Abstract
Background
Fecal incontinence (FI) is a prevalent but poorly recognized problem in the general population with profound negative effects on daily life. The prevalence of FI in IBS and its association with clinical, demographic and pathophysiological factors are largely unknown.
Methods
One US (n=304) and one Swedish (n=168) patient cohort fulfilling Rome III criteria for IBS completed Rome III diagnostic questions on FI and IBS symptoms, and questionnaires on IBS symptom severity, quality of life, anxiety and depression, and work productivity impairment. The patients also underwent assessments of colorectal sensitivity and motility.
Key Results
FI ≥ one day per month was reported by 19.7% (USA) and 13.7% (Sweden) of IBS patients. These proportions rose to 43.4% and 29.8% if patients with less frequent FI were included. FI prevalence was higher in older age groups, with a clear increase above age 40. IBS patients with FI reported greater overall IBS symptom severity, more frequent and loose stools, and greater urgency. Negative effects of FI on quality of life, psychological distress, and work productivity were demonstrated. No associations were found between colorectal physiology and FI.
Conclusions & Inferences
FI is common in IBS patients, and similar to previous general population reports, the major risk factors for FI in IBS are older age, rectal urgency, and loose, frequent stools. When IBS patients have comorbid FI, the impact on quality of life, psychological symptoms, and work impairment appears greater.
Keywords: Irritable bowel syndrome, fecal incontinence, functional GI disorders
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders (FGIDs) with a pooled world-wide prevalence of 11% in existing studies 1, but with large differences in prevalence figures due to methodological variance and heterogeneity between studies 2. IBS is defined by the Rome III criteria and is characterized by a combination of abdominal pain or discomfort and abnormal bowel habits 3. Apart from the characteristic IBS symptoms included in the diagnostic criteria, many patients with IBS also suffer from other troublesome symptoms and syndromes, including other FGIDs, non-gastrointestinal symptoms and psychiatric comorbid disorders 4-6. Among FGIDs, especially the overlap with functional dyspepsia is well documented 7-9, whereas the overlap with other conditions with disturbed gastrointestinal (GI) function is less well studied. Despite being a benign condition from a medical point of view, quality of life is substantially reduced in IBS patients 10, 11, and comorbid GI and non-GI conditions adds further to the disease burden and quality of life impairment 12.
Fecal incontinence, defined as unintentional loss of solid or liquid stool 13, is a common but often neglected problem in the population. Prevalence estimates for community-dwelling subjects range from 7 to 15%, with discrepancies between studies partly being explained by differences in survey methods, age range of included subjects, and definitions of fecal incontinence 13. In several studies increasing age, burden of general illness, rectal urgency and diarrhea have been identified as risk factors for fecal incontinence 14-17. When fecal incontinence is frequent or associated with larger amounts of stool loss, it can have a profound negative impact on quality of life 18-20, and often leads to embarrassment, isolation, reduced scope of life activities such as travel or social events, and psychological distress. Moreover, only a minority of individuals with fecal incontinence discuss this problem with their physician, leaving the problem unaddressed and untreated for many of those who suffer substantial adverse consequences 21-23.
In population-based studies IBS has been found to be a risk factor for fecal incontinence 14, 15. However, despite the fact that these two prevalent conditions constitute a substantial burden of the workload for gastroenterologists and GI surgeons, and lead to high costs for society 1, 2, 13, 24, 25, surprisingly few studies have assessed the overlap between IBS and fecal incontinence. Moreover, factors associated with coexistence of the conditions and their combined additive effect on the overall disease burden are incompletely understood. In the existing studies that have evaluated the prevalence of fecal incontinence in patients with IBS, the rates differ substantially, ranging from 6.2% to 57% 21, 26, 27. These seemingly discrepant results are likely in part attributable to differences in patient selection, definition of fecal incontinence and screening questions. These studies have also yielded mixed results regarding the association between fecal incontinence in IBS, IBS symptom severity, demographic factors, health care seeking, psychological factors, bowel habits and quality of life 21, 26, 27. Moreover, none of these studies have evaluated the association with key pathophysiological factors in IBS, such as visceral hypersensitivity and GI motor abnormalities 28-32.
Therefore, in this study we used two large, well-characterized cohorts of IBS patients from two western countries with different healthcare systems but comparable prevalence of IBS and fecal incontinence 1, 2, 17, 33, 34 to estimate and compare: 1) the prevalence of fecal incontinence in IBS; 2) the impact of fecal incontinence on quality of life and other measures of disease burden; and 3) demographic, clinical, and physiological predictors of fecal incontinence.
Materials and Methods
Subjects
We included subjects with IBS according to the Rome III criteria 3, who participated in two prospective studies assessing the relevance of pathophysiological factors for symptoms in IBS 28, 35-38. The patients in the first of these study cohorts were recruited from the outpatient clinic specialized in functional GI disorders at Sahlgrenska University Hospital in Gothenburg, Sweden. The majority of them were referred to the unit by their general practitioner or through self-referral. The diagnosis was based on a typical clinical presentation and additional investigations if considered necessary by the gastroenterologist (HT or MS), but most patients had already undergone sufficient examinations by the referring physician, and all patients met the Rome III criteria for IBS. IBS patients between 18 and 65 years were included. Exclusion criteria were: other GI disease(s) explaining the patient's symptoms; other severe disease(s) such as malignancy, severe heart disease, kidney disease, or neurological disease; symptoms indicating other severe disease(s) such as GI bleeding, weight loss or fever; severe psychiatric disease; a history of drug or alcohol abuse within 6 months prior to enrollment; or pregnancy at the time of the study.
The second cohort was patients in a research clinic specialized in functional GI disorders at the University of North Carolina in Chapel Hill, USA. The patients were 18 years or older (no upper limit), and were recruited by advertisements or physician referrals and screened by telephone before inclusion. They met the Rome III criteria for IBS, and had been diagnosed with IBS by a physician before enrollment, which was confirmed by means of an interview and examination conducted by a gastroenterologist. Exclusion criteria were: other GI disease(s) explaining the patient's symptoms, including GI resection (other than appendectomy or cholecystectomy), known inflammatory bowel disease, coeliac disease, lactose malabsorption; heart disease; diabetes mellitus; or pregnancy at the time of the study.
All patients in both study cohorts were given study-specific verbal and written information before giving their written consent to participate in the studies. The Regional Ethical Review Board in Gothenburg, and the Institutional Review Board of the University of North Carolina, respectively, approved the study prior to the start of patient enrollment.
Study Measures
Questionnaires
Demographics
Basic demographic information about participants was obtained by a study nurse / research coordinator at both sites, using a standardized case report forms. Of these variables, age, gender, height and weight (for calculation of body mass index) were used in the analyses in this study.
GI symptoms
The severity of IBS symptoms was evaluated at both sites with the widely used and validated questionnaire, IBS Severity Scoring System (IBS-SSS) 39. This questionnaire is based on five items; frequency and severity of abdominal pain, severity of abdominal distension, bowel habit dissatisfaction and interference of IBS with daily life. The questionnaire uses visual analogue scales and each item is scored 0-100, which yields a total score ranging from 0 to 500, with higher scores reflecting more severe symptoms. According to validated cut-off levels, the patients were divided into IBS severity groups of mild IBS (score of <175), moderate IBS (175-300), or severe IBS (>300). The patients also completed the Rome III diagnostic questionnaire for the adult functional GI disorders 40. For this study, we included select questions from this questionnaire with three purposes: 1) Questions in the IBS module to verify that the patients fulfilled the Rome III criteria for IBS; 2) Questions to characterize the presence and type of fecal incontinence / accidental bowel leakage in the last three months: frequency (never – less than one day a month – one day a month – two to three days a month – one day a week – more than one day a week – every day), amount (small - moderate - large), and composition of leakage (liquid/mucus only – stool only – both liquid/mucus and stool); and 3) Questions to characterize the bowel habit of the patient: frequent (≥ 4 / day) or infrequent (<3 / week) bowel movements, hard or lumpy stools, loose or watery stools and urgency (have to rush to the toilet to have a bowel movement) (response alternatives for all these questions: never or rarely – sometimes – often – most of the time – always). The information about the frequency of hard and lumpy stools, and loose and watery stools was also used for subgrouping of the IBS patients with the response of “sometimes” being used as the cut-off level (this is the appropriate equivalent of 25% of the time according to the Rome III committee) 3, 40.
Disease-specific quality of life
Different validated disease-specific quality of life instruments were used in the two cohorts. In the Swedish study, the Hahn et al Irritable Bowel Syndrome Quality of Life Questionnaire (IBSQOL) was used 41. This 30-item questionnaire measures nine quality of life domains found to be of relevance for IBS: emotional health, mental health, sleep, energy, physical functioning, food/diet, social functioning, physical role and sexual relations. Each scale score is transformed to a scale of 0-100, with 100 representing the best possible quality of life. In the US cohort, the Patrick and Drossman Irritable Bowel Syndrome Quality of Life scale (IBS-QOL) was used 42. This is a 34-item questionnaire that measures the impact of IBS on quality of life in eight domains; dysphoria, interference with activity, body image , health worry, food avoidance , social reaction, sexual, and relationships. The scores are transformed to a 0-100 scale, where 100 represents the best possible quality of life.
Psychological distress
Different validated questionnaires were also used to assess psychological distress by measuring the severity of anxiety and depression in the two countries. In the Swedish cohort, the widely used Hospital Anxiety and Depression scale (HADS) was used. This is a mood scale developed for use in non-psychiatric clinical settings to identify patients with psychological distress. It consists of 14 items, evenly divided into two subscales, one for anxiety and one for depression. It uses a 4-point Likert scale (0-3), which provides a minimum score of 0 (no symptoms) and a maximum score of 21 (maximal severity of symptoms) on each subscale. In the US sample the anxiety and depression subscales of the Brief Symptom Inventory-18 (BSI-18) were used 43. Each of these subscales consist of six items asking how much the patients were bothered by each of the symptoms, on a 0-4 scale ranging from “not bothered at all” to “extremely bothered”. The sum scores of the subscales were transformed to standardized scores, where the mean for the healthy population is 50 and each standard deviation is 10 (T scores). The standardized scores also adjust for sex differences.
Work productivity and Activity
This was only measured in the Swedish sample by using the Work Productivity and Activity Index – irritable bowel syndrome version (WPAI:IBS) 44. This questionnaire consists of six questions, and from these four metrics can be derived: absenteeism (the percentage of work time missed due to IBS in the past 7 days), presenteeism (the percentage of impairment suffered while at work due to IBS in the past 7 days), overall work impairment (the total percentage of missed time due to IBS-related absenteeism or presenteeism in the past 7 days), and activity impairment (the percentage of impairment suffered due to IBS during daily activities in the past 7 days). Each metric varies from 0 to 100%, with higher scores indicating greater impairment.
Physiologic measures
Colorectal sensitivity
Balloon distensions using an electronic barostat (Dual Drive Barostat, Distender Series II; G&J Electronics INC, Toronto, Ontario, Canada) were performed at both sites to assess colorectal sensitivity. The Swedish patients underwent a rectal barostat study using an ascending methods of limits ramp distension protocol starting at 0 mmHg and increasing in steps of 4mmHg every minute up to the pain threshold or to a maximum balloon pressure of 60 mmHg. Thresholds for first sensation, urgency, discomfort and pain were assessed 45. In the US patients, a colonic barostat test (where the test balloon was placed in the sigmoid colon with fluoroscopy guidance) was performed in the earliest of the consecutive patients (n=68), whereas the remainder underwent a rectal barostat study 28. Both tests used an ascending methods of limits paradigm with phasic distensions of 30 sec duration separated by 30 sec rest intervals with the balloon at the operating pressure (i.e., the minimum pressure needed for inflating the balloon). The distensions started 2 mmHg above the operating pressure and increased progressively in 2 mmHg steps until the subject reported pain of moderate intensity (3 on a 0-4 scale) or until a balloon pressure of 48 mmHg was reached. Thresholds for defecatory urge and pain were determined. To be able to combine the thresholds from the colonic and rectal barostat studies in the US sample, Z-scores (defining how many standard deviations a value is from the mean) were calculated. For the analyses in this study only the urge and pain thresholds from both the Swedish and US cohorts were used. Furthermore, as a measure of smooth muscle tone in the US cohort, average balloon volume at the operating pressure was analyzed (Z-scores)28, and in the Swedish cohort the balloon pressure at half of the maximum observed volume (P1/2) was used as a measure of compliance45.
Colorectal motility
The Swedish patients underwent a colonic transit time measurement by ingesting 10 radiopaque markers per day during six consecutive days, and on the morning of the seventh day the remaining markers were counted using fluoroscopy (Exposcop 7000 Compact; Ziemh GmbH; Nüremberg, Germany) 30. The colonic transit time in days was obtained by dividing the number of retained markers with the daily dose, i.e. 10. In the US IBS cohort colorectal motility was studied as part of colon and rectal barostat protocols. A water-perfused manometry catheter (Model C7-CB-00256, Mui Scientific, Ontario, Canada) and a physiologic recorder (Sandhill Scientific, Highlands Ranch, Colorado, USA) was used to record phasic motility 2.5 and 5 cm both proximal and distal to the balloon during 10 min balloon distension at 20 mmHg above the operating pressure, and for 30 min following an 810 kcal meal 28. A motility index averaging the phasic contractility during the distension and after the meal, respectively, was calculated using dedicated software (Polygram, Lower GI Edition, Version 5.06; Synectics Medical, now Medtronic Inc., Minneapolis, MN).
Data analysis
Statistical analyses were performed with the software package IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY). Data are presented as mean ± SD and proportions (%). As a first step we analyzed the proportion of IBS subjects who reported accidental bowel leakage with different frequencies, the amount leaked and the composition of the bowel leakage. Thereafter, we compared IBS patients with fecal incontinence (accidental bowel leakage ≥ one day per month) 17 to IBS patients without fecal incontinence (accidental bowel leakage never or < one day/month). Chi-squared test was used to analyze the association of fecal incontinence to categorical data: gender, age groups (≤30, 31-40, 41-50, >50 years); IBS subgroup; and bowel habits (Rome III questionnaire – categories based on frequencies and binned into three groups: never or rarely; sometimes or often; most of the time or always). Continuous data from questionnaires and physiological tests were analyzed with Student's t-test. In case of borderline significance, further exploratory analyses were performed using other cut-offs to define fecal incontinence. In order to determine factors independently associated with fecal incontinence (“risk factors”), we performed binary logistic regression analyses in the two cohorts with fecal incontinence (accidental bowel leakage ≥ one day per month) as the dependent variable, and factors univariately associated with fecal incontinence as independent variables. Statistical significance was accepted at p<0.05.
Results
Subjects
We included 168 IBS patients fulfilling the Rome III criteria 3 in the Swedish cohort and 304 Rome III positive IBS patients in the US cohort. Demographic and disease related information for the two cohorts is displayed in table 1. As can be seen, the gender distribution and the mean age were comparable between cohorts, but the age range was wider in the US cohort as only subjects aged 18-65 years were included in the Swedish cohort. The patients in the Swedish cohort had more severe IBS symptoms and slightly lower BMI than the US patients, but the distribution of IBS subtypes based on the Rome III diagnostic questionnaire was similar in both cohorts with a clear predominance of patients with IBS-M. In the Swedish cohort all patients were Caucasian, whereas the US cohort was 68.5% Caucasian, 28.1% African American, 1.3% Asian, and 2.0% American Indian / Alaska natives.
Table 1.
Demographic and disease related information.
| US Cohort (n=304) | Swedish cohort (n=168) | |
|---|---|---|
| Gender (Female/Male, %) | 77% / 23% | 70% / 30% |
| Age (mean±SD, range) | 36±14 (18-73) | 34±11 (18-60) |
| Body Mass Index (kg/m2) | 26.8±6.1 | 23.5±4.0 |
| IBS-SSS (mean±SD) | 271±89 | 304±91 |
| IBS severity (IBS-SSS; %) | ||
| Mild | 12.0% | 6.8% |
| Moderate | 48.7% | 39.8% |
| Severe | 39.3% | 53.4% |
| IBS Subtype (Rome III; %) | ||
| IBS-C | 17.3% | 10.1% |
| IBS-D | 23.3% | 23.8% |
| IBS-M | 59.3% | 66.1% |
Fecal incontinence in IBS
As can be seen in Figure 1, accidental bowel leakage ≥ one day a month was reported by 19.7% of IBS patients in the US and 13.7% in Sweden. These proportions rose to 43.4% in the US and 29.8% in Sweden if patients with bowel leakage less than one day a month in the last three months were included. The majority of the subjects with accidental bowel leakage reported leaking only small amounts (staining only) (US 79%; Sweden 86%), whereas 19% in the US and 12% in Sweden reported moderate amounts of leaking (more than staining but less than a full bowel movement). In both cohorts only 2% of subjects reporting accidental bowel leakage lost large amounts, i.e. a full bowel movement. Among those with any bowel leakage, the composition of the leakage was described as liquid/mucus only by 47% in the US and 30% in Sweden, as stool only by 18% in the US and 24% in Sweden, and as both liquid/mucus and stool by 35% in the US and 46% in Sweden. For further analyses fecal incontinence was defined as accidental bowel leakage ≥ one day a month 17, unless stated otherwise.
Figure 1.
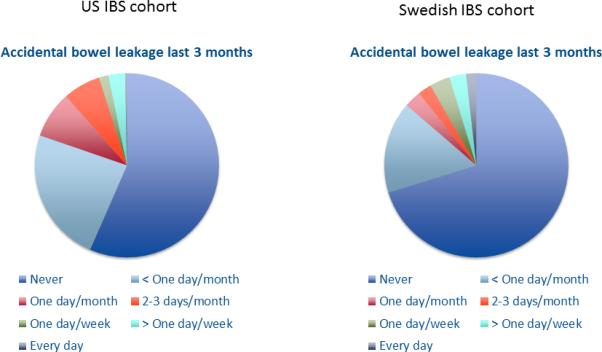
Response to the question: “In the last three months, how often have you accidently leaked liquid or solid stool?” in the US and Swedish IBS cohorts.
Fecal incontinence and demographics in IBS
The mean age was higher in US IBS patients with fecal incontinence than in patients without fecal incontinence (44.7±13.0 vs. 34.1±12.7 years; p>0.0001), with a non-significant tendency in the same direction in the Swedish cohort (37.0±12.2 vs 33.2±11.2 years; p=0.1). However, when comparing Swedish IBS patients who reported accidental bowel leakage ever (including “less than one day a month”) with patients who never reported leakage, patients with leakage were older than those without leakage (38.0±12.4 vs. 32.0±10.6 years; p<0.002). Dividing the patients into age groups demonstrated a clear increase in prevalence of fecal incontinence above age 40 in both the US (p<0.0001) and Sweden (p<0.05) (Figure 2), and again the differences were more prominent in the Swedish sample if a less restrictive cut-off for defining fecal incontinence (ever vs. never accidental bowel leakage) was used (ever FI prevalence: <30 years: 20.0%; 31-40 years: 28.2 %; 41-50 years 57.7%; >50 years: 38.9%; p=0.002). Fecal incontinence with moderate or large amounts of leakage rarely occurred below age 40 (Figure 2). There were no gender differences in fecal incontinence prevalence in the US (19% of females vs. 21% of males; p=0.69) or Swedish IBS patients (13% of females vs. 16% of males; p=0.69). In the US patients body mass index was higher in IBS patients with fecal incontinence compared to those without fecal incontinence (28.4±6.8 vs. 26.4±5.9 kg/m2; p=0.03), but this was not the case in the Swedish cohort (24.5±2.9 vs. 23.4±4.2 kg/m2; p=0.22). In the US cohort fecal incontinence was equally common in Caucasian (42/165; 20.3%) and African American (17/68; 20.0%) IBS patients.
Figure 2.
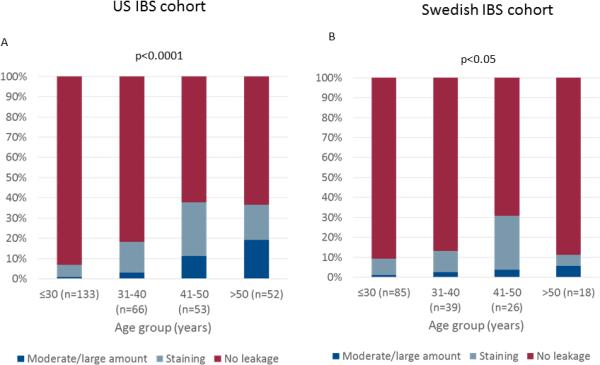
Association between fecal incontinence, including the amount of leakage, and age groups in US (A) and Swedish (B) IBS patients.
Fecal incontinence and other GI symptoms in IBS
IBS patients with fecal incontinence reported greater overall IBS symptom severity on the IBS-SSS in both the US (293±90 vs 265±88; p=0.03) and Swedish (348±86 vs. 290±90; p=0.02) cohorts. Assessment of bowel habit characteristics (Figure 3) showed that in both cohorts, fecal incontinence was significantly associated with heightened frequency of diarrhea-type symptoms: having more than 4 bowel movements per day (US: p<0.0001; Sweden: p=0.04), loose/watery stools (US: p<0.0001; Sweden: p=0.002), and urgency (US: p<0.0001; Sweden: p=0.005). Conversely, a tendency was seen only in US patients for IBS patients with fecal incontinence to have less frequent constipation-type symptoms: less than 3 bowel movements / week (US: p=0.07; Sweden: p=0.86) and hard/lumpy stools (US p=0.03; Sweden: p=0.76). Fecal incontinence was numerically most common in patients with IBS-D subtype, but this reached significance only in the US cohort (US: IBS-D: 31.4%; IBS-M 19.7%; IBS-C: 5.8%; p=0.002; Sweden: IBS-D: 17.5%; IBS-M: 13.5%; IBS-C 5.9%; p=0.5).
Figure 3.
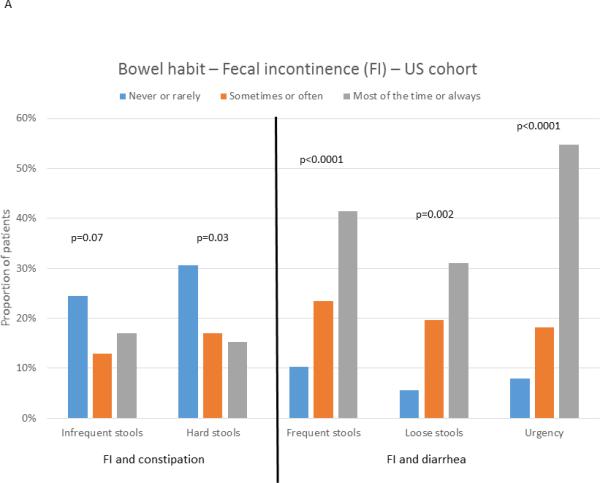
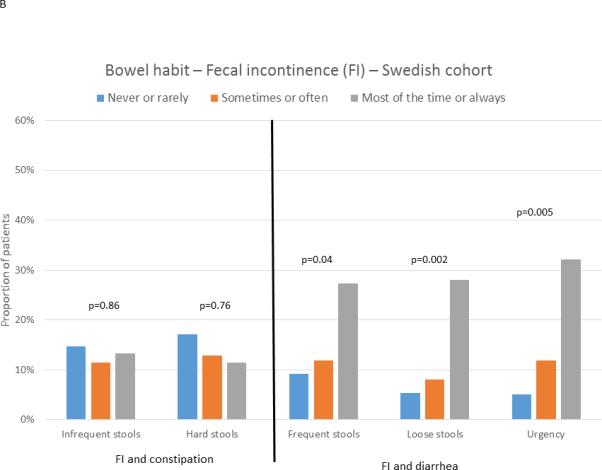
Association between the presence of fecal incontinence (FI) and frequency of bowel habit characteristics in US (A) and Swedish (B) IBS patients. The bars show the proportion of subjects reporting a specific frequency of a bowel habit characteristics who also report fecal incontinence (accidental bowel leakage at least one day a month).
Fecal incontinence and psychological distress, quality of life and work productivity in IBS
In the Swedish cohort, IBS patients with fecal incontinence had more anxiety (11.3±4.9 vs. 7.9±4.2; p=0.001) and depression (7.7±3.9 vs. 4.9±3.4; p<0.0001) on the HADS compared to patients without fecal incontinence, but this was not seen in the US cohort (BSI-18 anxiety: 50.9±9.9 vs. 51.9±10.4; p=0.5; BSI-18 depression: 51.8±10.9 vs. 52.6±10.8; p=0.6). Disease specific quality of life was lower for seven of ten quality of life domains on the IBSQOL in Swedish IBS patients with fecal incontinence, and for interference with activities and body image domains on the IBS-QOL in US IBS patients, as can be seen in Figure 4. In the Swedish cohort work productivity and activity measures of the WPAI:IBS showed greater impairment in patients with fecal incontinence (Figure 5); this information was not available in the US cohort.
Figure 4.
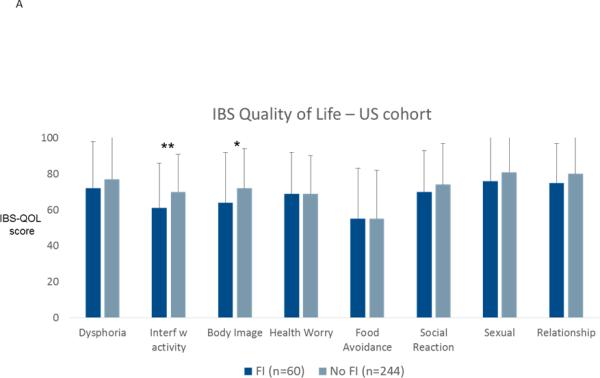
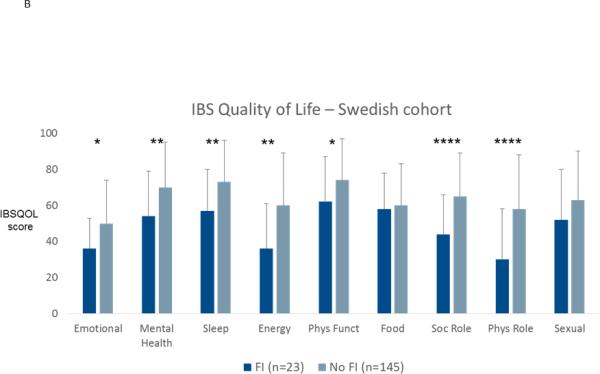
Disease-specific quality of life in patients with or without fecal incontinence (FI) in the US cohort (A) and in the Swedish cohort (B). * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Figure 5.
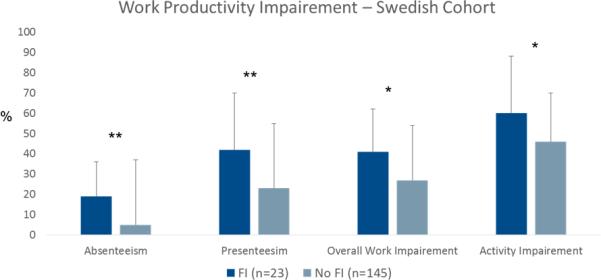
Work productivity and activity measured with WPAI:IBS in Swedish IBS patients with or without fecal incontinence (FI). * p<0.05; ** p<0.01;
Fecal incontinence and colorectal physiology in IBS
- In both cohorts patients underwent barostat tests to evaluate colorectal sensitivity (Table 2). There were no differences in urge or pain thresholds between patients with vs. without fecal incontinence in either sample. In the US cohort we also measured colorectal phasic motility response to stimuli and found no differences between patients with and without fecal incontinence. Moreover, smooth muscle tone (US cohort) and compliance (Swedish patients) were similar in both patient groups (Table 2). Colonic transit time was measured in the Swedish IBS patients and it did not differ between patients with and without fecal incontinence.
Table 2.
Colorectal physiologic measures
| US Cohort | FI (n=60) | No FI (n=244) | p value |
|---|---|---|---|
| Colorectal urge threshold (Z-score) | 0.02±1.2 | −0.03±1.0 | 0.9 |
| Colorectal pain threshold (Z-score) | −0.11±1.1 | −0.04±1.0 | 0.7 |
| Smooth muscle tone (Z-score) | −0.12±0.9 | −0.02±0.9 | 0.5 |
| Motility index, distention | 964±960 | 961±574 | 0.9 |
| Motility index, post-meal | 692±465 | 594±501 | 0.2 |
| Swedish cohort | FI (n=23) | No FI (n=145) | p-value |
|---|---|---|---|
| Rectal urge threshold (mmHg) | 18.5±7.2 | 19.5±7.0 | 0.5 |
| Rectal pain threshold (mmHg) | 26.7±10.2 | 27.2±8.6 | 0.8 |
| Compliance (P1/2) (mmHg) | 16.1±6.0 | 15.3±5.5 | 0.5 |
| Colonic transit time (days) | 1.4±1.1 | 1.5±1.0 | 0.7 |
Factors independently associated with fecal incontinence
Factors univariately associated with reporting fecal incontinence (accidental bowel leakage ≥ one day a month) were thereafter entered into binary logistic regression models in the two cohorts (US cohort: age; body mass index; IBS severity (IBS-SSS); frequent stools, loose stools and urgency (Rome III questionnaire). Swedish cohort: age; IBS severity (IBS-SSS); frequent stools, loose stools and urgency (Rome III questionnaire); anxiety and depression (HAD). In the US cohort urgency (β=1.80 (95% CI 1.14-2.83), p=0.01) and age (β=1.06 (95% CI 1.04-1.09) were found to be independently associated with fecal incontinence (Nagelkerke R2=0.30), whereas only urgency (β=1.99 (95% CI 1.14-2.83), p<0.05) made a unique statistically significant contribution to the model in Swedish IBS patients (Nagelkerke R2=0.32).
Discussion
In this study we have demonstrated that a substantial proportion, 14-20%, of Swedish and US IBS patients, who predominantly seek health care for their IBS symptoms, also suffer from fecal incontinence, which appears higher than the rates typically reported in general population samples 17, 33, 34. Moreover, we found that fecal incontinence adds to the burden of IBS, with significant adverse impact seen on quality of life, psychological symptoms, and work productivity. The risk factors for fecal incontinence in both US and Swedish patients with IBS were similar to those reported for the general population 14-17.
One of the problems when comparing fecal incontinence prevalence between studies is the use of different definitions and different questionnaires. In our study, both cohorts used the same questionnaire, Rome III diagnostic questionnaire for the adult functional GI disorders 40, and fecal incontinence was defined as accidental bowel leakage at least once per month, a definition that has been used in a recent US national survey 17 and also fits with the current definition of FGIDs 3, 40, 46, 47. The prevalence of fecal incontinence using this definition differed between the cohorts, with a somewhat higher prevalence in US patients with IBS. This is hard to explain with the existing data, but a wider age range in the US cohort with more older subjects may be one explanation. Even though prevalence of fecal incontinence was associated with IBS symptom severity, differences in prevalence rates between the cohorts cannot be explained by more severe IBS symptoms in the US cohort because the pattern was the opposite. Differences in health care systems, health care seeking behavior and willingness to participate in studies may have influenced our results, since our data are not population-based, but rather collected from IBS patients who were willing to participate in research studies on IBS pathophysiology. When our prevalence rates are compared with relevant studies of general population samples with of equivalent age, the prevalence of fecal incontinence appears higher than can be expected for a random population sample. In the US sample, the 19.7% rate of fecal incontinence in our IBS patients was more than double the 8.3% rate reported in a nation-wide U.S. population survey of 4,308 individuals that used the same definition of fecal incontinence, i.e. accidental bowel leakage at least one day a month 17. Although, slightly different definitions and questions were used in a Swedish population-based survey, the fecal incontinence rates in the Swedish IBS patients in the present study also appear higher than in the general population 34. From a clinical point of view it seems highly relevant that one in every five to six IBS patients accidentally leaks stool each month, and if a less strict definition is used (also including accidental bowel leakage less than one day a month in the last three months), three to four out of every ten patients have fecal incontinence. Based on the authors’ experiences, these high prevalence rates are likely unknown to many clinicians managing IBS patients.
The few existing studies that have addressed the prevalence of fecal incontinence in IBS have reported broadly varied prevalence rates 21, 26, 27. In our study we found that 14-20% of our IBS patients reported accidental bowel leakage at least one day a month in the last three months. This seems very different from the prevalence rate of fecal incontinence of 57% in a large clinical IBS sample in the study by Atarodi and colleagues 21. However, in that study patients with accidental bowel leakage less than once a year were included in their definition of fecal incontinence, and 24% of those had mild (“once a year or less”), 35% had moderate (“once a month or less but more than once a year”) , and 32% had severe fecal incontinence (“once a week or more or nocturnal”). Therefore, these findings are actually not so different from the findings in the present study, where we used a more stringent minimal frequency definition for identifying fecal incontinence cases (i.e., at least once in the last month). The other two studies that have addressed the overlap between IBS and fecal incontinence found that 18-23% of patients with IBS in a health maintenance organization (HMO) 27, and 6-20% of subjects with “bowel dysfunction compatible with IBS” in a survey of students reported that they “ever lost control of their bowels (including soiling of underwear)” 26. These studies are also difficult to compare directly with our findings, as the age range of subjects, the study settings, and incontinence definitions are very different
Perhaps of even greater importance than the prevalence rates per se, is the finding that coexistence of IBS and fecal incontinence negatively influence quality of life, and work productivity and activity, i.e. the presence of fecal incontinence seem to potentiate the already substantial disease burden in IBS. Work productivity and activity is reduced in IBS patients 48, 49, which leads to high costs for society 50 and reduced quality of life for patients 48. In our Swedish sample we used the validated WPAI:IBS questionnaire to assess work productivity and activity 44 and could demonstrate increased impairment in all four outcome measures with this questionnaire (absenteeism, presenteeism, overall work impairment and activity impairment) in patients who had both IBS and fecal incontinence. Moreover, although there is no mortality or medically severe complications associated with IBS, the quality of life in IBS patients is reduced to the same extent as in many other chronic conditions associated with severe morbidity and mortality 10. In both our IBS cohorts, the presence of fecal incontinence was associated with even greater impairment in quality of life, and in the US cohort this impairment was associated with quantity of incontinence – i.e., leaking moderate to large amounts of fecal material. These findings are in agreement with a study on patients with fecal incontinence where the presence of IBS was found to substantially impair quality of life 51, and also consistent with several other studies demonstrating that fecal incontinence is associated with reduced quality of life 18, 19. Regarding the association with psychological symptoms, there were differences between our cohorts. In the Swedish cohort, patients with fecal incontinence and IBS had more severe psychological distress than patients with IBS only, but this was not seen in the US cohort. Unfortunately, different questionnaires to assess psychological distress were used in the two studies, so direct comparisons are not possible, but factors related to the questionnaires per se, may explain differences between the cohorts. Taken together, the findings on quality of life, work productivity and psychological distress in our cohorts clearly demonstrates the additive burden fecal incontinence poses for patients with IBS, and highlights the importance of addressing and managing this symptom adequately in IBS patients in order to improve quality of life, psychological well-being, and work productivity.
In population-based studies several risk factors, or factors associated with fecal incontinence have been identified. Bowel habit disturbances, particularly diarrhea, rectal urgency, increasing age, being overweight, decreased physical activity, obstetrical injury, and poor general health are all factors that have been found to be associated with the presence and severity of fecal incontinence in several studies 14, 15, 17, 52. In our study we could confirm that several of these factors also seem to be associated with fecal incontinence in IBS. Loose and frequent stools, as well as urgency were clearly associated with fecal incontinence in both cohorts. Increasing age was also associated with fecal incontinence, with a clear increase in the prevalence above age 40, and moderate or large amount of leakage starting to appear above that age. Based on these data, it clearly seems as if having IBS is a risk factor for developing fecal incontinence when you grow older. In the Swedish cohort a seemingly paradoxical decrease in the proportion of subjects with fecal incontinence was seen in the group of patients > 50 years of age (Figure 2), which can be explained by the small number of subjects in this group (n=18), as well as by a smaller proportion of patients in this age group reporting diarrhea predominance (2/18 patients) compared with the other age groups (p<0.05). Moreover, the oldest patient in the Swedish cohort was 60 years compared with 73 years in the US cohort, which may be one explanation for the stronger association between age and fecal incontinence in US patients. Less consistent results were seen for body mass index, with an association with fecal incontinence only in the US sample. No gender differences in fecal incontinence prevalence were noted, which is consistent with previous population based studies 17, 49. Moreover, we could not detect an association with colorectal sensorimotor function in either of the two cohort.
There are obvious strengths with our studies, such as large patient cohorts, largely similar findings from studying patients in two different countries with different healthcare systems, careful and comprehensive phenotypic characterization with widely used and validated questionnaires, and uniform methodology. However, there are also important limitations. The patients were seen at secondary / tertiary care centers, so the findings cannot be generalized to IBS patients in primary care. Moreover, the patients were primarily included because of their IBS symptoms, which may have led to an underestimation of the true prevalence and severity of fecal incontinence in patients with IBS, since IBS patients with more dominant and severe fecal incontinence may not volunteer for studies where the main focus lies on IBS symptoms and the underlying pathophysiology. Furthermore, our failure to see physiological differences between IBS patients with fecal incontinence compared to those without may be a consequence of the fact that we did not assess anal function with anorectal manometry, and we do not have information about obstetrical injuries. Moreover, as the IBS patients were from two different countries with different health care systems and social conventions, it is possible that cultural taboos about discussing toileting behavior and incontinence may differ between the countries and influence our prevalence estimates for fecal incontinence. However, there are no major differences in prevalence rates in population-based studies on fecal incontinence between Sweden and the US 1, 2, 17, 33, 34. Therefore, we see the inclusion of two cohorts from different countries as a strength and an opportunity to test the replicability of the results across two large samples. The fact that there were many similarities between the findings in the two cohorts supports the generalizability of our findings in IBS – at least in regard to secondary or tertiary care patients.
To conclude, we have demonstrated that fecal incontinence is present in a sizeable proportion of patients who primarily seek health care for IBS in Sweden and the US. The major risk factors for fecal incontinence seem to be similar in IBS and in the general population: older age, rectal urgency, and loose, frequent stools. When IBS patients have comorbid fecal incontinence, the impact on quality of life, psychological symptoms, and work impairment appears greater than in IBS alone. Therefore, including questions about fecal incontinence when taking clinical history in IBS may guide management and improve clinical outcomes. Future studies should determine the underlying physiologic abnormalities for fecal incontinence in IBS through careful anorectal function assessment, and ideally be population-based.
Key Points.
Fecal incontinence is prevalent in the general population and has profound negative effects on daily life. Few studies have assessed the overlap between IBS and fecal incontinence.
Fecal incontinence was reported by 14 to 20% of IBS patients, and it was associated with loose, frequent stools, urgency, and adverse impact on quality of life, psychological symptoms, and work productivity.
Clinicians managing patients with IBS should include questions about fecal incontinence when taking the clinical history since this may guide management and improve clinical outcomes.
Acknowledgments
Funding:
This study was supported by NIDDK (grant RO1 DK31369), the Swedish Medical Research Council (grants 13409, 21691 and 21692), AFA Insurance, an unrestricted grant from Ferring Pharmaceuticals, and by the Faculty of Medicine, University of Gothenburg.
Abbreviations
- IBS
Irritable bowel syndrome
- FGIDs
Functional Gastrointestinal Disorders
- FI
Fecal Incontinence
- GI
Gastrointestinal
- IBS-SSS
IBS Severity Scoring System
- IBSQOL
Irritable Bowel Syndrome Quality of Life Questionnaire
- IBS-QOL
Irritable Bowel Syndrome Quality of Life scale
- HADS
Hospital Anxiety and Depression scale
- BSI-18
Brief Symptom Inventory-18
- WPAI:IBS
Work Productivity and Activity Index – irritable bowel syndrome version
Footnotes
Author contributions:
Guarantor of article: MS
MS performed the statistical analysis of the data, and wrote the manuscript.
WEW, OSP & MS designed the study and interpreted the results
All authors collected the data and revised the manuscript critically.
All authors have approved the final version of the article, including the authorship list.
Disclosures:
Hans Törnblom has served as Consultant/Advisory Board member for Almirall, Danone and Shire. Magnus Simrén has received unrestricted research grants from Danone, and Ferring Pharmaceuticals, and served as a Consultant/ Advisory Board member for AstraZeneca, Danone, Nestlé, Chr Hansen, Almirall, Allergan, Albireo, Glycom and Shire, and as a speaker for Tillotts, Takeda, Shire and Almirall.
Olafur Palsson has received salary support from a research grants from Takeda Pharmaceuticals and Salix Pharmaceuticals and from a consulting agreement with Ironwood Pharmaceuticals and an educational grant provided by Takeda Pharmaceuticals, and received a speaker honorarium in an educational program supported by Ironwood Pharmaceuticals and Takeda Pharmaceuticals. Hans Törnblom has served as Consultant/Advisory Board member for Almirall, Danone and Shire. William Whitehead received research grants from Takeda, Ironwood, Salix, and the Rome Foundation; served as a consultant to Biomerica USA, Ono Pharmaceuticals, and Ferring; and received unrestricted educational grants from Takeda and Ferring.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721. e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2016 doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Henningsen P, Herzog W. Irritable bowel syndrome and somatoform disorders. J Psychosom Res. 2008;64(6):625–9. doi: 10.1016/j.jpsychores.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Jerndal P, Ringstrom G, Agerforz P, et al. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22(6):646–e179. doi: 10.1111/j.1365-2982.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 7.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99(6):1152–9. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut. 1998;42(5):690–5. doi: 10.1136/gut.42.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Oudenhove L, Tornblom H, Storsrud S, Tack J, Simren M. Depression and Somatization Are Associated With Increased Postprandial Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119(3):654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 11.Motzer SA, Hertig V, Jarrett M, Heitkemper MM. Sense of coherence and quality of life in women with and without irritable bowel syndrome. Nurs Res. 2003;52(5):329–37. doi: 10.1097/00006199-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Vu J, Kushnir V, Cassell B, Gyawali CP, Sayuk GS. The impact of psychiatric and extraintestinal comorbidity on quality of life and bowel symptom burden in functional GI disorders. Neurogastroenterol Motil. 2014;26(9):1323–32. doi: 10.1111/nmo.12396. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110(1):127–36. doi: 10.1038/ajg.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101(6):1305–12. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139(5):1559–66. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De La Luz Nieto M, Wu JM, Matthews C, Whitehead WE, Markland AD. Factors associated with fecal incontinence in a nationally representative sample of diabetic women. Int Urogynecol J. 2015;26(10):1483–8. doi: 10.1007/s00192-015-2730-9. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–7. 517, e1–2. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4(8):1004–9. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Damon H, Schott AM, Barth X, et al. Clinical characteristics and quality of life in a cohort of 621 patients with faecal incontinence. Int J Colorectal Dis. 2008;23(9):845–51. doi: 10.1007/s00384-008-0489-x. [DOI] [PubMed] [Google Scholar]
- 20.Kunduru L, Kim SM, Heymen S, Whitehead WE. Factors that affect consultation and screening for fecal incontinence. Clin Gastroenterol Hepatol. 2015;13(4):709–16. doi: 10.1016/j.cgh.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atarodi S, Rafieian S, Whorwell PJ. Faecal incontinence-the hidden scourge of irritable bowel syndrome: a cross-sectional study. BMJ Open Gastroenterol. 2014;1(1):e000002. doi: 10.1136/bmjgast-2014-000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunivan GC, Heymen S, Palsson OS, et al. Fecal incontinence in primary care: prevalence, diagnosis, and health care utilization. Am J Obstet Gynecol. 2010;202(5):493, e1–6. doi: 10.1016/j.ajog.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawes SK, Ahmad A. Fecal Incontinence: a woman's view. Am J Gastroenterol. 2006;101(12 Suppl):S610–7. doi: 10.1111/j.1572-0241.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 24.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149(7):1731–1741. e3. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Menees SB, Zochowski MK, Fenner DE. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55(5):586–98. doi: 10.1097/DCR.0b013e31823dfd6d. [DOI] [PubMed] [Google Scholar]
- 26.Drossman DA, Sandler RS, Broom CM, McKee DC. Urgency and fecal soiling in people with bowel dysfunction. Dig Dis Sci. 1986;31(11):1221–5. doi: 10.1007/BF01296523. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth GF, Wolde-Tsadik G. Irritable bowel-type symptoms in HMO examinees. Prevalence, demographics, and clinical correlates. Dig Dis Sci. 1993;38(9):1581–9. doi: 10.1007/BF01303163. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa M, Palsson OS, Thiwan SI, et al. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103(10):2550–61. doi: 10.1111/j.1572-0241.2008.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133(4):1113–23. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Tornblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simren M. Colonic transit time and IBS symptoms: what's the link? Am J Gastroenterol. 2012;107(5):754–60. doi: 10.1038/ajg.2012.5. [DOI] [PubMed] [Google Scholar]
- 31.Tornblom H, Van Oudenhove L, Tack J, Simren M. Interaction between preprandial and postprandial rectal sensory and motor abnormalities in IBS. Gut. 2014;63(9):1441–9. doi: 10.1136/gutjnl-2013-305853. [DOI] [PubMed] [Google Scholar]
- 32.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25(6):404–13. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 33.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129(1):42–9. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Walter S, Hallbook O, Gotthard R, Bergmark M, Sjodahl R. A population-based study on bowel habits in a Swedish community: prevalence of faecal incontinence and constipation. Scand J Gastroenterol. 2002;37(8):911–6. doi: 10.1080/003655202760230865. [DOI] [PubMed] [Google Scholar]
- 35.Grover M, Kanazawa M, Palsson OS, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20(9):998–1008. doi: 10.1111/j.1365-2982.2008.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanazawa M, Palsson OS, van Tilburg MA, Gangarosa LM, Fukudo S, Whitehead WE. Motility response to colonic distention is increased in postinfectious irritable bowel syndrome (PI IBS). Neurogastroenterol Motil. 2014;26(5):696–704. doi: 10.1111/nmo.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Neve B, Brazeilles R, Derrien M, et al. Lactulose Challenge Determines Visceral Sensitivity and Severity of Symptoms in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2016;14(2):226–233. e3. doi: 10.1016/j.cgh.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 38.van Tilburg MA, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74(6):486–92. doi: 10.1016/j.jpsychores.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 40.Whitehead W,E, Team VW, Committe RQ. Development and Validation of the Rome III Diagnostic Questionnaire. In: Drossman DA, Corazziari E, Delvaux M, et al.Rome III, editors. The Functional Gastrointestinal Disorders. Third ed Degnon Associates, Inc.; McLean, Virginia: 2006. pp. 835–853. [Google Scholar]
- 41.Hahn BA, Kirchdoerfer LJ, Fullerton S, Mayer E. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(3):547–52. doi: 10.1046/j.1365-2036.1997.00168.x. [DOI] [PubMed] [Google Scholar]
- 42.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 43.Derogatis LR. BSI 18 Brief Symptom Inventory 18: administration, scoring, and procedures manual. NCSPearson Inc; 2000. [Google Scholar]
- 44.Reilly MC, Bracco A, Ricci JF, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire--irritable bowel syndrome version (WPAI:IBS). Aliment Pharmacol Ther. 2004;20(4):459–67. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 45.Cremonini F, Houghton LA, Camilleri M, et al. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil. 2005;17(6):810–20. doi: 10.1111/j.1365-2982.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- 46.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130(5):1510–8. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 47.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Dean BB, Aguilar D, Barghout V, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11(1 Suppl):S17–26. [PubMed] [Google Scholar]
- 49.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 50.Cash B, Sullivan S, Barghout V. Total costs of IBS: employer and managed care perspective. Am J Manag Care. 2005;11(1 Suppl):S7–16. [PubMed] [Google Scholar]
- 51.Walter S, Hjortswang H, Holmgren K, Hallbook O. Association between bowel symptoms, symptom severity, and quality of life in Swedish patients with fecal incontinence. Scand J Gastroenterol. 2011;46(1):6–12. doi: 10.3109/00365521.2010.513059. [DOI] [PubMed] [Google Scholar]
- 52.Bharucha AE, Seide BM, Zinsmeister AR, Melton LJ., 3rd Relation of bowel habits to fecal incontinence in women. Am J Gastroenterol. 2008;103(6):1470–5. doi: 10.1111/j.1572-0241.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


