Most of the recent advances in medical genetics have been in basic science and technology. Experience of translating these into effective, population based interventions is limited, but the potential is great, especially for lower resource countries.1,2 This is well illustrated by the experience of the national thalassaemia screening programme in Iran (p 1134), a comprehensive, primary care based programme for screening and genetic counselling. Since the programme's inception in 1996 premarital screening of 2.7 million couples has been carried out over five years, followed by genetic counselling of more than 10 000 couples who were found to be positive. This has resulted in a 70% reduction in the expected annual birth rate of affected infants.3 For a vast, lower-resource country with a population of 68 million, this is a considerable achievement.
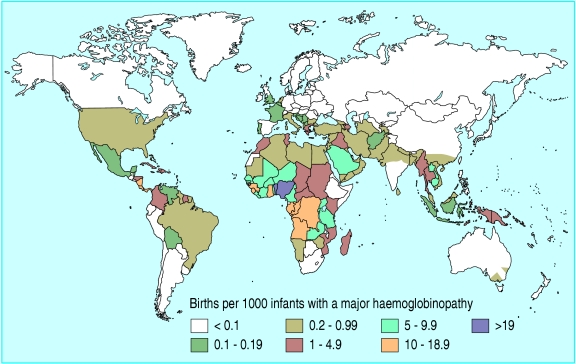
As low and middle income countries undergo demographic and epidemiological transition and infant mortality falls below 50/1000 live births, congenital disorders become increasingly visible and costly, contributing up to 30% of infant mortality in countries in the eastern Mediterranean region. Sickle cell disease and thalassaemia are a natural “point of entry” to genetics services for many such countries.2 The thalassaemias are prevalent in the countries of the eastern Mediterranean region, South Asia, and South East Asia (figure). Thalassaemia major results in profound anaemia and death in infancy if untreated.
Figure 1.
Distribution of haemoglobin disorders around the world
Credit: WHO GENOMIC RESOURCE CENTRE
Regular monthly transfusions of red cells require much organisation and resources, and if death from iron overload is to be avoided in teenagers very expensive iron chelation treatment has to be offered in parallel. The burden on affected families is immense and the costs of treatments can overwhelm the health budgets of many countries where thalassaemia is common. In Iran, the estimated cost of treating 15 000 patients in 2000 was $200m.5 Screening and offering termination of pregnancy provides a feasible practical option for such countries, although the challenge of organising comprehensive, effective programmes and gaining public acceptance is huge. The Iranian experience thus provides a good model to learn from.
Iran started its primary care thalassaemia prevention programme in 1996. The programme aimed to identify carrier couples before marriage and to offer counselling, thus providing them with the opportunity to separate. Prenatal diagnosis and the option of selective abortion were not available in the country at that time. By 1999, an audit showed that couples were still opting to marry rather than separate (which had been assumed would happen), and that the people affected wanted prenatal diagnosis and selective abortion. Widespread discussions ensued, and the law was amended in 2001 to allow the option of selective abortion up to 15 weeks' gestation for thalassaemia (a policy that is likely to be extended to include other serious congenital disorders). A national DNA laboratory network was therefore developed to offer prenatal diagnosis on chorionic villus samples.3
A key aspect of the programme was the holistic way in which the service was developed, with widespread public education, public health surveillance, and services developing in response to needs and wishes of the affected population and society in general. For example, the counsel of religious scholars informed the decision to accept 15 weeks as the limit at which a pregnancy could be terminated. The advice, based on the deliberations of Muslim scholars versed in Islamic law, is that the soul of the baby enters the womb at 120 days.6 The debate regarding termination of pregnancy for thalassaemia is still in progress in several other Muslim countries including Egypt, Tunisia, and Maldives; Saudi Arabia as well as Pakistan have concluded that 17 weeks is the appropriate cut-off point.2,7
The Iranian programme provides lessons for high income countries too—for example, countries with minority ethnic communities that originate from areas of high prevalence, such as the United Kingdom, and countries with indigenous populations with high carrier rates, such as Italy. Western countries have been slow to adopt population based genetic screening. One reason for this may be that status and interest focus on “cutting edge” science rather than applying existing knowledge. Another reason may be the emphasis on the libertarian and individualistic meaning of “informed choice” without linking the societal debate to issues of the public's health and resources.
Services for haemoglobin disorders need to be equitable, accessible, and understood and accepted by all the subgroups involved. Services that are determined locally in a piecemeal fashion and vary according to the priorities of providers are far from ideal. In the United Kingdom late identification of risk and hence variable access to prenatal diagnosis has in effect limited choice to decide early in pregnancy whether to continue an affected pregnancy.8 Iran adopted a well coordinated national approach in which screening is integrated with services for patients—an important lesson.
Although the results of Iran's programme are comparable with island based screening programmes in Greece, Cyprus, Sardinia, and Sicily9,10 the feasibility of implementing similar national screening programmes for haemoglobin disorders in countries whose geographical, economic, social, and political milieu is very different to Iran remains to be determined.
At national level, the Iranian study raises many interesting questions that warrant exploration, for example, what happened to couples who separated? What is the impact of being identified as a carrier? How are carriers viewed by society? Other options for screening need to be considered too, for example testing for carriers in early pregnancy to avoid women being stigmatised or concealing their carrier status.11,12
It is already evident, however, that screening programmes for haemoglobin disorders must comply with societal values. The messages conveyed in community education need to be clear for the public to understand the programme's aims and objectives. The sickle cell screening campaign in the United States in the 1970s failed because it was insufficiently planned and poorly communicated. The differences between carrier status and the homozygous condition were misunderstood, resulting in carriers being stigmatised and screening being rejected by the populations targeted.13
Samavat and Modell's understated article stands as a model by which to consider implementing genetic screening programmes in lower resource countries, working through primary care. Iran is now planning to develop genetic services building on this model of service.3 Perhaps the message to wealthier countries is that much can be achieved by adopting a holistic approach with an integrated community perspective that responds to community needs, and that such countries should consider seriously investing more in the public health application of basic genetic science and technology for the population's benefit.
Primary care p 1134
Competing interests: AS is currently seconded as the programme director for the NHS Sickle Cell and Thalassaemia Screening Programme. AC has an academic link with Bernadette Modell and has recently coauthored a book chapter with her on medical genetics in developing countries.
References
- 1.Christianson AL, Modell B. Medical genetics in developing countries. Ann Rev Genomics Hum Genet 2004;5: 219-65. [DOI] [PubMed] [Google Scholar]
- 2.Alwan A, Modell B. Recommendations for introducing genetic services in developing countries. Nature Rev Genet 2003;4: 61-8. [DOI] [PubMed] [Google Scholar]
- 3.Samavat A, Modell B. Iranian national thalassaemia screening programme. BMJ 2004;329: 1134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwan A, Modell B. 1997. Community control of genetic and congenital disorders. Eastern Mediterranean Regional Office Technical Publication Series 24. 1997. WHO: Regional Office of the Eastern Mediterranean, Alexandria, Egypt.
- 5.World Health Organization. Primary health care approaches for prevention and control of congenital and genetic disorders. Geneva: WHO, 2000.
- 6.National Screening Committee (NSC). Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. March 2003. http://rms.nelh.nhs.uk/screening/ (accessed 4 Nov 2004).
- 7.Abdel Haleem MAS. Medical ethics in Islam. In: Grubb A, ed. Choices and decisions in health care. Chichester: John Wiley, 1993: 1-20.
- 8.Ahmed S, Saleem M, Modell B, Petrou M. Screening extended families for genetic haemoglobin disorders in Pakistan. N Engl J Med 2002;347: 1162-8. [DOI] [PubMed] [Google Scholar]
- 9.Angastiniotis M, Modell B, Englezos P, Boulyjenkov. Prevention and control of haemoglobinopathies. Bull WHO 1995:73: 375-86. [PMC free article] [PubMed] [Google Scholar]
- 10.Maggio A, Caronia F, Orlandi F. Prenatal diagnosis of haemoglobinopathies in Sicily. Lancet 1992;339: 1361-2. [DOI] [PubMed] [Google Scholar]
- 11.Modell B, Harris R, Lane B, Khan M, Darlinson M, Petrou M, et al. Informed choice in genetic screening for thalassaemia during pregnancy: audit from a national confidential enquiry. BMJ 2000;320; 325-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murtz J. Matching scheme solves Tay Sachs problems. JAMA 1987;258: 2636-7. [DOI] [PubMed] [Google Scholar]
- 13.Atkin K, Ahmad WIU. Genetic screening and haemaglobinopathies: ethics, politics and practice. Soc Sci Med 1998;46: 445-58. [DOI] [PubMed] [Google Scholar]