have shown that H2S promoted the differentiation of certain hematopoietic stem/progenitor cells in the bone marrow [96]. This gave rise to an idea that H2S might promote hematopoiesis. In fact, H2S promoted the generation of megakaryocytes, increased platelet levels, ameliorate menorrhagia, and improved survival. H2S is a novel promoter for megakaryopoiesis by acting on the TPO receptors but not TPO to generate megakaryocytes/platelets [96]. Moreover, it was shown that in several cell types, including human inducible pluripotent stem cell (hiPSC)-derived neurons, H2S inhibited complex IV of the mitochondrial respiratory chain and induced apoptosis [97]. H2S increased hydroxyl radical production in isolated mouse heart mitochondria and F2-isoprostanes in brains and hearts of mice [98]. H2S plays an important role in the proliferation and differentiation of human periodontal ligament stem cells (PDLSCs) [99, 100]. Human PDLSCs express both CBS and CSE and produce H2S. Blocking the generation of endogenous H2S with CBS inhibitor hydroxylamine significantly attenuated PDLSC proliferation and reduced the osteogenic and adipogenic differentiation capacity of PDLSCs [101]. In contrast, CSE inhibitor DL-propargylglycine had no effect on PDLSC function [101]. Exogenous H2S could inhibit the production of endogenous H2S and impair PDLSC function in a dose-dependent manner. Thus, physiologic levels of endogenous H2S maintain the proliferation and differentiation capacity of PDLSCs, and CBS may be the main source of endogenous H2S in PDLSCs [101]. NaHS pretreatment can increase the survival of therapeutically used human adipose tissue-derived stem cells via increased antioxidant defense [102]. The underlying mechanisms involve enhanced ERK-phosphorylation and decreased AKT-phosphorylation [102]. Pretreatment with NaHS may represent a simple pharmacological step that may enhance the efficacy of cell-based therapies.
Accumulating evidence has suggested that H2S acts as a novel neuron-modulator and neuroprotective agent; however, it remains to be investigated whether H2S has a direct effect on neural stem cells (NSCs). Recently it was shown that NaHS promoted proliferation and neuronal differentiation of NSCs [103]. Further analysis revealed that NaHS-induced proliferation was associated with phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and neuronal differentiation was linked to altered expression of differentiation-related genes [104]. Moreover, administration of NaHS could increase the number of proliferating cells in the dentate gyrus of the hippocampus in the mice after hypoxia [104]. These findings suggest that H2S may afford a novel therapeutic strategy to intervene in the progression of brain diseases.
Pathological perspectives
Impaired angiogenesis and its induced refractory wound lesions are common complications of diabetes. H2S has been reported to have proangiogenic effects. We hypothesize that H2S improves diabetic wound healing by restoring endothelial progenitor cell (EPC) function in type 2 diabetes [105]. EPC functions of db/db mice were significantly improved after in vitro NaHS treatment [105]. The expressions of Ang-1 in wound skin tissue and in EPCs were upregulated in the NaHS and HTB groups compared with db/db controls but were downregulated by in vivo PAG and in vitro siCSE treatment compared with normal controls [105]. Chronic exposure to high glucose induces the expression of CSE in pancreatic beta-cells, thereby suppressing apoptosis. After 8 weeks of HFD, blood glucose levels were markedly increased in middle-aged CSE-KO mice, insulin responses were significantly reduced, and DNA fragmentation of the islet cells was increased along with an increase in thioredoxin binding protein-2 (TBP-2). Administration of NaHS, reduced TBP-2 gene levels in isolated islets from CSE-KO mice [106]. Diabetic EPC tube formation capacity was significantly inhibited by Ang-1 small interfering RNA before NaHS treatment compared with db/db EPCs treated with NaHS only [105]. Taken together, these results show that H2S improves wound healing by restoration of EPC functions and activation of Ang-1 in type 2 diabetic mice.
Inflammation and H2S signaling
Physiological perspective
H2S has been shown to serve as an important endogenous anti-inflammatory molecule and blocks leukocyte adherence to the vascular endothelium [107]. This was supported by studies where it was shown that H2S treatment attenuates adherence of leukocytes to the vascular endothelium and impairs extravasation of leukocytes; however Inhibition of endogenous H2S rescued the phenotype [107, 108]. Recent studies related to sub-dermal inflammation, H2S treatment provides a protective function by suppressing leukocyte infiltration and edema formation [109]. Moreover, several groups have shown that H2S suppress the expression of cell adhesion molecules on both the endothelium (e.g., intercellular adhesion molecule (ICAM)-1 and P-selectin) and on the leukocyte (lymphocyte function-associated antigen (LFA)-1) [110, 111]. This finding was further supported by in vivo data where CBS heterozygous mice show increased vascular permeability and increased numbers of adherent leukocytes in mesenteric venules [112]. Other than leukocyte adhesion, H2S inhibits the activity of the granulocyte enzyme myeloperoxidase (MPO) which catalyzes the conversion of hydrogen peroxide to hypochlorous acid and scavenges peroxynitrite, superoxide anion, hypochlorous acid and hydrogen peroxide [113].
Induction of neutrophil death and conversion of M1 macrophages to M2 by phagocytosis of dying neutrophil are critical for suppressing inflammation. H2S stimulates both processes and causes a reduction in inflammatory response [114]. Exposure to H2S also rendered macrophages hyporesponsive to inflammatory stimuli such as bacterial endotoxin or TNF-α [83]. Recently, it was shown that annexin- A1 serves as a pro-resolution mediator and H2S treatment facilitates this process by translocating annexin-A1 from cytosol to plasma membrane [83]. In annexin-A1 deficient mice, where the expression of CBS and CSE was increased, granulocyte adhesion was inhibited along with a reduction in expression of COX-2 and inducible NO synthase in macrophages [115]. In GI tract, H2S maintains mucosal integrity by regulating the expression of COX-2 and prostaglandin synthesis [116]. On the other hand, during chronic inflammation, H2S suppresses upregulation of pro-inflammatory cytokines such as IL-1β, tumor necrosis factor TNF-α, interferon IFNγ, IL-12, IL-23 and COX-2 and upregulates the expression of antiinflammatory cytokines such as IL-10 [117, 118]. Although it is anticipated that activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) can explain the diversified role of H2S in inflammation [119], the exact mechanism is still unknown.
Pathological perspective
Despite its role as an anti-inflammatory molecule, H2S repairs tissue injury and restores tissue function [120]. As an example, H2S treatment accelerated healing of ulcers in the stomach of rats, while suppression of H2S synthesis resulted in a marked delay in healing [121]. This study was further supported by the fact that H2S-releasing NSAID (ATB-346) provides faster healing of gastric ulcers compared to only NSAID treatment [120, 122]. This occurred despite the compound markedly suppressing gastric prostaglandin synthesis, which is believed to be responsible for COX-2-driven angiogenesis and repair. Thus, H2S appears to have additional pro-healing mechanisms to those mediated via induction of COX-2 expression [2].
Bowel disease
The pathogenesis of inflammatory bowel disease (IBD) including Crohn's disease and ulcerative colitis is mostly about an augmentation in the inflammatory response including an increase in the level of pro-inflammatory cytokines such as TNFα. Despite the fact that targeting TNFα provides an efficient therapeutic strategy, the adverse effect of this strategy raised significant concerns. Recently, mesalamine (5-aminosalicylic acid) has been considering for the treatment of IBD, but it has limited antiinflammatory effects. However, a derivative of mesalamine which can release H2S exhibits significantly enhanced anti-inflammatory, pro-resolution and pro-healing effects in rodent models of colitis by inhibiting tissue expression of a number of pro-inflammatory cytokines and chemokines (IL-1, IL-8, IL-12, TNFα, RANTES) [123, 124]. Similarly, nonsteroidal anti-inflammatory drugs have been using as anti-inflammatory drugs to reduce acute and chronic pain associated with osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, however, long-term use of these compounds cause ulceration and bleeding in the GI tract. In contrast, H2S-releasing NSAIDs provide anti-inflammatory effects by inhibiting COX-1 and COX-2 with similar potency as the parent NSAIDs but without ulceration and bleeding in the GI tract [125, 126].
Multiple sclerosis (MS)
MS is generally considered as an autoimmune disease affecting central nervous system and is characterized by dysfunctional immune system in damaging the brain, impairment of differentiation of oligodendrocyte progenitor cells (OPCs) into mature oligodendrocytes to produce myelin, attenuation of the formation of fibronectin aggregates by astrocytes to inhibit scar formation, and downregulation of the function of healthy endothelial cells (ECs). H2S upregulated proteins involved in immune system response and downregulated PBMNCs- and EC-related adhesion molecules (LFA-1 and VCAM-1) [127]. Furthermore, it had a cell expansion-inducing effect, altering EC morphology. The effects of H2S on OPCs and astrocytes were studied compared to mTOR inhibitor rapamycin [127]. In NaHS treated astrocytes the induced fibronectin production was partially inhibited while rapamycin almost fully inhibited fibronectin production [127]. NaHS slowed but did not inhibit the differentiation of OCPs or the production of myelin compared to rapamycin [127]. Thus these data suggest that H2S may provide potential therapeutic application against MS.
Hydrogen sulfide on cell cycle
Physiological perspective
The cell cycle is the series of events that take place in a cell leading to its division and duplication of its DNA (DNA replication) to produce two daughter cells. In eukaryotic cells, the cell cycle includes four conventional phases: Gap phase 1 (G1); DNA synthesis phase (S); Gap phase 2 (G2), during which the cell prepares itself for division; and mitosis phase (M), during which the chromosomes separate and the cell divides. However, during the development of cancers, the cell cycle process is disrupted. The G proteins, G(s) (stimulatory) and G(i) (inhibitory), are involved in calcium regulation; overexpression has pathological consequences. Interestingly, G(s) G protein was downregulated in cardiac tissue of heterozygous CBS KO mice to 46% that of control hearts. However, the intracellular G(i) G protein content remained the same in heterozygous CBS KO mice [128]. H2S treatment accelerates cell cycle progression in cell carcinoma through increasing phosphorylation of Akt and ERK [129]. Moreover, H2S causes a decrease in the Go-G1 population and an increase in S phase by regulating protein expression of p21, one of the cyclin-dependent kinase (CDK) inhibitory proteins [130]. Moreover, depletion of CSE protein induces G1/G0 phase arrest and decreases the cell population in S phase in hepatoma cells but the processed can be reversed by administration of H2S [131, 132]. These data collectively suggest that H2S is a pro-proliferative factor in human cancer cells partly by accelerating the progression of the cell cycle.
Cell cycle arrest can be classified into four groups, namely G1-to-S, G2-to-M, and M-to-G1 and H2S have shown to influence these processes in cancer cells. Treatment with SPARC blocks the proliferation and migration of cancer cells by inducing cell cycle arrest at G1/S phase [133]. Moreover, administration of GYY4137 inhibits G1/S cell cycle transition by causing downregulation of cyclin D1 [134]. Similarly, NaHS treatment induces G1arrest via up-regulation of CDK inhibitor p21Cip1 in colon cancer cells [135]. Interestingly, treatment with GYY4137 elicits G2/M arrest in MCF-7 cells after 5–8 days of treatment [136, 137]. However, GYY4137 does not induce cell death indicating that the influence of H2S on cell cycle is cell type specific but very selective.
H2S is also involved in modulating cell proliferation of smooth muscle cells where G1 phase are not affected by the treatment of H2S and as a result, asynchronized cells become more sensitive than synchronized cells [138]. Other groups have shown that treatment with H2S results in cell death via stabilization of p53 and an induction of p21 and Bax without affecting antiapoptotic protein level of Bcl2 [138]. However, in synchronized cells, H2S treatment stimulates cell growth instead of cell death [139]. Interestingly, it was shown that H2S treatment leads to cell death via DNA damage and alteration in the cell cycle [130]. Consistent with this result, H2S caused free radical associated DNA damage in Chinese hamster ovary (CHO) cells which was evidenced by using single-cell gel electrophoresis assay (comet assay) that quantitatively measures genomic DNA damage as single and double-strand breaks [140]. H2S also functions as an inhibitor of CDK which is the key factor for cell cycle progression of the smooth muscle and lung fibroblast [141, 142].
Pathological perspective
Injury
Moreover, H2S treatment influences cellular injury and apoptotic cell death induced by rotenone [143]. Treatment with H2S alters the expression of extracellular signal-regulated kinase (ERK), p38 MAPK and p21 with down-regulation of cyclin D that leads to cell cycle arrest [144]. Other groups have shown that H2S treatment induced cell death by inducing cytochrome C release and induction of Bax in cancer cells [145]. H2S treatment causes an inhibition of cell proliferation at G1 phase and these cells were eliminated by the process of apoptosis as indicated by an increase in the number of cells arrested at sub-G1/S phase [49]. Activation of p53 and p21 will be followed by the up-regulation of key cell cycle proteins cyclin-E, CDC-6 (cell division cycle- 6) and decrease in cyclin D1 and p27 involved in cell growth after H2S treatment suggesting that H2S controls cell growth regulation. In keratinocytes, H2S regulates cell proliferation and cell adhesion by influencing MAPK and ERK pathways and modulating the expression of β4, α2, and α6 integrins [139]. Evidence suggests that aberrant p38 MAPK signaling undermines the repair process after injury in aged mice. An interesting study shows that CBS−/+ mice, exhibited compromised regenerative function and cell proliferation upon injury. However, there was no significant difference in Pax7 expression levels in the satellite cells from CBS−/+ mouse skeletal muscles. In addition, there was enhanced p38 MAPK activation as well as p16 and p21 expression in the CBS−/+ mouse satellite cells. Tissue engraftment potential and regeneration after injury were restored to some extent upon treatment with the p38-MAPK inhibitor, SB203580, in the CBS−/+ mice [146].
Cell death and H2S
Physiolgical perspective
Apoptosis or programmed cell death is a highly regulated cellular suicide program that is critical for the several biological processes such as normal development and maintenance of tissue homeostasis in multicellular organisms. In tumor cells, apoptosis allows these cells to survive against oncogene activation, uncontrolled proliferation, and chemotherapy. It has been shown that H2S signaling pathway is critical in maintaining the proliferation of hepatoma cells. Inhibition of this pathway prevents the growth of these cells may be due to mitochondrial apoptosis. In support of these studies, it was shown that treatment with NaHS augments cell viability in PLC/PRF/5 hepatoma cells [147]. Similarly, H2S could inhibit the apoptotic effects in colon cancer cells induced by β-phenyethyl isothiocyanate (PEITC) [148]. Administration of 6-hydroxydopamine also leads to apoptosis in a human neuroblastoma cell line, SH-SY5Y, however, overexpression of CBS or treatment with NaHS reduced cell death [149]. Thus these data indicates that H2S may serve as an anti-apoptosis agent in the development of diseases that involve excessive cell growth and division, such as cancer and neuroblastoma cells, but the mechanism is not clear. One recent study has shown that in the liver of CBS−/− mice mitochondrial oxidative stress is reduced along with a reduction of a serine/threonine kinase, DYRK1A as an antiapoptotic factor to counteract these pro-apoptotic signals [70]. Moreover, NaHS treatment also mitigated mitochondrial oxidative stress (NOX4, ROS, and NO) and restored ATP that indicates its protective effects against mitochondrial toxicity [150].
Pathological perspective
Cancer
The balance between the generation of cells and cell death is critical in multicellular organisms and disruption of this balance leads to cancer development. Thus the reactivation of cell death programs by exposure of H2S could serve as an effective strategy for cancer prevention through increasing the apoptotic percentage of cancer cells such as CA9–22, but signs for apoptosis in normal keratinocytes are not observed [141, 151]. Similarly, overexpression of CSE induces cell death in human melanoma cells by suppressing the activity of nuclear factor-κB (NF-κB) and decreasing the expression of anti-apoptotic proteins. This result is in contrary with our results where we have shown that the antiapoptotic actions of NF-κB are mediated by H2S synthesized by CSE [28]. H2S generated by CSE stimulates DNA binding and gene activation of NF-κB, processes that are abolished in CSE deleted mice. H2S acts by sulfhydration the p65 subunit of NF-κB, which promotes its binding to the coactivator ribosomal protein S3 (RPS3). Anti-apoptotic influences of NF-κB, which are markedly diminished in CSE mutant mice [152] (Figure 3, box 3). Thus, sulfhydration of NF-κB appears to be a physiologic determinant of its anti-apoptotic transcriptional activity. These results suggest that the effectivity of H2S on cell death depends on the context how cell death is induced and the nature of the cells which is functionally different from one to another. The administration of an H2S donor, GYY4137 exhibit anticancer activities by stimulating cell death although it has no effect on cell survival of normal lung fibroblasts [153].
Diabetic cardiomyopathy (DCM)
An induction of ER-stress leads to an activation of protein kinase RNA-like ER kinase (PERK) by increasing its phosphorylation at Tyr916 residues. Activation of PERK affects protein synthesis by increasing the phosphorylation level of eukaryotic translational initiation factor 2α (eIF2α) [154–156]. The phosphorylation of PERK depends on the activity of phosphodiesterase, PTP1B. H2S sulfhydrates PTP1B (Figure 3, box 6) and impairs the catalytic activity of this enzyme [157]. As a result, the phosphorylation of PERK is increased and causes an induction of ER-stress inside cells. In contrary, it was shown that H2S inhibits endoplasmic ER-Stress in a rat model of diabetic cardiomyopathy (DCM). H2S exerts cardioprotective effects against AMI-induced apoptosis through the GSK-3β/β-catenin signaling pathway [158]. NaHS significantly ameliorated hemorrhagic shock caused hemodynamic deterioration, decreased myocardial enzymes elevation, protected myocardial ultrastructure, and inhibited the expression of apoptosis-relevant proteins [159]. It suggested that H2S might exert its cardioprotective roles via both the extrinsic Fas/FasL/caspase-8/caspase-3 pathway and the intrinsic mitochondria-involved pathways [159].
Ischemia/reperfusion (IR)
The protective effect of H2S against myocardial ischemia/reperfusion (IR) injury via anti-apoptotic signaling is well established, but the underlying mechanism remains unclear. Recently, miRNAs have been identified as important mediators of myocardial injury by regulating apoptosis-related genes [160]. It was found that HR injury increased apoptosis of cardiac myocytes, upregulated the expression of miR-1, and down-regulated the expression of Bcl-2 [160]. H2S preconditioning attenuated cardiomyocyte apoptosis and LDH release, as well as enhanced cell viability following HR injury. MiR-1 was up-regulated by HR and down-regulated by H2S preconditioning. H2S also attenuated IR-induced cardiomyocyte apoptosis in vivo. MiR-1 regulated H2S protection of cardiomyocytes against IR-induced apoptosis by stimulating Bcl-2. These results implicate miR-1 as an important regulator of H2S on the IR myocardium [160].
Stroke
The N-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel protein found in nerve cells. It is activated when glutamate and glycine (or D-serine) bind to it, and when activated it allows positively charged ions to flow through the cell membrane [161]. The NMDA receptor is very important for controlling synaptic plasticity and memory function [162]. The cysteine disulfide bond at the hinge of the ligand binding domain of N-methyl daspartate (NMDA) receptors is important for its activity. However, it was discovered that the reduction of this bond by a reducing agent DTT [20] or treatment with NaHS enhances the activity of NMDA receptors [22, 163]. Thus the intracellular concentrations of H2S and the redox balance is important to maintain the disulfide bond of NMDAR. Other reports have shown that NaHS improved cerebral energy metabolism after cerebral global ischemia and prolonged survival time of animals with acute cerebral anoxia. In cultured neurons, sodium sulfide increased cell viability and decreased cell apoptosis induced by oxygen-glucose deprivation [164]. Thus, NaHS presents protective effect on acute cerebral ischemia and might be a promising therapeutic drug [164]. Mechanistic studies demonstrated that NaHS-treated rats displayed a significant reduction of malondialdehyde content, and strikingly increased the activity of superoxide dismutases and glutathione peroxidase in the brain tissues compared with I/R group [165, 166]. DATS protects B35 neural cells against OGD-induced cell injury by inhibiting ROS production via upregulating the PI3K/Akt-mediated Nrf2 pathway, which further activates HO-1 [167]. Based on our results, DATS may be a potential candidate for intervention in hypoxic-ischemic brain injuries such as stroke. In addition, Na2S also enhanced H2S level and CSE expression associated with upregulation of SIRT6 expression and activity in OGD/R-stimulated brain endothelial cells, whereas CSE inhibitor DL-propargylglycine further deteriorated the decrease of SIRT6 expression and activity as well as the reduction of H2S level and CSE expression caused by OGD/R [168]. Furthermore, SIRT6 knockdown abolished Na2S-mediated CSE expression and cytoprotection action in OGD/R-stimulated cells. Na2S protected brain endothelial cells against simulated ischemic injury through SIRT6-dependent mechanisms [168]. A recent study shows that a topical treatment with DL-propargylglycine (PAG, an inhibitor for cystathionine γ-lyase (CSE)) and aspartate (ASP, inhibitor for cysteine aminotransferase/3-mercaptopyruvate sulfurtransferase (CAT/3-MST)), but not O-(Carboxymethyl) hydroxylamine hemihydrochloride (CHH, an inhibitor for cystathionine β-synthase (CBS)), abolished postischemic cerebral vasodilation/hyperemia. Moreover, CSE knockout (CSE−/−) reduced postischemic cerebral vasodilation/hyperemia along with an increase in H2S production in ischemic side of the cerebral cortex [169].
Injury
H2S attenuates brain edema in early brain injury after subarachnoid hemorrhage in rats and this could be due to an involvement of MMP-9 induced blood-brain barrier disruption and AQP4 expression [170]. NaHS treatment significantly improved brain edema and neurobehavioral function, and attenuated neuronal cell death in the prefrontal cortex, associated with a decrease in Bax/Bcl-2 ratio and suppression of caspase-3 activation after SAH [171].
Alzheimer’s Disease (AD)
Beta-amyloid (Aβ), a neurotoxic peptide, accumulates in the brain of Alzheimer's disease (AD) subjects to initiate neuroinflammation eventually leading to memory impairment. NaHS exerted a beneficial effect on inhibition of IκB-α degradation and subsequent activation of transcription factor nuclear factor κB (NF-κB), as well as inhibition of extracellular signal-regulated kinase (ERK1/2) activity and p38 MAPK activity but not c-Jun N-terminal kinase (JNK) activity induced by Aβ [172]. These results demonstrate that NaHS might be a potential agent for treatment of neuroinflammation-related AD. Exogenous stress-induced neuronal cell death in the hippocampus is closely associated with the pathogenesis of depression which is related to the disturbance of endogenous H2S generation and ER stress in the hippocampus and suggested that endogenous H2S and ER stress are novel treatment targets of depression [173, 174]. In addition, cytoprotective effect of H2S-releasing NMDAR antagonists correlated with their ability to increase intracellular sulfane sulfur, but not H2S, levels [175]. These studies suggest that H2S-donor compounds that increase intracellular sulfane sulfur are potentially useful neuroprotective agents against neurodegenerative diseases.
Parkinson’s Disease (PD)
Another study shows that NaHS administration improves the survival rate and significantly ameliorates the weight loss of MPTP-treated mice [176]. NaHS treatment attenuated MPTP-induced neuronal damage, restored the diminution of DA neurons, and suppressed the overactivation of astrocytes in the mouse striatum [176]. Additionally, NaHS upregulated striatal serotonin levels and modulated the balance of excitatory glutamate and the inhibitory γ-aminobutyric acid system in response to MPTP challenge. The current study indicates that H2S may function as an effective neuromodulator to regulate striatal neurotransmission and provides insight into the potential of H2S for PD therapy. Recently it was shown that an E3 ligase, parkin protein is sulfhydrated at the physiological conditions and enhances its catalytic activity [177] (Figure 3, box 2). However, the level of sulfhydration of parkin is decreased in the brains of patients with Parkinson's disease and causes an accumulation of parkin targets such as α-synuclein, a constituent of the characteristic Lewy bodies, suggesting that this loss may be pathologic [177]. H2S exerts neuroprotection against hypoxia-induced neurotoxicity through its anti-inflammatory effect in microglia. This effect appears to be attributable to inhibition of iNOS, NF-κβ, ERK and p38 MAPK signaling pathways. These results suggest a potential therapeutic application of H2S releasing drugs in hypoxic brain damage treatment.
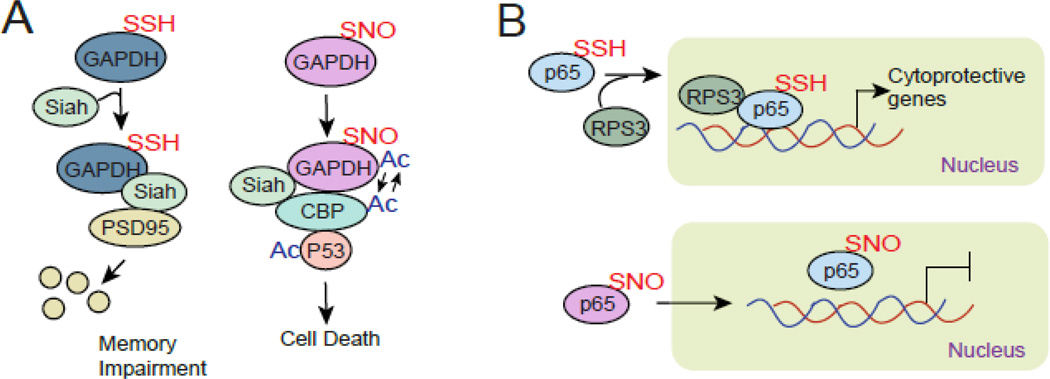
Induction of a proinflammatory cytokine, interleukin-1β (IL-1β) plays a role in memory impairment associated with various neurological disorders such as AD and PD and brain injury. Previously we have shown that IL-1β-induced memory impairment in the brain is mediated by H2S synthesized by CBS. H2S modifies GAPDH essentially via sulfhydration in dendrites, which promotes its binding to the E3 ligase protein, Siah. Then Siah binds to a critical synaptic scaffolding molecule, PSD95, and leads it to degradation via ubiquitination. In CBS−/− mice, IL-1β-induced loss of PSD95 was rescued significantly. Thus, our findings reveal a mechanism where GAPDH sulfhydration appears to be a physiologic determinant of cytokine-induced memory impairment in the brain [27] (Figure 3, box 4).
A crosstalk between Nitric oxide and hydrogen sulfide
Nitric Oxide (NO) is a well-known gaseous transmitter and is synthesized by the enzymatic activity of nNOS, eNOS and iNOS. Thionitrous acid (HSNO), a potential key intermediate in biological signaling pathways, has been proposed to link NO and H2S biochemistries, but its existence and stability in vivo remain controversial. It was shown that HSNO is spontaneously formed in high concentration when NO and H2S gases are mixed at room temperature in the presence of metallic surfaces [178]. In vivo, HSNO can be formed if H2S reacts with S-nitrosothiols [[179]. At the cellular level, HSNO can be metabolized to afford NO(+), NO, and NO(−) species, all of which have distinct physiological consequences of their own. HSNO can freely diffuse through membranes, facilitating transnitrosation of proteins such as hemoglobin. Thus these data introduce a new signaling molecule, HSNO, and suggest that it may play a key role in cellular redox regulation [180]. An interesting study shows that physiological concentrations of H2S abrogated peroxynitrite-induced cell damage as demonstrated by the: (i) inhibition of apoptosis and necrosis caused by peroxynitrite; (ii) prevention of protein nitration; and (iii) inhibition of PARP-1 [poly(ADP-ribose) polymerase 1] activation in cellular models. This implies that a major part of the cytoprotective effects of H2S may be mediated by modulation of peroxynitrite chemistry, in particular under inflammatory conditions [181]. Glutamate-induced neurotoxicity involves in overproduction of nitric oxide (NO) and oxidative stress. Treatment with NOS inhibitor, ADMA suppressed glutamate-induced neurotoxicity is by promoting endogenous H2S generation, resulting from suppression in NOS excessive activation and NO overproduction. These findings provide a novel mechanism underlying the protection of ADMA against glutamate-induced neurotoxicity [182]. H2S is known to inhibit high glucose-induced protein synthesis in kidney podocytes. It was shown that treatment with tadalafil, a phosphodiesterase 5 inhibitor ameliorates high glucose stimulation of matrix proteins by generating H2S in podocytes through NO-H2S-AMPK axis [183]. Moreover, the anti-thrombotic efficacy of H2S involves the NOS-pathway and may be of preventive and therapeutic value for clinical disorders with increased risk of thrombotic events [184].
The interplay between sulfhydration and nitrosylation has well been elicited in studies related with TNFα-induced transcriptional activation of the p65 subunit of NFkB [108]. In this study it was shown that nitrosylation of p65 is followed by sulfhydration of p65; however nitrosylation of p65 inactivates transcriptional activity of p65 although sulfhydration of p65 stimulates its activity. Upon sulfhydration of p65 , it binds with RPS3 and facilitates its transcriptional activity. The other great example of how sulfhydration and nitrosylation affect functional aspects of protein is GAPDH. Nitrosylation of GAPDH leads to cell death by activating p53 through autoacetylation of acetyltransferase p300/CBP [185]; however, sulfhydration of GAPDH prevents its interaction with p300/CBP but induces degradation of PSD95 and facilitates memory impairment [27].
Concluding remarks
Several years ago when nitric oxide was discovered as a gasotransmitter, several investigators had appreciated its importance in both physiology and pathological conditions. However, H2S is comparatively newly identified exciting gasotransmitter and have already shown its influence in several signaling pathways which are critical for biological processes as discussed here. Unlike nitric oxide, H2S present as a bound form such as sulfane sulfur and free form inside cells. Most importantly, the discovery of sulfhydration of proteins provides an edge to the field to explain the functions of H2S in biological processes. Sulfhydration has been proposed as a mode of action of H2S that causes conformational changes in the target proteins to modify their activity. Interestingly under oxidative conditions cysteine residues are oxidized (SOH, -SO2H and SO3H) to cysteine sulfenic acid and in the presence of NO they are oxidized to cysteine S-nitrosothiol (-SNO). The red maleimide assay can detect the sulfhydration of protein, however in a combination of green maleimide the assay can also differentiate the nitrosylated species from sulfhydrated species in vitro and in vivo [28]. The oxidized species will be unable to interact with red maleimide, thus this assay can exclude the oxidized species from the same sample.
The current literature suggest that H2S provides a promising target for therapeutic intervention. One possible option would be either activate or inhibit CSE/CBS to influence the related signaling pathways. However, it is important to keep in mind that modulation of the physiological level of H2S may cause adverse effects than anticipated. Moreover, the specificity and selectivity of these compounds are in question too. Instead, targeting the sulfhydrated proteins may provide more specificity towards therapeutic intervention based on H2S signaling although this could be more challenging.
Figure 4.
Two contrasting examples how nitrosylation and sulfhydration of proteins affect the biological processes. (A) sulfhydration of GAPDH leads to degradation of PSD95 via Siah. In contrast nitrosylation of GAPDH leads to cell death via activating p300/CBP and p53 signaling axis. (B) sulfhydration of p65 interacts with RPS3 and facilitates transcriptional activation of p65. On the other hand, nitrosylation of p65 prevents its transcriptional activation.
Research Highlights.
H2S, a newly discovered gaso-neurotransmitter, signals inside cells mostly via sulfhydration of proteins.
Modulation of autophagy by H2S signaling pathway is implicated in ischemia, myocardial fibrosis, metabolic disorders and TBI.
H2S affects cellular metabolism which affects tumor growth and angiogenesis.
The maintenance, proliferation, and differentiation of stem cells are regulated by H2S.
The influence of H2S signaling in cell cycle and cell death pathways is associated with cancer and neurodegenerative diseases.
Acknowledgments
This work was supported by the NIH funding (1R01NS094516 and 1R01EY025622) awarded to N.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szabo C. Hydrogen sulphide and its therapeutic potential. Nature reviews Drug discovery. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 2.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxidants & redox signaling. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nature reviews Molecular cell biology. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3- mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. Journal of biochemistry. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Banerjee R. PLP-dependent H(2)S biogenesis. Biochimica et biophysica acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. The Journal of biological chemistry. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 8.Stipanuk MH. Metabolism of sulfur-containing amino acids. Annual review of nutrition. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. The Journal of biological chemistry. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacological research. 2016;113:300–312. doi: 10.1016/j.phrs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxidants & redox signaling. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Wang K, Li MX, He W, Chang JR, Liao CC, et al. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Scientific reports. 2015;5:14971. doi: 10.1038/srep14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beard RS, Jr, Bearden SE. Vascular complications of cystathionine beta-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. American journal of physiology Heart and circulatory physiology. 2011;300:H13–H26. doi: 10.1152/ajpheart.00598.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen U, Mishra PK, Tyagi N, Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell biochemistry and biophysics. 2010;57:49–58. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taoka S, Ohja S, Shan X, Kruger WD, Banerjee R. Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. The Journal of biological chemistry. 1998;273:25179–25184. doi: 10.1074/jbc.273.39.25179. [DOI] [PubMed] [Google Scholar]
- 17.Taoka S, West M, Banerjee R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine beta-synthase reveals nonequivalent active sites. Biochemistry. 1999;38:2738–2744. doi: 10.1021/bi9826052. [DOI] [PubMed] [Google Scholar]
- 18.Kabil O, Weeks CL, Carballal S, Gherasim C, Alvarez B, Spiro TG, et al. Reversible heme-dependent regulation of human cystathionine beta-synthase by a flavoprotein oxidoreductase. Biochemistry. 2011;50:8261–8263. doi: 10.1021/bi201270q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkelstein JD, Kyle WE, Martin JL, Pick AM. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochemical and biophysical research communications. 1975;66:81–87. doi: 10.1016/s0006-291x(75)80297-x. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitvitsky V, Kabil O, Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxidants & redox signaling. 2012;17:22–31. doi: 10.1089/ars.2011.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toohey JI. Sulfur signaling: is the agent sulfide or sulfane? Analytical biochemistry. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto J, Sato W, Kosugi T, Yamamoto T, Kimura T, Taniguchi S, et al. Distribution of hydrogen sulfide (H(2)S)-producing enzymes and the roles of the H(2)S donor sodium hydrosulfide in diabetic nephropathy. Clinical and experimental nephrology. 2013;17:32–40. doi: 10.1007/s10157-012-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. The Journal of clinical investigation. 1999;104:1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, et al. H2S signals through protein S-sulfhydration. Science signaling. 2009;2 doi: 10.1126/scisignal.2000464. ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen N, Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends in neurosciences. 2010;33:493–502. doi: 10.1016/j.tins.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mir S, Sen T, Sen N. Cytokine-Induced GAPDH Sulfhydration Affects PSD95 Degradation and Memory. Molecular cell. 2014;56:786–795. doi: 10.1016/j.molcel.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, et al. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Molecular cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino SM, Gladyshev VN. Analysis and functional prediction of reactive cysteine residues. The Journal of biological chemistry. 2012;287:4419–4425. doi: 10.1074/jbc.R111.275578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Current opinion in chemical biology. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxidants & redox signaling. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annual review of pathology. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy S, Debnath J. Autophagy and tumorigenesis. Seminars in immunopathology. 2010;32:383–396. doi: 10.1007/s00281-010-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Qin ZH. Coordination of autophagy with other cellular activities. Acta pharmacologica Sinica. 2013;34:585–594. doi: 10.1038/aps.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penaloza C, Orlanski S, Ye Y, Entezari-Zaher T, Javdan M, Zakeri Z. Cell death in mammalian development. Current pharmaceutical design. 2008;14:184–196. doi: 10.2174/138161208783378789. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Bao C, Dong Y, Liu X. Activation of autophagy in rat brain cells following focal cerebral ischemia reperfusion through enhanced expression of Atg1/pULK and LC3. Molecular medicine reports. 2015;12:3339–3344. doi: 10.3892/mmr.2015.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. International journal of biological sciences. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shui M, Liu X, Zhu Y, Wang Y. Exogenous hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by inhibiting autophagy in mice. Canadian journal of physiology and pharmacology. 2016:1–6. doi: 10.1139/cjpp-2016-0100. [DOI] [PubMed] [Google Scholar]
- 41.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao J, Zhu X, Kang B, Xu J, Wu L, Hong J, et al. Hydrogen Sulfide Attenuates Myocardial Hypoxia-Reoxygenation Injury by Inhibiting Autophagy via mTOR Activation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;37:2444–2453. doi: 10.1159/000438597. [DOI] [PubMed] [Google Scholar]
- 43.Sheng R, Qin ZH. The divergent roles of autophagy in ischemia and preconditioning. Acta pharmacologica Sinica. 2015;36:411–420. doi: 10.1038/aps.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H, Xiao J, Kang B, Zhu X, Xin N, Wang Z. PI3K/SGK1/GSK3beta signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Experimental cell research. 2016;345:134–140. doi: 10.1016/j.yexcr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Xie H, Xu Q, Jia J, Ao G, Sun Y, Hu L, et al. Hydrogen sulfide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux. Biochemical and biophysical research communications. 2015;458:632–638. doi: 10.1016/j.bbrc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Jiang HK, Li YP, Guo YP. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. Journal of biomedical science. 2015;22:50. doi: 10.1186/s12929-015-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyagi N, Qipshidze N, Munjal C, Vacek JC, Metreveli N, Givvimani S, et al. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. Journal of molecular neuroscience : MN. 2012;47:128–138. doi: 10.1007/s12031-011-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H, et al. Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3beta pathway in isolated aging rat hearts. Cell & bioscience. 2015;5:11. doi: 10.1186/s13578-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu YC, Wang XJ, Yu L, Chan FK, Cheng AS, Yu J, et al. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PloS one. 2012;7:e37572. doi: 10.1371/journal.pone.0037572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giricz Z, Mentzer RM, Jr, Gottlieb RA. Autophagy, myocardial protection, and the metabolic syndrome. Journal of cardiovascular pharmacology. 2012;60:125–132. doi: 10.1097/FJC.0b013e318256ce10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao F, Zhang M, Chen L. 5'-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta pharmaceutica Sinica B. 2016;6:20–25. doi: 10.1016/j.apsb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji L, Li L, Qu F, Zhang G, Wang Y, Bai X, et al. Hydrogen sulphide exacerbates acute pancreatitis by over-activating autophagy via AMPK/mTOR pathway. Journal of cellular and molecular medicine. 2016 doi: 10.1111/jcmm.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Wu J, Sun A, Sun Y, Yu X, Liu N, et al. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell & bioscience. 2016;6:33. doi: 10.1186/s13578-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao T, Luo J, Wu Z, Li F, Zeng O, Yang J. Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Molecular medicine reports. 2016;13:1765–1773. doi: 10.3892/mmr.2015.4689. [DOI] [PubMed] [Google Scholar]
- 55.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochimica et biophysica acta. 2014;1843:2816–2826. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, et al. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. American journal of physiology Cell physiology. 2012;303:C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talaei F, Van Praag VM, Shishavan MH, Landheer SW, Buikema H, Henning RH. Increased protein aggregation in Zucker diabetic fatty rat brain: identification of key mechanistic targets and the therapeutic application of hydrogen sulfide. BMC cell biology. 2014;15:1. doi: 10.1186/1471-2121-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao J, et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. American journal of physiology Endocrinology and metabolism. 2015;309:E925–E935. doi: 10.1152/ajpendo.00294.2015. [DOI] [PubMed] [Google Scholar]
- 60.Cheng P, Chen K, Xia Y, Dai W, Wang F, Shen M, et al. Hydrogen sulfide, a potential novel drug, attenuates concanavalin A-induced hepatitis. Drug design, development and therapy. 2014;8:1277–1286. doi: 10.2147/DDDT.S66573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng P, Wang F, Chen K, Shen M, Dai W, Xu L, et al. Hydrogen sulfide ameliorates ischemia/reperfusion-induced hepatitis by inhibiting apoptosis and autophagy pathways. Mediators of inflammation. 2014;2014:935251. doi: 10.1155/2014/935251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Napolitano G, Johnson JL, He J, Rocca CJ, Monfregola J, Pestonjamasp K, et al. Impairment of chaperone-mediated autophagy leads to selective lysosomal degradation defects in the lysosomal storage disease cystinosis. EMBO molecular medicine. 2015;7:158–174. doi: 10.15252/emmm.201404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Shan H, Chang P, Ma L, Chu Y, Shen X, et al. Upregulation of 3-MST Relates to Neuronal Autophagy After Traumatic Brain Injury in Mice. Cellular and molecular neurobiology. 2016 doi: 10.1007/s10571-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Shan H, Chang P, Wang T, Dong W, Chen X, et al. Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice. PloS one. 2014;9:e87241. doi: 10.1371/journal.pone.0087241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sen U, Pushpakumar SB, Amin MA, Tyagi SC. Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2014;41:27–37. doi: 10.1016/j.niox.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino acids. 2010;39:1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 69.Tan H, Shi C, Jiang X, Lavelle M, Yu C, Yang X, et al. Hyperhomocysteinemia promotes vascular remodeling in vein graph in mice. Front Biosci (Landmark Ed) 2014;19:958–966. doi: 10.2741/4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamelet J, Noll C, Ripoll C, Paul JL, Janel N, Delabar JM. Effect of hyperhomocysteinemia on the protein kinase DYRK1A in liver of mice. Biochemical and biophysical research communications. 2009;378:673–677. doi: 10.1016/j.bbrc.2008.11.126. [DOI] [PubMed] [Google Scholar]
- 71.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. American journal of physiology Renal physiology. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang R, Liu Y, Shi S. Hydrogen Sulfide Regulates Homeostasis of Mesenchymal Stem Cells and Regulatory T Cells. Journal of dental research. 2016 doi: 10.1177/0022034516659041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barr LA, Shimizu Y, Lambert JP, Nicholson CK, Calvert JW. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2015;46:145–156. doi: 10.1016/j.niox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Investigative ophthalmology & visual science. 2009;50:4460–4470. doi: 10.1167/iovs.09-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circulation research. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veeranki S, Winchester LJ, Tyagi SC. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochimica et biophysica acta. 2015;1852:732–741. doi: 10.1016/j.bbadis.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British journal of pharmacology. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, et al. Cystathionine betasynthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PloS one. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torraco A, Diaz F, Vempati UD, Moraes CT. Mouse models of oxidative phosphorylation defects: powerful tools to study the pathobiology of mitochondrial diseases. Biochimica et biophysica acta. 2009;1793:171–180. doi: 10.1016/j.bbamcr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Yang G. Hydrogen Sulfide Maintains Mitochondrial DNA Replication via Demethylation of TFAM. Antioxidants & redox signaling. 2015;23:630–642. doi: 10.1089/ars.2014.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veeranki S, Tyagi SC. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2015;46:66–71. doi: 10.1016/j.niox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. Journal of the American Heart Association. 2012;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paul BD, Snyder SH. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxidants & redox signaling. 2015;22:411–423. doi: 10.1089/ars.2014.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madurga A, Golec A, Pozarska A, Ishii I, Mizikova I, Nardiello C, et al. The H2S-generating enzymes cystathionine beta-synthase and cystathionine gamma-lyase play a role in vascular development during normal lung alveolarization. American journal of physiology Lung cellular and molecular physiology. 2015;309:L710–L724. doi: 10.1152/ajplung.00134.2015. [DOI] [PubMed] [Google Scholar]
- 85.Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, et al. Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1212–1222. doi: 10.1038/jcbfm.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pushpakumar S, Kundu S, Narayanan N, Sen U. DNA hypermethylation in hyperhomocysteinemia contributes to abnormal extracellular matrix metabolism in the kidney. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:4713–4725. doi: 10.1096/fj.15-272443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, et al. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovascular research. 2006;69:253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan WS, Sideris A, Sutachan JJ, Montoya GJ, Blanck TJ, Recio-Pinto E. Differential regulation of proliferation and neuronal differentiation in adult rat spinal cord neural stem/progenitors by ERK1/2, Akt, PLCgamma. Frontiers in molecular neuroscience. 2013;6:23. doi: 10.3389/fnmol.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Guo Z, Guo B, Xie Y, Yang J, Wang A. Inhibition of the endogenous CSE/H(2)S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Molecular medicine reports. 2014;9:2467–2472. doi: 10.3892/mmr.2014.2111. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Wang CD, Zhang N, Tong WX, Zhang YF, Shan SZ, et al. Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell death & disease. 2016;7:e2221. doi: 10.1038/cddis.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell stem cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gambari L, Lisignoli G, Cattini L, Manferdini C, Facchini A, Grassi F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacological research. 2014;87:99–112. doi: 10.1016/j.phrs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxidants & redox signaling. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 94.Aykan A, Ozturk S, Sahin I, Avcu F, Sagkan RI, Isik S. The Effects of Hydrogen Sulfide on Adipocyte Viability in Human Adipocyte and Adipocyte-Derived Mesenchymal Stem Cell Cultures Under Ischemic Conditions. Annals of plastic surgery. 2015;75:657–665. doi: 10.1097/SAP.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 95.Guo Z, Li CS, Wang CM, Xie YJ, Wang AL. CSE/H2S system protects mesenchymal stem cells from hypoxia and serum deprivationinduced apoptosis via mitochondrial injury, endoplasmic reticulum stress and PI3K/Akt activation pathways. Molecular medicine reports. 2015;12:2128–2134. doi: 10.3892/mmr.2015.3651. [DOI] [PubMed] [Google Scholar]
- 96.Liu HD, Zhang AJ, Xu JJ, Chen Y, Zhu YC. H2S protects against fatal myelosuppression by promoting the generation of megakaryocytes/platelets. Journal of hematology & oncology. 2016;9:13. doi: 10.1186/s13045-016-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion. 2012;12:180–189. doi: 10.1016/j.mito.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang J, Chan A, Ali S, Saha A, Haushalter KJ, Lam WL, et al. Hydrogen Sulfide--Mechanisms of Toxicity and Development of an Antidote. Scientific reports. 2016;6:20831. doi: 10.1038/srep20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greabu M, Totan A, Miricescu D, Radulescu R, Virlan J, Calenic B. Hydrogen Sulfide, Oxidative Stress and Periodontal Diseases: A Concise Review. Antioxidants. 2016:5. doi: 10.3390/antiox5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang L, Zhou W, Yang N, Yu J, Liu H. ET-1 Promotes Differentiation of Periodontal Ligament Stem Cells into Osteoblasts through ETR, MAPK, and Wnt/beta-Catenin Signaling Pathways under Inflammatory Microenvironment. Mediators of inflammation. 2016;2016:8467849. doi: 10.1155/2016/8467849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su Y, Liu D, Liu Y, Zhang C, Wang J, Wang S. Physiologic Levels of Endogenous Hydrogen Sulfide Maintain the Proliferation and Differentiation Capacity of Periodontal Ligament Stem Cells. Journal of periodontology. 2015;86:1276–1286. doi: 10.1902/jop.2015.150240. [DOI] [PubMed] [Google Scholar]
- 102.Dongo E, Benko Z, Csizmazia A, Marosi G, Grottke A, Jucker M, et al. H2S preconditioning of human adipose tissue-derived stem cells increases their efficacy in an in vitro model of cell therapy for simulated ischemia. Life sciences. 2014;113:14–21. doi: 10.1016/j.lfs.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z, Liu DX, Wang FW, Zhang Q, Du ZX, Zhan JM, et al. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H(2)S pathway. Neuroscience. 2013;237:106–117. doi: 10.1016/j.neuroscience.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 104.Liu D, Wang Z, Zhan J, Zhang Q, Wang J, Zhang Q, et al. Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells and protects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacology, biochemistry, and behavior. 2014;116:55–63. doi: 10.1016/j.pbb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63:1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamoto M, Yamaoka M, Takei M, Ando T, Taniguchi S, Ishii I, et al. Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochemical and biophysical research communications. 2013;442:227–233. doi: 10.1016/j.bbrc.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 107.Wallace JL, Ferraz JG, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxidants & redox signaling. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. Journal of cellular and molecular medicine. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zayachkivska O, Havryluk O, Hrycevych N, Bula N, Grushka O, Wallace JL. Cytoprotective effects of hydrogen sulfide in novel rat models of non-erosive esophagitis. PloS one. 2014;9:e110688. doi: 10.1371/journal.pone.0110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guan Q, Wang X, Gao L, Chen J, Liu Y, Yu C, et al. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 in endothelial cells. Journal of cardiovascular pharmacology. 2013;62:278–284. doi: 10.1097/FJC.0b013e31829875ef. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, et al. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PloS one. 2012;7:e41147. doi: 10.1371/journal.pone.0041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palinkas Z, Furtmuller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, et al. Interactions of hydrogen sulfide with myeloperoxidase. British journal of pharmacology. 2015;172:1516–1532. doi: 10.1111/bph.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhatia M. Role of hydrogen sulfide in the pathology of inflammation. Scientifica (Cairo) 2012;2012:159680. doi: 10.6064/2012/159680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brancaleone V, Mitidieri E, Flower RJ, Cirino G, Perretti M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. The Journal of pharmacology and experimental therapeutics. 2014;351:96–104. doi: 10.1124/jpet.114.217034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20:9099–9123. doi: 10.3390/molecules20059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical pharmacology. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Annals of translational medicine. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annual review of pharmacology and toxicology. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 120.Gemici B, Wallace JL. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods in enzymology. 2015;555:169–193. doi: 10.1016/bs.mie.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 121.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 122.Blackler R, Syer S, Bolla M, Ongini E, Wallace JL. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PloS one. 2012;7:e35196. doi: 10.1371/journal.pone.0035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. British journal of pharmacology. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Triantafillidis JK, Douvi G, Agrogiannis G, Patsouris E, Gikas A, Papalois AE. Effect of mesalamine and prednisolone on TNBS experimental colitis, following various doses of orally administered iron. Biomed Res Int. 2014;2014:648535. doi: 10.1155/2014/648535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fiorucci S, Santucci L. Hydrogen sulfide-based therapies: focus on H2S releasing NSAIDs. Inflammation & allergy drug targets. 2011;10:133–140. doi: 10.2174/187152811794776213. [DOI] [PubMed] [Google Scholar]
- 126.Wallace JL. Building a better aspirin: gaseous solutions to a century-old problem. British journal of pharmacology. 2007;152:421–428. doi: 10.1038/sj.bjp.0707396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Talaei F. Pathophysiological Concepts in Multiple Sclerosis and the Therapeutic Effects of Hydrogen Sulfide. Basic and clinical neuroscience. 2016;7:121–136. doi: 10.15412/J.BCN.03070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vacek TP, Sen U, Tyagi N, Vacek JC, Kumar M, Hughes WM, et al. Differential expression of Gs in a murine model of homocysteinemic heart failure. Vascular health and risk management. 2009;5:79–84. [PMC free article] [PubMed] [Google Scholar]
- 129.Ma Z, Bi Q, Wang Y. Hydrogen sulfide accelerates cell cycle progression in oral squamous cell carcinoma cell lines. Oral diseases. 2015;21:156–162. doi: 10.1111/odi.12223. [DOI] [PubMed] [Google Scholar]
- 130.Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:247–255. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 131.Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA. Activation of p21-Dependent G1/G2 Arrest in the Absence of DNA Damage as an Antiapoptotic Response to Metabolic Stress. Genes & cancer. 2011;2:889–899. doi: 10.1177/1947601911432495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lv M, Li Y, Ji MH, Zhuang M, Tang JH. Inhibition of invasion and epithelial-mesenchymal transition of human breast cancer cells by hydrogen sulfide through decreased phospho-p38 expression. Molecular medicine reports. 2014;10:341–346. doi: 10.3892/mmr.2014.2161. [DOI] [PubMed] [Google Scholar]
- 133.Thomas SL, Alam R, Lemke N, Schultz LR, Gutierrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro-oncology. 2010;12:941–955. doi: 10.1093/neuonc/noq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H(2)S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. International journal of oncology. 2014;44:1259–1267. doi: 10.3892/ijo.2014.2305. [DOI] [PubMed] [Google Scholar]
- 135.Bhattacharya S, Ray RM, Johnson LR. Cyclin-dependent kinases regulate apoptosis of intestinal epithelial cells. Apoptosis : an international journal on programmed cell death. 2014;19:451–466. doi: 10.1007/s10495-013-0942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang R, Zhu L, Zhang L, Xu A, Li Z, Xu Y, et al. PTEN enhances G2/M arrest in etoposide-treated MCF7 cells through activation of the ATM pathway. Oncology reports. 2016;35:2707–2714. doi: 10.3892/or.2016.4674. [DOI] [PubMed] [Google Scholar]
- 137.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PloS one. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie X, Dai H, Zhuang B, Chai L, Xie Y, Li Y. Exogenous hydrogen sulfide promotes cell proliferation and differentiation by modulating autophagy in human keratinocytes. Biochemical and biophysical research communications. 2016;472:437–443. doi: 10.1016/j.bbrc.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 139.Gobbi G, Ricci F, Malinverno C, Carubbi C, Pambianco M, Panfilis G, et al. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Laboratory investigation a journal of technical methods and pathology. 2009;89:994–1006. doi: 10.1038/labinvest.2009.61. [DOI] [PubMed] [Google Scholar]
- 140.Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Molecular cancer research : MCR. 2007;5:455–459. doi: 10.1158/1541-7786.MCR-06-0439. [DOI] [PubMed] [Google Scholar]
- 141.Takeuchi H, Setoguchi T, Machigashira M, Kanbara K, Izumi Y. Hydrogen sulfide inhibits cell proliferation and induces cell cycle arrest via an elevated p21 Cip1 level in Ca9-22 cells. Journal of periodontal research. 2008;43:90–95. doi: 10.1111/j.1600-0765.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 142.Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. The Journal of biological chemistry. 2004;279:49199–49205. doi: 10.1074/jbc.M408997200. [DOI] [PubMed] [Google Scholar]
- 143.Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Molecular pharmacology. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 144.Perry MM, Hui CK, Whiteman M, Wood ME, Adcock I, Kirkham P, et al. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. American journal of respiratory cell and molecular biology. 2011;45:746–752. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Adhikari S, Bhatia M. H2S-induced pancreatic acinar cell apoptosis is mediated via JNK and p38 MAP kinase. Journal of cellular and molecular medicine. 2008;12:1374–1383. doi: 10.1111/j.1582-4934.2008.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Veeranki S, Lominadze D, Tyagi SC. Hyperhomocysteinemia inhibits satellite cell regenerative capacity through p38 alpha/beta MAPK signaling. American journal of physiology Heart and circulatory physiology. 2015;309:H325–H334. doi: 10.1152/ajpheart.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhen Y, Pan W, Hu F, Wu H, Feng J, Zhang Y, et al. Exogenous hydrogen sulfide exerts proliferation/anti-apoptosis/angiogenesis/migration effects via amplifying the activation of NF-kappaB pathway in PLC/PRF/5 hepatoma cells. International journal of oncology. 2015;46:2194–2204. doi: 10.3892/ijo.2015.2914. [DOI] [PubMed] [Google Scholar]
- 148.Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World journal of gastroenterology : WJG. 2005;11:3990–3997. doi: 10.3748/wjg.v11.i26.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie L, Tiong CX, Bian JS. Hydrogen sulfide protects SH-SY5Y cells against 6-hydroxydopamine-induced endoplasmic reticulum stress. American journal of physiology Cell physiology. 2012;303:C81–C91. doi: 10.1152/ajpcell.00281.2011. [DOI] [PubMed] [Google Scholar]
- 150.Kamat PK, Kalani A, Tyagi SC, Tyagi N. Hydrogen Sulfide Epigenetically Attenuates Homocysteine-Induced Mitochondrial Toxicity Mediated Through NMDA Receptor in Mouse Brain Endothelial (bEnd3) Cells. Journal of cellular physiology. 2015;230:378–394. doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murata T, Sato T, Kamoda T, Moriyama H, Kumazawa Y, Hanada N. Differential susceptibility to hydrogen sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer cell lines and oral keratinocytes: role of PHLDA1 as an apoptosis suppressor. Experimental cell research. 2014;320:247–257. doi: 10.1016/j.yexcr.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 152.Ostrakhovitch EA, Akakura S, Sanokawa-Akakura R, Goodwin S, Tabibzadeh S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Experimental cell research. 2015;330:135–150. doi: 10.1016/j.yexcr.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 153.Lee ZW, Teo XY, Tay EY, Tan CH, Hagen T, Moore PK, et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. British journal of pharmacology. 2014;171:4322–4336. doi: 10.1111/bph.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su Q, Wang S, Gao HQ, Kazemi S, Harding HP, Ron D, et al. Modulation of the eukaryotic initiation factor 2 alpha-subunit kinase PERK by tyrosine phosphorylation. The Journal of biological chemistry. 2008;283:469–475. doi: 10.1074/jbc.M704612200. [DOI] [PubMed] [Google Scholar]
- 155.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Molecular biology of the cell. 2011;22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxidants & redox signaling. 2007;9:2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 157.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Science signaling. 2011;4 doi: 10.1126/scisignal.2002329. ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ge N, Liu C, Li G, Xie L, Zhang Q, Li L, et al. Hydrosulfide attenuates acute myocardial ischemic injury through the glycogen synthase kinase-3beta/beta-catenin signaling pathway. International journal of molecular medicine. 2016;37:1281–1289. doi: 10.3892/ijmm.2016.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y, Dai X, Zhu D, Xu X, Gao C, Wu C. An exogenous hydrogen sulphide donor, NaHS, inhibits the apoptosis signaling pathway to exert cardio-protective effects in a rat hemorrhagic shock model. International journal of clinical and experimental pathology. 2015;8:6245–6254. [PMC free article] [PubMed] [Google Scholar]
- 160.Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, et al. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Molecular biology reports. 2014;41:6845–6853. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 161.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 162.Li F, Tsien JZ. Memory and the NMDA receptors. The New England journal of medicine. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. The Journal of biological chemistry. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shi HQ, Zhang Y, Cheng MH, Fan BS, Tian JS, Yu JG, et al. Sodium Sulfide, a Hydrogen Sulfide-Releasing Molecule, Attenuates Acute Cerebral Ischemia in Rats. CNS neuroscience & therapeutics. 2016;22:625–632. doi: 10.1111/cns.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu Q, Lu Z, Tao L, Yang L, Guo Y, Yang Y, et al. ROS-Dependent Neuroprotective Effects of NaHS in Ischemia Brain Injury Involves the PARP/AIF Pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;36:1539–1551. doi: 10.1159/000430317. [DOI] [PubMed] [Google Scholar]
- 166.Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, et al. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain research. 2013;1491:188–196. doi: 10.1016/j.brainres.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 167.Xu XH, Li GL, Wang BA, Qin Y, Bai SR, Rong J, et al. Diallyl trisufide protects against oxygen glucose deprivation-induced apoptosis by scavenging free radicals via the PI3K/Akt-mediated Nrf2/HO-signaling pathway in B35 neural cells. Brain research. 2015;1614:38–50. doi: 10.1016/j.brainres.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 168.Hu Y, Li R, Yang H, Luo H, Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2015;24:601–609. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 169.Jiang Z, Li C, Manuel ML, Yuan S, Kevil CG, McCarter KD, et al. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PloS one. 2015;10:e0117982. doi: 10.1371/journal.pone.0117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cao S, Zhu P, Yu X, Chen J, Li J, Yan F, et al. Hydrogen sulfide attenuates brain edema in early brain injury after subarachnoid hemorrhage in rats: Possible involvement of MMP-9 induced blood-brain barrier disruption and AQP4 expression. Neuroscience letters. 2016;621:88–97. doi: 10.1016/j.neulet.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 171.Li T, Liu H, Xue H, Zhang J, Han X, Yan S, et al. Neuroprotective effects of hydrogen sulfide against early brain injury and secondary cognitive deficits following subarachnoid hemorrhage. Brain pathology. 2016 doi: 10.1111/bpa.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu H, Deng Y, Gao J, Liu Y, Li W, Shi J, et al. Sodium Hydrosulfide Attenuates Beta-Amyloid-Induced Cognitive Deficits and Neuroinflammation via Modulation of MAPK/NF-kappaB Pathway in Rats. Current Alzheimer research. 2015;12:673–683. doi: 10.2174/1567205012666150713102326. [DOI] [PubMed] [Google Scholar]
- 173.Tan H, Zou W, Jiang J, Tian Y, Xiao Z, Bi L, et al. Disturbance of hippocampal H2S generation contributes to CUMS-induced depression-like behavior: involvement in endoplasmic reticulum stress of hippocampus. Acta biochimica et biophysica Sinica. 2015;47:285–291. doi: 10.1093/abbs/gmv009. [DOI] [PubMed] [Google Scholar]
- 174.Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues in clinical neuroscience. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marutani E, Sakaguchi M, Chen W, Sasakura K, Liu J, Xian M, et al. Cytoprotective effects of hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonists are mediated by intracellular sulfane sulfur. MedChemComm. 2014;5:1577–1583. doi: 10.1039/C4MD00180J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang M, Zhu J, Pan Y, Dong J, Zhang L, Zhang X, et al. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson's disease. Journal of neuroscience research. 2015;93:487–494. doi: 10.1002/jnr.23504. [DOI] [PubMed] [Google Scholar]
- 177.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, et al. Sulfhydration mediates neuroprotective actions of parkin. Nature communications. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nava M, Martin-Drumel MA, Lopez CA, Crabtree KN, Womack CC, Nguyen TL, et al. Spontaneous and Selective Formation of HSNO, a Crucial Intermediate Linking H2S and Nitroso Chemistries. Journal of the American Chemical Society. 2016;138:11441–11444. doi: 10.1021/jacs.6b05886. [DOI] [PubMed] [Google Scholar]
- 179.Beltowski J. Hydrogen sulfide in pharmacology and medicine--An update. Pharmacological reports : PR. 2015;67:647–658. doi: 10.1016/j.pharep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 180.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, et al. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. Journal of the American Chemical Society. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, et al. Biochemical insight into physiological effects of H(2)S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. The Biochemical journal. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 182.Wang XY, Yang HW. Upregulation of CBS/H2S system contributes to asymmetric dimethylarginine-triggered protection against the neurotoxicity of glutamate to PC12 cells by inhibiting NOS/NO pathway. Experimental cell research. 2016;346:111–118. doi: 10.1016/j.yexcr.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 183.Lee HJ, Feliers D, Mariappan MM, Sataranatarajan K, Choudhury GG, Gorin Y, et al. Tadalafil Integrates Nitric Oxide-Hydrogen Sulfide Signaling to Inhibit High Glucose-induced Matrix Protein Synthesis in Podocytes. The Journal of biological chemistry. 2015;290:12014–12026. doi: 10.1074/jbc.M114.615377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kram L, Grambow E, Mueller-Graf F, Sorg H, Vollmar B. The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thrombosis research. 2013;132:e112–e117. doi: 10.1016/j.thromres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 185.Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nature cell biology. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]