Abstract
Background
Sleep-disordered breathing, particularly central sleep apnea (CSA), is highly prevalent in heart failure (HF) and an independent prognostic marker. We assessed the hypothesis that an increased hypoxemic burden during sleep may have greater prognostic value than the frequency of apneic and hypopneic episodes.
Methods and Results
We prospectively conducted overnight cardiorespiratory polygraphy on consecutive HF patients referred to our hospital between 2008 and 2011. We studied CSA defined by an apnea-hypopnea index (AHI) of ≥5 events/h, whereby >75% of all events were central in origin. We determined the AHI, proportion of the sleep time with an SpO2<90% (T90%), and percent of the recording time that 4% desaturation events occurred (4%POD). We studied 112 HF patients with either systolic or diastolic dysfunction. During a follow-up period of 37±25 months, 32 patients (29%) died. Non-survivors had a higher 4%POD compared to survivors (11±6.4 vs. 19±13%, p=0.001), but did not differ significantly regarding the AHI and T90% compared to survivors. An adjusted logistic regression analysis revealed that the 4%POD was the best independent predictor of mortality.
Conclusion
The 4%POD, a novel metric for the nocturnal hypoxemic burden, is an independent prognostic marker in HF patients affected by CSA.
Keywords: sleep, heart failure, hypoxia and mortality
Introduction
Heart failure is a major and increasing public health burden, and carries significant morbidity and mortality (1). Better risk stratification and targeting of clinical resources might lead to an improvement in survival. Sleep-disordered breathing (SDB), both in the form of obstructive sleep apnea (OSA) and central sleep apnea (CSA) are comorbid conditions in up to 50% of heart failure patients (2–4) and have implications for heart failure progression and prognosis (5–8).
In CSA, recurrent episodes of hypoxemic followed by hyperpnea are associated with periodic ischemia/reoxygenation, arousals, and elevations in sympathetic activity (9–11). Unlike OSA, changes in intrathoracic pressure are only modest in CSA. Thus, the adverse effects on CSA are mainly mediated by increased sympathetic activity rather than the ventricular afterload. In an early report by Naughton et al., (12) increased sympathetic activation in patients with heart failure and CSA was shown to be related to the severity of the nocturnal oxygen desaturation rather than the frequency of apnea and hypopnea episodes. Mansfield et al., (13) reported that cardiac norepinephrine spillover correlated with a reduced SpO2, but not with the apnea-hypopnea index (AHI). These studies suggest that the magnitude or severity of hypoxemia during sleep may have a greater detrimental effect on the heart, than the number of apnea or hypopnea episodes.
The severity of SDB is traditionally determined by the number of apnea and hypopnea episodes per hour of sleep, i.e., the AHI. However, the duration of the apnea episode and subsequent ventilation phase are inversely proportional to the cardiac function, so the AHI may not directly reflect the severity of the heart failure (5). While there seems little doubt that cardiovascular morbidity is associated with oxygen desaturation, few studies have explored or offered robust metrics for the nocturnal hypoxemic burden. The aim of this study was to explore the novel oxygen desaturation metrics for risk stratification in patients with heart failure affected by CSA.
Materials and Methods
Patients
From January 2008 to December 2011, we prospectively enrolled patients who were consecutively referred to the hospital for the evaluation or treatment of either systolic or diastolic heart failure (3). Patients were excluded if they satisfied one or more of the following criteria: an ongoing treatment for SDB, chronic obstructive pulmonary disease (a forced expiratory volume per second of <70%), hypercapnia (PaCO2 >45 mmHg), pregnancy, use of sedatives/sleeping medications, those ≥95 years or <20 years of age, active myocarditis, renal failure requiring dialysis, or malignancy. In addition, patients were excluded if, within the previous 2 months, they had undergone coronary revascularization or had had an acute myocardial infarction, unstable angina, or a stroke. The study protocol conformed to the Declaration of Helsinki. The study was approved by the Ethics Committee of Fujita Health University and patients gave their informed consent to participate.
Sleep studies and group definitions
We used type 3 in-hospital unattended cardiorespiratory polygraphy (Fukuda Denshi, LS-300, Tokyo), with recording of the body position, electrocardiography, oronasal airflow, chest and abdominal effort, and pulse oximetry (14, 15). The sampling rate of the pulse oximetry was 1 Hz. Apnea was defined as a cessation of airflow that lasted at least 10 s, and hypopnea was defined as a ≥ 30% decrease in the sum of the thoracoabdominal movements lasting ≥ 10 seconds, followed by a reduction in the SpO2 of at least 4%. CSA was defined as an absence of oronasal airflow during sleep for ≥10 s associated with an absent respiratory effort or any reduction in the oronasal airflow for ≥10 s associated with in-phase thoracoabdominal movement and a ≥4% fall in the SpO2. Cheyne-Stokes respiration was defined as at least three episodes of continuous cycles of waxing and waning tidal volumes with periods of hyperventilation separated by CSA or hypopnea episodes. In this study, Cheyne-Stokes respirations were included in CSA. OSA was defined as cessation of the oronasal airflow for ≥10 s in the presence of an out-of-phase thoracoabdominal effort or as a fall in the oronasal airflow for ≥10 s with an out-of-phase thoracoabdominal movement associated with a ≥4% fall in the SpO2. We studied CSA defined by an AHI of ≥5 events/h, whereby ≥ 75% of all events were central in origin. We excluded OSA defined by an AHI of ≥5 events/h with ≥25% non-central events. The sleep studies were performed while patients were in stable heart failure. We studied the data recorded from 22:00 h to 06:00 h, expressed as the total recording time. The sleep study analyses were performed and scored by an SDB technician (SF) and a board-certified sleep specialist (YM) who were blinded to this study.
Desaturation index
To quantify the SDB-related variation in the SpO2 during the cardiorespiratory polygraphy, the following measures were obtained:
Oxygen desaturation index (ODI) defined as the number of oxygen desaturation events per hour during the sleep duration, where an event was detected if the oxygen level dropped by 3% and 4% (3%ODI and 4%ODI, respectively) from the baseline oxygen saturation.
The proportion of the sleep time with an SpO2 of <90% (T90%), and area under the T90% (AUT90%) were determined. Also, the mean SpO2, minimum SpO2, and standard deviation of the SpO2 during sleep were analyzed.
- The percentage of the oxygen desaturation events (POD) was defined as
where τi was the duration of each oxygen desaturation event. The 3%POD and 4%POD were defined corresponding to the 3%ODI and 4%ODI, respectively. That value was normalized by the total recording time and presented as the % (Figure 1). - The area under the oxygen desaturation (AOD) event was defined as
where Si was the accumulation of (threshold −SpO2) × (sampling interval) of each oxygen desaturation event. In this definition, the sum of Si (numerator) could be proportional to the total recording time. Therefore, the AOD was normalized by the total recording time. The AOD value primarily reflects the depth of the apnea-induced desaturations averaged over the total recording time. The 3%AOD and 4%AOD were defined corresponding to the 3%ODI and 4%ODI, respectively. That value was measured for the total recording time and presented as the % (Figure 1).
Figure 1. Schematic diagram of the sleep parameters considered in this study.
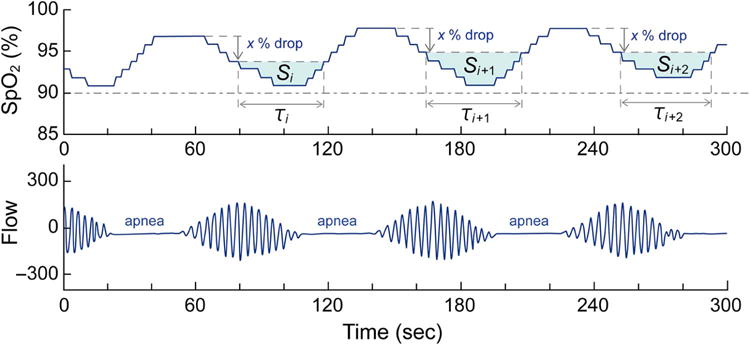
The upper panel indicates the SpO2, and the lower panel indicates the nasal flow. See the text for the details.
Follow-Up and documentation of the end point
Patients were followed up at our hospital, and their status was determined from the medical records. The follow-up data of those who did not visit our hospital was obtained by a telephone interview of the patient, patient’s family, or primary care physician. The end point was death due to any cause. Members of the events verification committee who were blinded to the sleep study results reviewed the medical records and ascertained the death endpoint. Patients were censored at death or the last follow-up.
Statistical analysis
Differences in the frequency were tested using a χ2 test for categorical data and Student’s t-test for continuous variables. Relationships between clinical factors and the sleep apnea measures were assessed by a Spearman’s rank correlation analysis. A multivariate logistic regression analysis was used to identify the oxygen desaturation measures that were independently linked to mortality during a period of 1 year after the follow up. We constructed 3 separate models; Model 1 was adjusted for the age, sex, ischemic heart disease, and left ventricular ejection fraction (LVEF). Model 2 was adjusted for the age, sex, chronic kidney disease, and left atrial diameter, and Model 3 was adjusted for the age, sex, diabetes mellitus, and left atrial diameter. The odds ratio (OR) per increment of 1 standard deviation (SD) and 95% confidence interval (CI) are shown. A time-to-event curve describing the proportion of patients remaining free from death was calculated by the Kaplan-Meier method and compared with the log-rank test. Receiver operating characteristic (ROC) curves were constructed for each sleep study parameter and the sensitivities and specificities, and areas under curves (AUCs) were determined. Quantitative data are expressed as means±SD. For variables with a skewed distribution, the values were transformed to natural logarithms. A two-tailed P-value of <0.05 was considered significant. The statistical analyses were performed using JMP 10.0.2 software (SAS Institute, USA).
Results
Clinical characteristics
The baseline characteristics of the patients are summarized in Table 1. There were no significant differences with regard to the age, body mass index, and New York Heart Association classification between survivors and non-survivors. Non-survivors were more likely to be male, had higher rates of ischemic heart disease, diabetes, chronic kidney disease, a reduced LVEF, and enlarged left atrial diameter as compared to survivors.
Table 1.
Baseline clinical characteristics.
| All patients (n = 112) |
Survivors (n = 80) |
Non-survivors (n = 32) |
P-value | |
|---|---|---|---|---|
| Age, years | 72±12 | 71±12 | 77±6 | 0.06 |
| Male, n (%) | 78 (70) | 61 (66) | 17 (89) | 0.04 |
| BMI, kg/m2 | 23±4 | 23±4 | 23±3 | 0.78 |
| NYHA class II, III/IV, n | 104/8 | 74/6 | 30/2 | 0.53 |
| Atrial fibrillation, n (%) | 55 (49) | 42 (53) | 13 (41) | 0.26 |
| ICD/CRT-D | 2/4 (5) | 1/3 (5) | 1/1 (6) | 0.79 |
| Medical history, n (%) | ||||
| Diabetes mellitus | 54 (48) | 41 (44) | 13 (68) | 0.05 |
| Hypertension | 56 (50) | 47 (51) | 9 (47) | 0.80 |
| Ischemic heart disease | 51 (46) | 37 (40) | 14 (74) | 0.01 |
| Dyslipidemia | 43 (38) | 37 (40) | 6 (32) | 0.50 |
| Chronic kidney disease | 50 (45) | 38 (41) | 12 (63) | 0.07 |
| Systolic blood pressure, mmHg | 108±19 | 109±19 | 105±21 | 0.51 |
| Diastolic blood pressure, mmHg | 62±13 | 62±12 | 57±14 | 0.12 |
| Laboratory values | ||||
| Creatinine, mg/dl | 1.3±0.8 | 1.3±0.9 | 1.5±0.6 | 0.36 |
| Hemoglobin, g/dl | 13±2.1 | 13±2 | 12±2 | 0.13 |
| BNP, pg/ml | 349±428 | 315±296 | 501±788 | 0.09 |
| ln BNP | 5.4±1.0 | 5.3±1.0 | 5.7±1.0 | 0.17 |
| Echocardiography | ||||
| LVEF, % | 40±16 | 42±16 | 32±11 | 0.01 |
| ≤ 50% | 73 (65) | 49 (61) | 24 (75) | 0.09 |
| > 50% | 39 (35) | 32 (39) | 7 (25) | |
| LAD, mm | 42±7 | 41±7 | 47±6 | <0.01 |
| Medications at discharge, % | ||||
| β-Blocker | 95 (85) | 78 (84) | 17 (89) | 0.54 |
| ACE-I/ARB | 96 (86) | 79 (85) | 17 (89) | 0.61 |
| Loop diuretics | 98 (88) | 80 (86) | 18 (95) | 0.30 |
| Spironolactone | 77 (69) | 64 (69) | 13 (68) | 0.98 |
| Digitalis | 25 (22) | 19 (20) | 6 (32) | 0.29 |
| Statin | 50 (45) | 42 (45) | 8 (42) | 0.81 |
BMI: body mass index, NYHA: New York Heart Association functional class, ICD: implantable cardioverter-defibrillator, CRT-D: cardiac resynchronization therapy with defibrillator, BNP: B-type natriuretic peptide, ln: natural logarithm, LVEF: left ventricular ejection fraction, LAD: left atrial diameter, ACE-I: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker. Chronic kidney disease indicates the estimated glomerular filtration ratio <60 mL/1.73m2. Data represent the frequencies or means±SD.
Sleep study
The results of the sleep study are presented in Table 2. No significant differences were observed in regard to the AHI, central apnea index, and minimum SpO2 between survivors and non-survivors. Non-survivors tended to have a higher 3%ODI, SD SpO2, T90%, AUT90%, 3%AOD, and 4%AOD. Non-survivors also exhibited a lower mean SpO2 (95±2.2 vs. 96±2.1%, p=0.003) and higher 3%POD (19±11vs.30±18, p=0.002) and 4%POD (11±6.4 vs. 19±13%, p=0.001) than survivors. We further divided the survivors and non-survivors into 3 subgroups based on the LVEF (LVEF<30%, 30–40%, and >40%), but we found no significant difference in the 4%POD in the survivors and non-survivors across these 3 groups (data not shown).
Table 2.
Sleep study
| All patients (n = 112) |
Survivors (n = 80) |
Non-survivors (n = 32) |
P-value | |
|---|---|---|---|---|
| AHI, /h | 21±13 | 20±11 | 25±15 | 0.11 |
| OAHI, /h | 1.6±2.2 | 1.5±2.2 | 1.7±2.4 | 0.65 |
| CAI, /h | 18±12 | 16±10 | 20±11 | 0.16 |
| 3%ODI, /h | 17±8.4 | 16±6.6 | 20±11 | 0.06 |
| 4%ODI, /h | 13±8.1 | 12±5.9 | 17±11 | 0.045 |
| Mean SpO2, % | 96±2.2 | 96±2.1 | 95±2.2 | 0.003 |
| Minimum SpO2, % | 79±5.0 | 79±5.2 | 79±4.8 | 0.94 |
| SD SpO2, % | 2.5±0.9 | 2.4±0.8 | 2.7±1.1 | 0.15 |
| T90%, % | 5.3±11 | 4.0±9.8 | 8.7±13 | 0.06 |
| AUT90%, % | 0.2±0.5 | 0.15±0.5 | 0.3±0.6 | 0.18 |
| 3%AOD, % | 0.18±0.2 | 0.16±0.2 | 0.26±0.3 | 0.08 |
| 4%AOD, % | 0.1±0.1 | 0.08±0.1 | 0.15±0.2 | 0.11 |
| 3%POD, % | 23±14 | 19±11 | 30±18 | 0.002 |
| 4%POD, % | 14±9.4 | 11±6.4 | 19±13 | 0.001 |
AHI: apnea-hypopnea index, OAHI: obstructive apnea-hypopnea index, CAI: central apnea-hypopnea index, SD SpO2: standard deviation of SpO2 during sleep, T90%, proportion of the sleep time with an arterial oxygen saturation of <90%. AUT90%: area under T90%, ODI: oxygen desaturation index, AOD: area under oxygen desaturation events, POD: percentage of oxygen desaturation events.
Relationships between the clinical features and sleep apnea measures
In the entire patient population, close correlations were observed among the sleep apnea measures (Table 3). There were weak correlations between the SD SpO2, T90%, AUT90%, and age. The AHI had a weak association with the body mass index and chronic kidney disease. The 4%AOD and 4%POD were independent of the age, sex, body mass index, and LVEF. Also, none of the sleep apnea measures was associated with medications (data not shown).
Table 3.
Relationships between the clinical features and sleep apnea measures
| AHI | CAI | 4%ODI | SD SpO2 | T90% | AUT90% | 4%AOD | 4%POD | Age | Sex | BMI | LVEF | IHD | DM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAI | 0.88§ | |||||||||||||
| 4%ODI | 0.75§ | 0.63§ | ||||||||||||
| SD SpO2 | 0.48§ | 0.45§ | 0.59§ | |||||||||||
| T90% | 0.42§ | 0.37§ | 0.50§ | 0.89 | ||||||||||
| AUT90% | 0.41§ | 0.36§ | 0.46§ | 0.88§ | 0.97§ | |||||||||
| 4%AOD | 0.45§ | 0.38§ | 0.67§ | 0.88§ | 0.86§ | 0.84§ | ||||||||
| 4%POD | 0.47§ | 0.35§ | 0.64§ | 0.71§ | 0.77§ | 0.70§ | 0.82§ | |||||||
| Age | 0.10 | 0.08 | 0.17 | 0.20* | 0.22* | 0.22* | 0.16 | 0.17 | ||||||
| Sex | 0.24* | 0.14 | 0.15 | 0.14 | 0.07 | 0.06 | 0.10 | 0.11 | −0.02 | |||||
| BMI | 0.20* | 0.26† | 0.10 | 0.11 | 0.17 | 0.12 | 0.09 | 0.10 | −0.21* | 0.04 | ||||
| LVEF | 0.05 | 0.08 | −0.05 | −0.01 | 0.10 | 0.09 | −0.04 | −0.05 | 0.21* | −0.30† | 0.16 | |||
| IHD | 0.08 | 0.14 | 0.14 | 0.13 | 0.13 | 0.08 | 0.12 | 0.15 | 0.22* | 0.14 | 0.22* | −0.08 | ||
| DM | 0.06 | 0.09 | 0.08 | 0.12 | 0.13 | 0.13 | 0.06 | 0.06 | 0.15 | 0.05 | 0.23* | −0.06 | 0.41§ | |
| CKD | 0.21* | 0.23* | 0.18 | 0.12 | 0.06 | 0.06 | 0.07 | 0.04 | 0.17 | 0.09 | 0.01 | −0.03 | 0.33† | 0.03 |
Endpoint
During the follow-up period of 37±25 months, 32 patients (29%) died due to heart failure (n=21), sudden death (n=8), major bleeding (n=2), and stroke (n=1). Twenty nine patients died in our hospital, and the primary care physicians confirmed the remaining 3 patients’ deaths. We could not ascertain the outcome in 7 patients (8.8%) in the survivors. There were no significant differences in the clinical background and sleep study parameters between those with completed follow up and those lost to follow up (data not shown). The multivariate logistic regression analysis is summarized in Table 4. In all 3 Models, the 4%POD was the best independent predictor of mortality. A Kaplan-Meier plot for cardiovascular death dichotomized using the median value of the 4%POD (11.9%) is shown in Figure 2. The prognostic accuracy of the sleep study parameters is presented in Table 5. Of the individual measures, the 4%POD was the best predictor of mortality with a sensitivity of 93.7%, specificity of 56.9%, and AUC of 0.777.
Table 4.
Logistic regression model
| Parameters | 1 SD | Univariate OR (95%CI) |
χ2 | Model 1 OR (95%CI) |
Model 2 OR (95%CI) |
Model 3 OR (95%CI) |
|---|---|---|---|---|---|---|
| ln AHI (ln /h) | 0.61 | 1.63 (0.68 – 4.20) | 1.16 | 1.76 (0.17 – 21.3) | 1.20 (0.45 – 3.36) | 1.12 (0.40 – 3.24) |
| ln CAI (ln /h) | 0.74 | 5.18 (0.47 – 72.9) | 1.77 | 1.30 (0.58 – 3.01) | 1.43 (0.60 – 3.66) | 1.31 (0.53 – 3.42) |
| ln 3%ODI (ln /h) | 0.22 | 2.81 (0.88 – 9.88) | 3.02 | 1.73 (0.45 – 7.21) | 1.95 (0.50 – 8.34) | 1.59 (0.38 – 7.12) |
| ln 4%ODI (ln /h) | 0.28 | 2.56 (0.97 – 7.33) | 3.59 | 1.60 (0.58 – 4.88) | 1.66 (0.57 – 5.31) | 1.21 (0.71 – 2.07) |
| ln Minimum SpO2 (%) | 0.06 | 0.23 (0.01 – 6.63) | 0.77 | 0.14 (0.01 – 6.26) | 0.69 (0.02 – 4.62) | 0.44 (0.01 – 3.24) |
| ln SD SpO2 (%) | 0.32 | 7.13 (1.40 – 41.5) | 5.59 | 3.22 (0.53 – 21.7) | 4.24 (0.63 – 33.1) | 3.67 (0.50 – 29.6) |
| ln T90% (%) | 1.70 | 1.49 (1.06 – 2.20) | 5.38 | 1.40 (0.96 – 2.14) | 1.39 (0.95 – 2.12) | 1.36 (0.92 – 2.13) |
| ln AUT90% (%) | 1.88 | 1.39 (1.03 – 1.95) | 4.78 | 1.31 (0.95 – 1.92) | 1.32 (0.93 – 1.91) | 1.30 (0.92 – 1.92) |
| ln 3%AOD (%) | 0.44 | 2.12 (1.18 – 4.15) | 6.64 | 1.85 (0.97 – 3.98) | 1.89 (0.95 – 4.08) | 1.75 (0.87 – 3.87) |
| ln 4%AOD (%) | 0.50 | 1.82 (1.10 – 3.24) | 5.50 | 1.43 (0.86 – 2.61) | 1.48 (0.85 – 2.77) | 1.40 (0.80 – 2.62) |
| ln 3%POD (%) | 0.27 | 4.17 (1.56 – 12.9) | 8.49 | 3.40 (1.12 – 12.9)* | 3.67 (1.15 – 14.6)* | 3.66 (1.16 – 14.3)* |
| ln 4%POD (%) | 0.31 | 3.63 (1.49 – 10.3) | 8.57 | 2.41 (1.01 – 6.73)* | 2.82 (1.05 – 9.21)* | 2.97 (1.09 – 9.67)* |
Figure 2. Kaplan-Meier survival curves for the cardiovascular mortality.
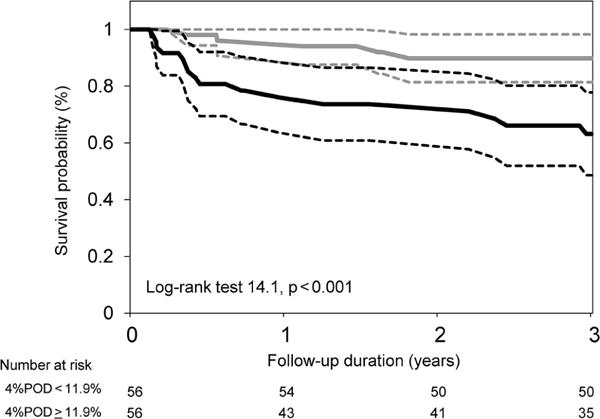
The patients were dichotomized by a median value of the 4%POD of 11.9%. The dotted lines indicate the 95% confidence interval. POD: percentage of oxygen desaturation events.
Table 5.
Prognostic accuracy of the sleep study parameters.
| Parameters | Sensitivity | Specificity | AUC |
|---|---|---|---|
| AHI (/h) | 0.375 | 0.937 | 0.583 |
| CAI (/h) | 0.312 | 0.968 | 0.601 |
| 3%ODI (/h) | 0.750 | 0.541 | 0.638 |
| 4%ODI (/h) | 0.812 | 0.458 | 0.656 |
| Minimum SpO2 (%) | 0.812 | 0.479 | 0.589 |
| SD SpO2 (%) | 0.687 | 0.687 | 0.659 |
| T90% (%) | 0.687 | 0.697 | 0.683 |
| AUT90% (%) | 0.625 | 0.687 | 0.667 |
| 3%AOD (%) | 0.625 | 0.740 | 0.687 |
| 4%AOD (%) | 0.625 | 0.687 | 0.664 |
| 3%POD (%) | 0.750 | 0.678 | 0.702 |
| 4%POD (%) | 0.937 | 0.569 | 0.777 |
AUC: area under the curve.
Discussion
Major findings
We conducted a detailed analysis of the degree of oxygen desaturation during sleep in patients with heart failure affected by CSA. We found that the percent of the recording time that 4% desaturation events occurred (4%POD), a novel prognostic metric to assess the nocturnal hypoxemia burden, predicted the mortality independent of other desaturation metrics such as the AHI or ODI. Our data suggest that a greater hypoxemic burden may have a more marked detrimental effect on the heart than the frequency of apnea-hypopnea episodes.
Heart failure and CSA frequently coexist, commonly resulting in serious adverse events. With both conditions increasing in prevalence, it is important to understand how the oxygen desaturation during sleep impacts the prognosis. In this study we examined patients with worsening heart failure treated in accordance with the Guideline-Directed Medical Therapy (GDMT) as shown in the 2013 ACCF/AHA guidelines for the management of heart failure (1) and they were compliant with the GDMT during the follow-up period.
Previous studies showed that the presence of CSA with an AHI of ≥ 5/h identified individuals at an increased risk of cardiovascular consequences in systolic heart failure (2, 16). However, several studies also reported conflicting results (17, 18). Unlike OSA, there are limited changes in the intrathoracic pressure during CSA and mild desaturations and hyperpnea. Further, oxygen desaturation is thought to be less predictive of mortality in CSA than in OSA (19, 20). In an early report by Javaheri et al., (2) the T90% was a significant univariate predictor of death in patients with systolic heart failure and CSA. Recently, Oldenburg et al. (21) reported the independent prognostic value of the T90% using a large population of systolic heart failure patients with a long-term follow up. Collectively, our data suggest that the prognostic information was greater with the hypoxemic burden than with the frequency of apnea episodes in patients with heart failure affected by CSA. In this study we found novel and independent hypoxemic burden metrics for cardiovascular mortality including the 4%POD followed by the 3%POD and T90%. We initially expected that the 4%AOD or AUT90%, which integrates the duration and magnitude of hypoxemia, would be more efficient prognostic markers than the 3%POD and T90%. This is probably due to the underpowering of the study because of the small sample size.
An adequate supply of oxygen is critical for the cardiac viability and function, and hypoxemia itself can have direct adverse effects (19). Ryan et al. showed that as oxygen levels decrease, either intermittently or sustained, gene expression patterns in cultured cells are significantly altered (22). Intermittent hypoxia activates NF-κB with downstream consequences such as increased production of inflammatory genes such as TNF-α. Sustained hypoxia stabilizes the HIF-1 with downstream consequences including production of BNP or erythropoietin. Induction of BNP in CSA patients was demonstrated by Gottlieb et al., (20) who performed serial measurements of the BNP levels during sleep and found that the changes in the BNP paralleled those for the T90%, rather than apneic episodes.
Cumulative oxygen desaturation may have detrimental effects on the heart, but therapy aimed to improve hypoxemia does not necessarily reduce the mortality. Nocturnal supplemental oxygen has been shown to reduce the AHI, and increase the cardiac function or quality of life but does not reduce the mortality (23, 24). Adaptive servo-ventilation (ASV), one of the promising positive pressure support devices, has been shown to be more effective than oxygen supplementation, continuous positive airway pressure, or bilevel positive airway pressure therapy at lowering the AHI in patients with heart failure and CSA (25). Previous studies reported that short-term treatment by ASV in SDB patients with heart failure can improve the central AHI, ejection fraction, BNP, and health-related quality of life (26, 27). However, a recent large randomized trial, the Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure (SERVE-HF) study (28), failed to show any attenuation of hospitalizations and death in patients randomized to ASV compared to those randomized to no ASV. Further, the all-cause mortality and cardiovascular mortality were significantly higher in the ASV group than in the control group. The implications of this outcome for the concept and application of ASV will motivate careful reflection and additional investigation.
Study limitations
This study was based on observations of a small cohort of patients who were referred to a tertiary university hospital. We observed 32 deaths, which may not be enough to draw final conclusions regarding the oxygen desaturation analysis in a prognostic stratification. We used ambulant polygraphy, which excluded the analysis of the sleep stages and was not the current gold standard diagnostic approach for SDB. We only used a pressure probe, but not that with a thermocouple. Thus, some of the events may conceivably have been misclassified. The study results also cannot be extrapolated to patients with predominantly OSA. We did not analyze the correlation between the 4%POD and diastolic dysfunction stages.
Conclusion
We conducted a detailed analysis of the degree of oxygen desaturation during sleep in patients with heart failure and CSA. We identified a novel prognostic metric to assess the nocturnal hypoxemic burden, which is an important pathophysiological feature of CSA, and which strongly predicted mortality independent of the other desaturation metrics. These findings have implications for improved risk stratification in patients with heart failure and CSA. Our findings, albeit interesting, should be validated prospectively in a large cohort of heart failure patients.
Highlights.
Central sleep apnea (CSA) is prevalent in heart failure and related to mortality.
We analyzed the hypoxemic burden during sleep in heart failure and CSA.
A percent of the recording time that 4% desaturation events occurred (4%POD) is an independent prognostic marker.
Assessing nocturnal hypoxemic burden has an implication for risk stratification.
Acknowledgments
The authors thank Ms. Shiho Fujita and Dr. Yuuki Mieno for analyzing the sleep study and Ms. Akemi Yamauchi for scrutinizing the outcome.
Disclosures: VKS: Grant support – Philips Respironics Foundation (gift to Mayo Foundation); Consultant for Respicardia, ResMed, Sorin Inc., U-Health, GlaxoSmithKline, Rhonda Grey ; Working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease.
EW is supported by a Grant from the Suzuken Memorial Foundation and JSPS KAKENHI Grant Number 26461094. KK is supported by JSPS KAKENHI 23700544.VKS is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL065176. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Other authors have no conflict of interest with regard to this study.
References
- 1.Writing Committee M. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49(20):2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 3.Sano K, Watanabe E, Hayano J, Mieno Y, Sobue Y, Yamamoto M, et al. Central sleep apnoea and inflammation are independently associated with arrhythmia in patients with heart failure. Eur J Heart Fail. 2013;15(9):1003–10. doi: 10.1093/eurjhf/hft066. [DOI] [PubMed] [Google Scholar]
- 4.Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–9. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107(13):1822–6. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 7.Kasai T. Sleep apnea and heart failure. J Cardiol. 2012;60(2):78–85. doi: 10.1016/j.jjcc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo MR, Khayat R, Ponikowski P, Augostini R, Stellbrink C, Mianulli M, et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol. 2015;65(1):72–84. doi: 10.1016/j.jacc.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Borne P, Oren R, Abouassaly C, Anderson E, Somers VK. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;81(4):432–6. doi: 10.1016/s0002-9149(97)00936-3. [DOI] [PubMed] [Google Scholar]
- 10.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–7. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 11.Spaak J, Egri ZJ, Kubo T, Yu E, Ando S, Kaneko Y, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46(6):1327–32. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 12.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152(2):473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield D. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107(10):1396–400. doi: 10.1161/01.cir.0000056520.17353.4f. [DOI] [PubMed] [Google Scholar]
- 14.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damy T, Margarit L, Noroc A, Bodez D, Guendouz S, Boyer L, et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail. 2012;14(9):1009–19. doi: 10.1093/eurjhf/hfs085. [DOI] [PubMed] [Google Scholar]
- 17.Grimm W, Sosnovskaya A, Timmesfeld N, Hildebrandt O, Koehler U. Prognostic impact of central sleep apnea in patients with heart failure. J Card Fail. 2015;21(2):126–33. doi: 10.1016/j.cardfail.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Roebuck T, Solin P, Kaye DM, Bergin P, Bailey M, Naughton MT. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J. 2004;23(5):735–40. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 19.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115(3):500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb JD, Schwartz AR, Marshall J, Ouyang P, Kern L, Shetty V, et al. Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol. 2009;54(18):1706–12. doi: 10.1016/j.jacc.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 22.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 23.Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H, Group C-HS Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J. 2006;70(1):1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 24.Sasayama S, Izumi T, Matsuzaki M, Matsumori A, Asanoi H, Momomura S, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J. 2009;73(7):1255–62. doi: 10.1253/circj.cj-08-1210. [DOI] [PubMed] [Google Scholar]
- 25.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 26.Kasai T, Usui Y, Yoshioka T, Yanagisawa N, Takata Y, Narui K, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3(1):140–8. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 27.Oldenburg O, Bitter T, Lehmann R, Korte S, Dimitriadis Z, Faber L, et al. Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol. 2011;100(2):107–15. doi: 10.1007/s00392-010-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]


