Abstract
We exist in a physical world, and cells within biological tissues must respond appropriately to both environmental forces and forces generated within the tissue to ensure normal development and homeostasis. Cell division is required for normal tissue growth and maintenance, but both the direction and rate of cell division must be tightly controlled to avoid diseases of over-proliferation such as cancer. Recent studies have shown that mechanical cues can cause mitotic entry and orient the mitotic spindle, suggesting that physical force could play a role in patterning tissue growth. However, to fully understand how mechanics guides cells in vivo, it is necessary to assess the interaction of mechanical strain and cell division in a whole tissue context. In this mini-review we first summarise the body of work linking mechanics and cell division, before looking at the advantages that the Xenopus embryo can offer as a model organism for understanding: 1) the mechanical environment during embryogenesis, and 2) factors important for cell division. Finally, we introduce a novel method for applying a reproducible strain to Xenopus embryonic tissue and assessing subsequent cell divisions.
Keywords: Biomechanics, mitosis, division orientation, Xenopus laevis
Introduction
The rate and orientation of cell division is crucial in both development and disease. Correctly orienting divisions in the plane of an epithelium is required for morphogenesis and organogenesis during development (Baena-Lopez et al., 2005; Quesada-Hernandez et al., 2010). In adult life, if cells over proliferate and/or misorient their divisions, diseases such as cancer can result (Pease and Tirnauer, 2011; Quyn et al., 2010). Therefore, understanding the cues used by cells to determine division rate and orientation is essential for modelling cell behaviour during development and disease. The processes of cell division and mitosis can be broken down into sequential stages, some of which are oriented. Mitosis starts with chromosome condensation at prophase. The nuclear envelope then breaks down and the chromosomes align in the centre of the cell as the bipolar mitotic spindle forms at metaphase. At anaphase the chromosomes are pulled apart towards the poles of the mitotic spindle, before the two daughter nuclei form during telophase and the cell divides into two daughter cells by cytokinesis. During metaphase the mitotic spindle often rotates or wobbles within the cell until it reaches a final orientation that determines the direction of chromosome separation during anaphase (Adams, 1996; Fink et al., 2011; Woolner et al., 2008). Once anaphase begins, spindle orientation is generally fixed and spindle poles undergo very little subsequent movement (Adams, 1996; Woolner et al., 2008). The position of the spindle in anaphase determines the position of cytokinetic ring formation and, therefore, determines the ultimate orientation of cell division. For this reason, when discussing the orientation of cell division, we generally refer to mechanisms that orient the mitotic spindle in the stages leading up to anaphase, focusing in particular on metaphase.
The external mechanical microenvironment has been convincingly shown to be an important cue for division, playing a role in determining both division rate and orientation in cells in culture. For example, individual cells grown on micropatterned fibronectin substrates that bias cell shape align their mitotic spindle with the longest axis of the cell (Thery et al., 2005), this spindle alignment is potentially due to biased placement of cortical cues. Indeed, a study in which micropatterns specifically biased the placement of stress fibres rather than cell shape suggested that spindle orientation could be a consequence of anisotropic forces produced by stress fibres attached to the substrate pattern (Thery et al., 2007). Moreover, the direct application of tensile force to a flexible substrate for HeLa cells and keratinocytes in culture has been shown to orient the mitotic spindle (Fink et al., 2011; Seldin et al., 2013). Furthermore, research on epithelial monolayers has shown that, when stretched, cells within the monolayer upregulate YAP/TAZ signalling and re-enter the cell cycle (Benham-Pyle et al., 2015). The decision to re-enter the cell cycle when a monolayer is stretched may be based on a decrease in cellular density: a stretched monolayer will re-enter the cell cycle via G1-S phase transition, whereas cell cycle progression is halted in a compressed monolayer if cell density is increased above a certain threshold (Streichan et al., 2014). All the of the examples discussed so far focus on cells cultured on a flexible substrate, therefore relying mainly on cell-matrix adhesions to transmit forces produced when the substrate is stretched. However, work on a suspended epithelial monolayer with only cell-cell adhesions, found that, similar to the cells grown on a substrate, cells elongated by stretching the suspended epithelium also orient divisions along their longest axis, easing tension across the monolayer (Wyatt et al., 2015). Together, these studies make a convincing case that cultured cells sense their mechanical environment and can respond to those cues.
How cells are sensing their mechanical environment to position the mitotic spindle is still unclear, but a number of non-exclusive hypotheses have been suggested (reviewed in (Nestor-Bergmann et al., 2014)). Furthermore, there is considerable discussion over whether cells are directly sensing force or simply responding to changes in cell shape upon stretching (Nestor-Bergmann et al., 2014; Wyatt et al., 2015). Tension on a cell can change its geometry and elongate it, and it has long been noted that elongated cells preferentially divide along their long axis, a phenomenon known as Hertwig’s rule (Hertwig, 1893). Both astral microtubules of the mitotic spindle and the actin cytoskeleton are thought to play roles in aligning the mitotic spindle with cell shape (Fink et al., 2011; Kunda and Baum, 2009; Minc et al., 2011). The importance of astral microtubules for orienting the spindle with cell shape has been demonstrated by pushing single-celled sea-urchin zygotes into differently shaped micro-fabricated wells (Minc et al., 2011). In this system it is possible to explain the orientation of the spindle to cell shape using a simple model where astral microtubules probe the cell space and exert pulling forces on the spindle proportional to their length (Minc et al., 2011). Spindles have also been shown to orient with 3D cell shape in the presumptive enveloping layer of the zebrafish embryo, though the molecular mechanism was not investigated (Xiong et al., 2014). However, cells within a tissue experiencing force from adhesions have more cues available than just cell shape. Subcortical actin structures are enriched in areas of cultured cells with tension bearing retraction fibres, and in turn the mitotic spindle aligns with the enrichment in subcortical actin (Fink et al., 2011). How actin is being enriched in areas of the cell cortex under greater tension is not clear, but tension sensor proteins such as talin and vinculin, which are known to link cell adhesions to the actin cytoskeleton, are intriguing candidates (Carisey and Ballestrem, 2011; Gomez et al., 2011; Nayal et al., 2004). Within a tissue many more cues are available to cells in addition to geometry, though geometry may be actively or passively involved in the spatial arrangement of these cues. NuMA, a protein known to be involved in orienting the mitotic spindle via interaction with the microtubule motor dynein (reviewed in (Kotak and Gonczy, 2013), is enriched at tricellular junctions within Drosophila pupal notum epithelium, and the position of tricellular junctions is a reliable determinant of division orientation (Bosveld et al., 2016). Therefore cell junctions could be important for spindle orientation, however, the mechanism by which NuMA is enriched at tricellular junctions is not yet clear. Separating the contribution of cell geometry and force to mitotic spindle orientation is challenging, especially within a complex 3D tissue with a mixture of chemical and mechanical cues. To do so requires both an ability to quantify the mechanical environment that cells experience in a tissue, and an ability to manipulate the molecular candidates that may be involved in mechanotransduction of these physical environmental cues.
There are currently only a few studies on the role of mechanical stress on cell division in native tissues. Anisotropic forces were mapped by laser ablation and mathematical modeling during zebrafish embryo epiboly to show that mitotic spindle orientation aligns with a global stress patterns in the tissue and that this aids epiboly progression (Campinho et al., 2013). Mechanical forces present during development and growth of the Drosophila wing imaginal disc have also been studied to understand how proliferation and growth of the wing disc can be tightly controlled to form the adult wing (Legoff et al., 2013; Mao et al., 2013). Strain levels across the wing disc were mapped and shown to correlate with cell shape and the orientation of cell divisions. This varying level and orientation of stresses has been hypothesised to be critical for normal growth and morphogenesis of the wing disc (Legoff et al., 2013). Although these studies make a very promising start to mapping mechanical stresses in vivo they focus on epithelial monolayers and the cellular mechanisms underlying these findings remain unclear. Therefore, there is a crucial requirement to develop systems in which the interaction between the mechanical environment and cell division within a complex 3D tissue or embryonic context can be fully revealed.
The Xenopus laevis embryo is an excellent model for investigating mechanical properties and cell division within tissues and the living organism, and offers a number of advantages for this. Female frogs can be induced to lay over 1,000 eggs at any time of year by hormone injection, meaning that applications are rarely limited by number of embryos available. These embryos then develop synchronously as eggs are fertilised in vitro. The embryos are large (~1.2mm diameter), allowing micro-dissection and manipulation of tissue explants from the embryo. Xenopus laevis is a well established model organism for research in developmental biology, therefore there are reliable protocols for embryo culture and manipulation (Sive et al., 2000). Microinjection of the embryos at 1 to 4 cell stages is relatively easy due to their large size. Genetic manipulation is possible by injection of morpholino oligonucleotides, TALENs or CRISPR/Cas9 constructs to knock down gene function (Tandon et al., 2016). In vitro synthesised mRNA coding for dominant negative proteins can also be injected to alter gene function, while mRNA coding for fluorescently labelled proteins can be injected to label cellular and sub-cellular structures (Kieserman et al., 2010; Sive et al., 2000; Woolner et al., 2009). It is also possible to produce transgenic lines, for example to fluorescently label a developing organ or cellular structures (Ishibashi et al., 2012a; Ishibashi et al., 2012b; Takagi et al., 2013). Pharmaceutical manipulation of embryonic development is very simple, as bioactive compounds can simply be added to the embryo media (Wheeler and Brandli, 2009). All these characteristics make Xenopus a highly suitable system for many biological studies, including those investigating mechanical stress and cell division.
In this review we will first summarise the use of Xenopus in studies of mechanical stress, and then look at studies that have worked on cell division and mitosis in Xenopus, before finally considering the advantages of using Xenopus as a model to study the intersection of mechanics and cell division.
Methods for investigating mechanical stress in Xenopus
There is a long tradition of using embryos of Xenopus and other amphibians to study morphogenetic movements and changes in cell shape during embryogenesis and gastrulation (Keller et al., 2003). However, many methods to quantify the mechanical properties and stresses of embryonic tissue have only been developed more recently (Campas, 2016). Now, many techniques are available to study the mechanical properties of Xenopus embryonic tissues (Summarised in Table 1). Here we will group them into methods that locate and compare the level of anisotropic stresses within and between tissues, methods to determine the mechanical properties of tissue, and methods to measure the force produced by tissues.
Table 1.
Overview of biomechanical methods used in Xenopus embryos
| Method and reference | Schematic | Overview | Mechanical property measured | Tissue studied | Quantitative? | Destructive? |
|---|---|---|---|---|---|---|
| Tissue dissection (Beloussov et al., 1975) |

|
Tissue cut with blade and immediate deformation observed | Map location and direction of mechanical stresses in embryo | Whole embryo | No | Yes |
| Laser Ablation (Hara et al., 2013) |
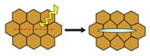
|
Wound tissue with laser and record recoil velocity | Map location and direction of mechanical stresses in embryo | Whole embryo or expant | No | Yes |
| Strain mapping (Kim et al., 2014; Feroze et al., 2015; Yamashita et al., 2016) |
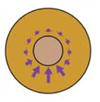
|
Observation of movement and deformation of cells in tissue allows forces to be inferred | Map location and direction of mechanical stresses in embryo and/or estimate the distance a mechanical signal propogates | Whole embryo or expant | No | No |
| FRET-based tension sensor (Yamashita et al., 2016) |
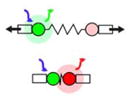
|
Measure FRET around an elastic linker within protein that can experience tension. | Tension at the molecular level | Whole embryo | No | No |
| nNewton Force Measurement (Moore et al., 1995; Davidson and Keller, 2007) |
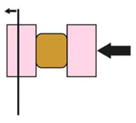
|
Tissue explant uniaxially compressed against a cantilever | Viscoelastic properties of explant | Brick shaped explant | Yes | Explant cut |
| Microaspiration (von Dassow et al., 2010) |
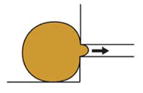
|
Measure length of tissue drawn into a channel of known diameter under negative pressure | Viscoelastic properties of embryo | Whole embryo | Yes | No |
| Axisymmetric drop shape analysis (Kalantarian et al., 2009; Luu et al., 2011; David et al., 2014) |
|
Measure deformation of an explant or cellular aggregate over time | Surface tension of the explant or aggregate | Explant or cellular aggregate | Yes | Yes |
| Cantilever based measurement of migration force (Hara et al., 2013) |
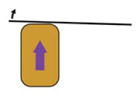
|
A migrating explant pushes against a cantilever | Force produced during explant migration | Leading edge mesoderm explant | Yes | Explant cut |
| Insertion of cantilevers into embryo (Feroze et al., 2015) |
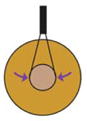
|
Morphogenetic movements of blastopore closure push directly on inserted cantilever | Force produced during blastopore closure | Whole embryo | Yes | No |
| Tractor pull assay (Pfister et al., 2016) |
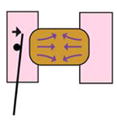
|
Covergence and extension of explant pulls on sled and moves cantilever | Force produced during convergence and extension | Giant explant | Yes | Explant cut |
| 3D Force Microscopy (Zhou et al., 2015) |
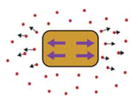
|
Force produced by explant embedded in gel measured by fluorescent bead displacement. | Force produced during morphogenetic movements | Dorsal explant | Yes | Explant cut |
Methods to map anisotropic forces in Xenopus
To begin to understand how forces act in the embryo or a developing tissue to pattern growth and morphogenesis, the location and magnitude of these forces needs to be mapped. Initially this was achieved by simply cutting into the embryo with a blade. The speed and direction that the tissue retracts gives an indication of the level and direction of tension in the tissue, and can be used to map global stresses in the embryo (Beloussov et al., 1975). More recently a similar effect has been achieved by using a laser to wound the tissue, a method known as laser ablation (Kiehart et al., 2000). The immediate recoil of cells around the laser ablation wound is recorded by microscopy; the speed of recoil indicates the level of tension. However, if the mechanical properties of the tissue are not known this method remains qualitative (Ma et al., 2009). In Xenopus, laser ablation has been used to assess force transmission by migrating cell populations during gastrulation (Hara et al., 2013). Although able to indicate the direction and relative magnitude of mechanical stress in tissue, both of these invasive methods damage the tissue, releasing cues to initiate a wound healing response and so perturbing normal development beyond the point of assessment.
Some aspects of mechanical stress can also be inferred without damaging tissue. Since forces on a tissue generate shape change and deformation, some information about mechanical cues can be inferred from quantitative maps of strain (a dimensionless measure of deformation). The levels of anisotropic strain in the tissue can be inferred by assessing the rate of changes in cell and tissue shape during morphogenesis, known as strain mapping or tissue tectonics (Blanchard et al., 2009). In Xenopus, strain mapping has been used for a number of different purposes. By assessing the speed and movement of cells during blastopore closure, the location, direction and relative magnitude of morphogenetic forces driving gastrulation have been mapped (Feroze et al., 2015). Similarly, comparing cell level strain rates between epithelial and neural precursor ectoderm suggests a greater level of tension in neural precursor ectoderm (Yamashita et al., 2016). Strain mapping can also be used to estimate the distance that a mechanical effect propagates through a tissue; after a subset of cells in a Xenopus epithelial explant were stimulated to contract by precise stimulation with ATP, the long-range contraction response was characterised by strain mapping (Kim et al., 2014). Strain mapping is limited since it cannot distinguish between internal and external sources of force. Still, with a priori assumptions about the tissue or the morphogenetic process, strain mapping may allow forces to be inferred when direct visualisation or measurement of these forces is not practical.
To directly observe stress within a tissue, Forster Resonance Energy Transfer (FRET) based tension sensors have been developed (reviewed in (Jurchenko and Salaita, 2015). FRET based tension sensors are molecular reporters of intramolecular strain. Tension sensors are commonly constructed from a protein that would normally transmit tension in the cell, such as proteins involved in cellular adhesion. The native protein is then genetically engineered to include a FRET-based strain sensor consisting of an exogenous elastic protein, such as one from spider silk. This technique allows tensions in molecular complexes or load-bearing structures in the cell to be mapped in vivo by calculating the FRET index, although quantitative readouts of tension have yet to be achieved in a tissue environment and it is not yet possible to determine the direction of FRET-sensed tension within a tissue. In Xenopus, a FRET-tension sensor inserted into α-actinin, an F-actin cross-linking protein, has been used to show that α-actinin complexes are under more tension in precursor neural ectoderm than in precursor epidermal ectoderm in the intact developing embryo (Yamashita et al., 2016). These methods to visualise and map patterns of tension provide valuable insights on the mechanical environment across a tissue, but to fully understand how mechanics affects cell behaviour we need to be able to quantify the mechanical properties of the tissue.
Methods to measure the mechanical properties of Xenopus embryonic tissues
The application of quantitative mechanical methods to understanding the function of biological tissues is known as biomechanics. The following methods all apply a known force to Xenopus embryos or dissected explants, and measure the response of the tissue over time. This allows fundamental mechanical properties, such as the viscoelastic behaviour of the tissue with regard to tissue stiffness and fluidity, to be calculated.
To measure the mechanical resistance of embryonic tissue to compressive force, a device known as the nanoNewton Force measurement device has been developed. A brick shaped embryonic explant is uniaxially compressed against a calibrated cantilever in the fashion of a creep-test (Findley, 1989). Compressive force, its effect on deforming the tissue, and tissue geometry allow the viscoelastic properties of the explant to be calculated (Davidson and Keller, 2007; Moore et al., 1995). The major advantage of nanoNewton force measurement is that the uniform stresses and resulting tissue deformation are easily interpreted by a time-dependent Young’s modulus, a universal measure of mechanical properties that does not rely on a priori structural mechanical models. However, this tool has several drawbacks in that it is only possible with regular, brick-shaped blocks, destructively isolated from the embryo; assumptions needed to interpret the mechanical test restricts the shape, size, and composition of the block. Several other approaches have been adapted to work around these restrictions and estimate the mechanical properties of irregular shaped tissues or of intact whole embryos.
To measure the mechanical properties of thinner tissues, or of cell aggregates, a technique based on relaxation of the explant over time, known as axisymmetric drop shape or sessile drop analysis, can be used. Dissected explants and cell aggregates are observed as they deform due to gravity, or after centrifugation. Briefly, an explant that retains the shape of a very round ball is thought to have a higher surface tension than an explant that flattens over time (David et al., 2009; Kalantarian et al., 2009; Luu et al., 2011). The change in shape under the uniform stress of centrifugation allows an equivalent surface tension of the explant or aggregate to be calculated. Additionally, active tension that restores the tissue to a spherical shape can be estimated by these experiments. This method can be extended by observing cell movement during explant relaxation, with cell neighbour exchange suggesting a higher level of “fluidity” in the tissue (David et al., 2014). This method also relies on cutting explants from the embryo, or dissociating embryonic cells to form aggregates, but can be used with early stage tissues that do not maintain their shape after isolation.
It is possible to non-destructively measure stiffness of the entire embryo by microaspiration of embryonic tissue into a channel of known diameter using a known negative pressure. The distance that tissue is drawn into the channel is used to calculate the tissue stiffness (Rolo et al., 2009; von Dassow et al., 2010). As the embryo is not damaged during microaspiration, the same embryo can be assessed later during development, to observe changes in mechanical properties over time. Microaspiration has also been used to change the geometry or apply directional strain to Xenopus epithelia (Chien et al., 2015).
Microaspiration-, centrifugation-, and creep-based methods all apply known forces to the embryonic tissue to measure its mechanical properties. Viscoelastic properties measured by these methods may be considered “passive” responses but still reflect biological processes active in the embryo. Since these measurements are typically made over a short time span they are not able to evaluate the ability of embryonic tissues to produce directed forces that drive morphogenetic movements.
Methods to measure forces produced by embryonic tissues
A Xenopus embryo develops without the aid of externally applied forces (except gravity and osmotic pressures) and so, to truly understand how the mechanical environment changes over developmental time, it is important to understand the forces produced by the embryo during growth and morphogenesis.
Force production over long time-scales can be accurately measured using a calibrated cantilever: if embryonic tissue pushes or pulls against the cantilever, the force produced by the morphogenetic movement can be determined by the distance the cantilever moves. Calibrated cantilevers have been inserted into Xenopus embryos to directly measure the forces driving blastopore closure (Feroze et al., 2015). Cantilevers have also been used to measure forces produced by extending Xenopus tissue explants (Moore, 1994). Additionally, a cantilever placed in the path of a migrating leading edge mesoderm explant was used to quantify the force produced by these cells during gastrulation (Hara et al., 2013). Both the previous studies measured pushing, or compressive forces; a pulling or tension force can be measured by the ‘tractor-pull’ assay, which was used to quantify the force produced by cell intercalation during convergent extension movements in a giant explant (Pfister et al., 2016). Cantilevers are flexible and precise tools for measuring forces of the magnitude that embryonic tissues can generate. However, they can only measure a force that acts linearly.
3-dimensional mapping and quantification of forces produced by a tissue is possible using 3D Force Microscopy (3D-FM). An embryonic explant is embedded in an agarose gel of known stiffness containing fluorescent beads. Deformation of the explant pushes on the gel, moving the beads embedded in it. The movement of the beads over time is tracked in 3D by microscopy, allowing the force responsible for their movement to be calculated. 3D-FM has been used to measure force produced in both anterior-posterior and dorsal-ventral directions during elongation and thickening of dorsal explants (Zhou et al., 2015).
All the methods described here can be used in combination to both map and quantify mechanical forces during development of the Xenopus embryo. While these methods are not restricted for use with Xenopus, the size of Xenopus embryos and their durable response to microsurgery enables application of the tools with greater ease than other developmental model organisms. This “mechanical tractability” combined with the manipulation of the embryo by genetic, biochemical, or microsurgical methods provides a powerful set of tools to ascertain the importance of different factors for creating and maintaining the mechanical tissue environment. Once the mechanical environment of the tissue is understood, the next logical step is to ask how this affects the behaviour of cells within that tissue. In the next section we discuss methods that allow one important aspect of cell behaviour to be studied in Xenopus: cell division.
Methods for investigating mitosis and cell division in Xenopus
Over many years, Xenopus has proved to be a key model system for the study of the cell cycle, mitotic and meiotic spindles and cell division. A great strength is the ability to combine in vitro Xenopus egg extract work with in vivo studies in the oocyte, egg and embryo. Moreover, in recent years, live imaging techniques in Xenopus have advanced considerably, allowing the dynamic analysis of spindles and cytokinesis in vivo. The techniques, knowledge and reagents provided by this rich history of cell cycle research makes Xenopus a perfect system for further analysis of mitosis and cell division, for example in relation to the role of external mechanical force in these processes. Here, we outline some of the major approaches and findings in the study of cell cycle and cell division in Xenopus, with a view to how they might contribute to furthering our understanding of force and cell division.
Use of in vitro Xenopus egg extracts
Xenopus embryos undergo their first 12 rounds of cell division before zygotic transcription, meaning that their eggs are loaded with all of the RNAs and proteins required to drive these divisions and build over 4000 nuclei. Extracts prepared by centrifugation of Xenopus eggs therefore provide a formidable self-contained platform to investigate many of the processes underlying the cell cycle and cell division: key aspects of cyclin-dependent kinase (cdk) activity, DNA replication, mitotic spindle assembly and nuclear envelope dynamics have all been uncovered using egg extracts (Cross and Powers, 2009; Philpott and Yew, 2008). Depending on how they are prepared, egg extracts can be induced to assemble nuclei or spindles around exogenously supplied chromatin (usually demembranated sperm chromatin). By adding fluorescent tubulin, assembly of the spindle can be followed dynamically (Sawin and Mitchison, 1991).
A great strength of the egg extract system is that individual factors can be depleted (e.g. by immunodepletion) or inhibited (e.g. by the addition of a dominant negative mutant form of the protein) in the extract and the effect of their loss on spindle or nuclei assembly can be assessed. In this way many of the key spindle assembly factors have been discovered and/or studied extensively using egg extracts. Examples include Ran GTPase, TPX2, Aurora A, Maskin and HURP (Brunet et al., 2004; Koffa et al., 2006; O’Brien et al., 2005; Ohba et al., 1999; Tsai et al., 2003). Moreover, much of our knowledge of the spindle kinesin, Eg5, has come from Xenopus egg extracts. Eg5 is a plus-end directed microtubule motor that plays a crucial role in the assembly of a bipolar spindle by pushing overlapping spindle microtubules apart: depletion or pharmacological inhibition of Eg5 in egg extracts leads to the formation of monopolar spindles (Mayer et al., 1999; Miyamoto et al., 2004; Sawin et al., 1992). In recent years, egg extracts have also been vital for understanding how cellular structures such as spindles and nuclei are appropriately scaled to the size of the cell they inhabit (Levy and Heald, 2012). The cells, eggs, nuclei and spindles of Xenopus tropicalis are all considerably smaller than Xenopus laevis, and this size difference is maintained for spindles and nuclei in extracts. By comparing these two systems, much has been learnt about the size control of these organelles, for example nuclei size is regulated by differing nuclear import rates between the two Xenopus species (Levy and Heald, 2010), while spindle size differences are due, at least in part, to differences in the phosphorylation state of the microtubule-severing enzyme, katanin (Loughlin et al., 2011).
As outlined here, the use of Xenopus egg extracts has been key to identifying the molecular components required for spindle and nuclei assembly and cell cycle progression. However, a next important step is to understand how these processes are controlled in vivo, especially within the context of a complex developing tissue.
Imaging mitosis and cell division in vivo using Xenopus
The vast knowledge and array of reagents (especially Xenopus specific antibodies) built up through the study of the cell cycle, spindle and nuclei assembly using egg extracts provides a great background for the study of these processes in vivo in Xenopus. Although Xenopus has previously been thought of as a system that is not ideally suited to live imaging, due to the opacity of the embryo, recent advances have demonstrated that mitotic spindles and cell division can be imaged live and at high resolution in these embryos (Kieserman et al., 2010; Woolner et al., 2009). The fact that Xenopus embryos develop externally at room temperature make them particularly amenable to live imaging, whilst the large size of their cells make them ideal for imaging subcellular processes. Moreover, live imaging can be combined with analysis of fixed tissue, and perturbations such as morpholino knockdown and/or the use of pharmacological inhibitors to study the cellular machinery underlying spindle assembly and cytokinesis in the Xenopus embryo, egg and oocyte.
Recent work in Xenopus has uncovered some key aspects of the regulation of spindle assembly and function. For example, live imaging in Xenopus embryos has revealed how the molecular motors, dynein and myosin-10, function to maintain spindle bipolarity during mitosis (Jones et al., 2014; Woolner et al., 2008). Moreover, Xenopus eggs and embryos have also uncovered how myosin-10, which can bind to both actin and microtubules, regulates spindle length, orientation and dynamics through interactions with both cytoskeletons (Sandquist et al., 2016; Weber et al., 2004; Woolner et al., 2008; Woolner and Papalopulu, 2012). In related work, live analysis revealed that highly dynamic F-actin cables are associated with the mitotic spindle as it assembles and proceeds through metaphase and anaphase. These cables reach between the spindle and cell cortex and may be involved in moving and positioning the spindle (Woolner et al., 2008). Similar subcortical actin structures have since been identified in cultured cells, where they are thought to be involved in aligning the mitotic spindle to externally applied force (Fink et al., 2011). Together these studies demonstrate the power of the Xenopus embryo to further our understanding of spindle assembly and function.
In a similar way, Xenopus has made key contributions to understanding regulation of the cytoskeleton during cytokinesis. The Xenopus oocyte has proved to be an excellent system for modelling the cytoskeletal regulation underlying cytokinesis, since small laser wounds made in the oocyte lead to the formation of contractile arrays of actomyosin analogous to those formed during cytokinesis (Bement et al., 2006). Live analysis of oocytes has revealed that assembly of these contractile arrays is controlled by spatially restricted zones of Rho GTPase activity, disruption of these zones interferes with array formation and wound healing (Benink and Bement, 2005; Burkel et al., 2012; Vaughan et al., 2011). Similar zones of active Rho have also been identified at the site of contractile ring formation during cytokinesis in the Xenopus embryo and other systems (Miller and Bement, 2009; Piekny et al., 2005; Yuce et al., 2005). In the case of cytokinesis, the tight spatial control of Rho activity is key for proper formation and maintenance of the contractile ring; this is controlled by the constant flux of Rho through its activation/deactivation cycle (Breznau et al., 2015; Miller and Bement, 2009). In this way, Xenopus continues to make key contributions to revealing how the cytoskeleton is regulated during cell division. A perfect complement to this molecular work is to use Xenopus to investigate the control of cell division in the context of complex tissues, as described below.
The orientation of cell division, determined by the orientation of the mitotic spindle, is crucial for cell fate determination and also for tissue shaping during embryonic development. The Xenopus embryo has made important contributions to our understanding of the regulation of division orientation. For example, in the Xenopus blastula, oriented divisions of polarised blastomeres generate two distinct populations of cells – the superficial and deep cell layers – that have different neuronal fates, crucial for the development of the nervous system (Chalmers et al., 2005; Chalmers et al., 2003; Muller and Hausen, 1995). The asymmetric divisions that produce these two cell populations are driven by the orientation of mitotic spindles in the dividing blastomeres, with spindles oriented perpendicular to the surface of the embryo giving asymmetric divisions. Live analysis and dissection of individual blastomeres has shown that spindle orientation is determined by the cell shape of the blastomeres, spindles align with the long axis of the cell, such that tall cells with a small apical surface produce perpendicular divisions (Strauss et al., 2006). Slightly later in development, at gastrulation, the majority of cells in the outer epithelial layer of the embryo undergo symmetric divisions in the plane of the tissue, which are thought to help spread the tissue during epiboly (Kieserman and Wallingford, 2009; Marsden and DeSimone, 2001; Tabler et al., 2010; Woolner and Papalopulu, 2012). Analysis of these divisions in live embryos has shown that, as well as being oriented with the plane of the epithelium, the spindles in these cells show a very specific apicobasal position. The spindles are positioned along the apicobasal axis through a balance between microtubule and actomyosin driven forces and the maintenance of this position appears to be closely linked to keeping the spindle level to produce a symmetric division (Woolner and Papalopulu, 2012). Xenopus embryos have also been used to study how the planar direction of division in epithelial cells is controlled. During neural tube closure, divisions in the neural plate of the Xenopus embryo show a stereotyped planer orientation, dividing in an alternating oblique pattern to the midline. Intriguingly, this planar orientation pattern does not appear to require PCP signalling but is instead regulated by the small GTPase, Cdc42 (Kieserman and Wallingford, 2009).
In this section we have described how Xenopus offers a powerful system to understand mitosis and cell division across biological scales, from molecular components and their regulation all the way up to roles for cell division in fate determination and tissue shaping in the developing embryo. Next we will discuss how this knowledge can be brought together with the biomechanical strengths of the Xenopus system to investigate how mechanical force and cell division are coordinated in tissues.
Conclusion: Using Xenopus as a model to investigate the role of force in cell division
We have described the strengths of Xenopus as a system to study both biomechanics and cell division. An obvious next step is to combine these strengths to investigate when and how mechanical force influences cell division, especially in complex developing tissues. Recent studies, the vast majority performed in cultured cells, have already indicated that mechanical strain alters division rate and division orientation. However, the molecular mechanisms linking strain and cell division remain unclear, even in single cells, and we understand even less how force and division are coordinated in vivo in complex tissues. The Xenopus embryo offers an excellent opportunity to cross these scales – with the ability to address both molecular mechanism and in vivo relevance in a single system.
An important first step in understanding how mechanical force regulates cell division in tissue is the development of tools to reproducibly deform 3D tissue and simultaneously image and analyse cell division. To date this has been achieved for simple cultured cell monolayers, but not complex tissues. Xenopus animal cap explants offer a great opportunity to bridge this gap, since they provide a resilient epithelial tissue that maintains its 3D multi-layered in vivo structure when cultured for short periods of time (we have tested up to 5 hours from dissection in our experiments). Using animal caps, we have developed a system to apply reproducible stretch or compression to tissue (Figure 1). Animal caps are dissected from early gastrula stage embryos and cultured on an elastomeric silicon-based (PDMS) membrane coated with fibronectin. Animal caps are dissected and adhered to the PDMS membrane using a protocol similar to that described previously (Joshi and Davidson, 2010), adherence takes only 2 hours and the 3D in vivo structure of the tissue is maintained. The PDMS substrate is then mounted on a computer controlled biaxial stretcher that can apply strain along one or both axes (Deben UK). Compression can also be applied using this system, by adhering the tissue to an already stretched PDMS membrane and then releasing the stretch once the animal cap is adhered. To analyse cell division in the stretched/compressed tissue, the stretch device can be mounted under a confocal microscope and cells in the apical layer of the epithelium recorded by time-lapse microscopy, allowing the effect of mechanics on cell shape, division rate and orientation to be assessed (Figure 1). To investigate potential molecular mechanism, the tissue can be manipulated prior to stretching, for example morpholino oligonucleotides can be injected into the embryo at the 2–4 cell stage to knock down candidates involved in mechanosensation. In this way, the role of mechanical regulation in cell division can be molecularly dissected in the context of a 3D tissue.
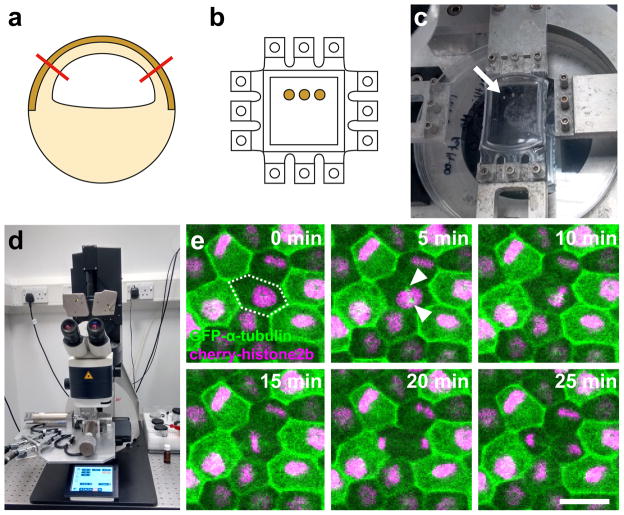
Figure 1. Method to simultaneously apply a reproducible strain and analyse cell division in the Xenopus animal cap.
a) The animal cap is dissected (red lines) from an early gastrula stage embryo that has been labeled by injection of GFP-α-tubulin and Cherry-histone2B mRNAs at the 2 cell stage. The dark brown area indicates the pigmented apical cell layer that will be imaged. b) Animal caps are cultured on a fibronectin coated flexible PDMS membrane until adhered. The PDMS membrane is cast in a custom mould with teeth on all four sides allowing either a uni-axial or biaxial stretch to be applied. c) PDMS membrane with cultured animal caps (arrow) attached to stretch apparatus. A uniaxial stretch is applied to stretch the animal caps by 35%; the teeth orthogonal to the stretch direction have been removed. d) The stretch apparatus is attached to the stage of an upright confocal microscope; a water-dipping objective is used to visualize cells in the apical layer. e) Example timelapse series using maximum intensity projections of cell division in the apical cell layer with frames 5 minutes apart. 35% stretch was applied in horizontal direction. The dividing cell is marked by dashed outline at 0 min, GFP-α-tubulin (green) labels the microtubule cytoskeleton and condensing centrosomes (arrowheads), Cherry-histone2B (magenta) labels nuclei. Scale bar: 30μm.
An ex vivo system such as that described above will be an important aid to understanding the molecular machinery involved in linking cell division and mechanical force in tissue. However, an ultimate goal will always be to take what we learn from these systems and explore how they apply in vivo. As described in the previous sections, the Xenopus embryo offers an ideal system to investigate both cell division and biomechanics in vivo and the significance of mechanical control in tissue self-assembly. A first step will be to use methods such as laser ablation and strain mapping to infer force across proliferating tissue in vivo and integrate this with information about division rate and division orientation obtained from live imaging. Knockdown of mechanosensing candidates gleaned from ex vivo work could then be used to test functionally how mechanical force and cell division are linked in vivo. Moreover, these types of investigation will also allow us to determine the importance of this link for embryonic events such as tissue morphogenesis. In this way, recent work combining cell culture studies with the Xenopus embryo has revealed that focal adhesion kinase (FAK) plays an important role in transducing extracellular force in order to orient the mitotic spindle (Petridou and Skourides 2014). In single cultured cells, knockout of FAK was shown to reduce the ability of mitotic spindles to align with cell shape and force when grown on micropatterned substrates. Moreover, in Xenopus gastrula stage embryos, morpholino knockdown of FAK prevented spindles from aligning with acute changes in tension when neighbouring cells in the outer epithelium were laser ablated. Selectively blocking FAK function using an inducible dominant negative construct led to failures in epiboly and pronephros development, suggesting that FAK-mediated spindle orientation plays an important role in morphogenesis and organogenesis (Petridou and Skourides, 2014). This work illustrates beautifully the potential for Xenopus to reveal how and why mechanical force and cell division are so intricately linked.
Overall, Xenopus offers many advantages to studies on the interaction of mechanics and cell division. Many powerful tools to measure and manipulate the mechanical environment, either in vivo or on tissue explants, were initially developed using Xenopus embryos. Similarly, there is a long history of using Xenopus to clarify the molecular and cellular pathways guiding mitosis and cell division. As methods for studying the mechanical environment and cell division are successfully established in this model organism, Xenopus is ideally placed as an experimental system for understanding how these processes are interlinked.
Acknowledgments
SW and GS-V were supported by a Wellcome Trust/Royal Society Sir Henry Dale Fellowship to SW [098390/Z/12/Z]. Further support for GS-V was provided by a Wellcome Trust ISSF award [105610/Z/14/Z]. LAD was supported by grants from the National Science Foundation (USA, CBET-1547790) and the National Institutes of Health (USA, R01HD044750; R56HL13495). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
References
- Adams RJ. Metaphase spindles rotate in the neuroepithelium of rat cerebral cortex. J Neurosci. 1996;16:7610–7618. doi: 10.1523/JNEUROSCI.16-23-07610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Beloussov LV, Dorfman JG, Cherdantzev VG. Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol. 1975;34:559–574. [PubMed] [Google Scholar]
- Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard GB, Kabla AJ, Schultz NL, Butler LC, Sanson B, Gorfinkiel N, Mahadevan L, Adams RJ. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nat Methods. 2009;6:458–464. doi: 10.1038/nmeth.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosveld F, Markova O, Guirao B, Martin C, Wang ZM, Pierre A, Balakireva M, Gaugue I, Ainslie A, Christophorou N, Lubensky DK, Minc N, Bellaiche Y. Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis (vol 530, pg 495, 2016) Nature. 2016:534. doi: 10.1038/nature16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznau EB, Semack AC, Higashi T, Miller AL. MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell-cell junctions in epithelial cells. Mol Biol Cell. 2015;26:2439–2455. doi: 10.1091/mbc.E14-11-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Sardon T, Zimmerman T, Wittmann T, Pepperkok R, Karsenti E, Vernos I. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol Biol Cell. 2004;15:5318–5328. doi: 10.1091/mbc.E04-05-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel BM, Benink HA, Vaughan EM, von Dassow G, Bement WM. A Rho GTPase signal treadmill backs a contractile array. Dev Cell. 2012;23:384–396. doi: 10.1016/j.devcel.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campas O. A toolbox to explore the mechanics of living embryonic tissues. Semin Cell Dev Biol. 2016;55:119–130. doi: 10.1016/j.semcdb.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg CP. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol. 2013;15:1405–1414. doi: 10.1038/ncb2869. [DOI] [PubMed] [Google Scholar]
- Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. European Journal of Cell Biology. 2011;90:157–163. doi: 10.1016/j.ejcb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development. 2005;132:977–986. doi: 10.1242/dev.01645. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Strauss B, Papalopulu N. Oriented cell divisions asymmetrically segregate aPKC and generate cell fate diversity in the early Xenopus embryo. Development. 2003;130:2657–2668. doi: 10.1242/dev.00490. [DOI] [PubMed] [Google Scholar]
- Chien YH, Keller R, Kintner C, Shook DR. Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr Biol. 2015;25:2774–2784. doi: 10.1016/j.cub.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MK, Powers MA. Learning about cancer from frogs: analysis of mitotic spindles in Xenopus egg extracts. Dis Model Mech. 2009;2:541–547. doi: 10.1242/dmm.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Luu O, Damm EW, Wen JW, Nagel M, Winklbauer R. Tissue cohesion and the mechanics of cell rearrangement. Development. 2014;141:3672–3682. doi: 10.1242/dev.104315. [DOI] [PubMed] [Google Scholar]
- David R, Ninomiya H, Winklbauer R, Neumann AW. Tissue surface tension measurement by rigorous axisymmetric drop shape analysis. Colloids Surf B Biointerfaces. 2009;72:236–240. doi: 10.1016/j.colsurfb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Davidson L, Keller R. Measuring mechanical properties of embryos and embryonic tissues. Methods Cell Biol. 2007;83:425–439. doi: 10.1016/S0091-679X(07)83018-4. [DOI] [PubMed] [Google Scholar]
- Feroze R, Shawky JH, von Dassow M, Davidson LA. Mechanics of blastopore closure during amphibian gastrulation. Dev Biol. 2015;398:57–67. doi: 10.1016/j.ydbio.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley WN, Lai JS, Onaran K. Creep and relaxation of nonlinear viscoelastic materials. New York: Dover Publications, Inc; 1989. [Google Scholar]
- Fink J, Carpi N, Betz T, Betard A, Chebah M, Azioune A, Bornens M, Sykes C, Fetler L, Cuvelier D, Piel M. External forces control mitotic spindle positioning. Nat Cell Biol. 2011;13:771–778. doi: 10.1038/ncb2269. [DOI] [PubMed] [Google Scholar]
- Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends in Cell Biology. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hara Y, Nagayama K, Yamamoto TS, Matsumoto T, Suzuki M, Ueno N. Directional migration of leading-edge mesoderm generates physical forces: Implication in Xenopus notochord formation during gastrulation. Dev Biol. 2013;382:482–495. doi: 10.1016/j.ydbio.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Hertwig O. Ueber den Werth der ersten Furchungszellen für die Organbildung des Embryo. Experimentelle Studien am Frosch- und Tritonei. Archiv für mikroskopische Anatomie. 1893;42:662–807. [Google Scholar]
- Ishibashi S, Kroll KL, Amaya E. Generating transgenic frog embryos by restriction enzyme mediated integration (REMI) Methods Mol Biol. 2012a;917:185–203. doi: 10.1007/978-1-61779-992-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Love NR, Amaya E. A simple method of transgenesis using I-SceI meganuclease in Xenopus. Methods Mol Biol. 2012b;917:205–218. doi: 10.1007/978-1-61779-992-1_12. [DOI] [PubMed] [Google Scholar]
- Jones LA, Villemant C, Starborg T, Salter A, Goddard G, Ruane P, Woodman PG, Papalopulu N, Woolner S, Allan VJ. Dynein light intermediate chains maintain spindle bipolarity by functioning in centriole cohesion. J Cell Biol. 2014;207:499–516. doi: 10.1083/jcb.201408025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SD, Davidson LA. Live-cell imaging and quantitative analysis of embryonic epithelial cells in Xenopus laevis. J Vis Exp. 2010 doi: 10.3791/1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurchenko C, Salaita KS. Lighting Up the Force: Investigating Mechanisms of Mechanotransduction Using Fluorescent Tension Probes. Mol Cell Biol. 2015;35:2570–2582. doi: 10.1128/MCB.00195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantarian A, Ninomiya H, Saad SM, David R, Winklbauer R, Neumann AW. Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. Biophys J. 2009;96:1606–1616. doi: 10.1016/j.bpj.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieserman EK, Lee C, Gray RS, Park TJ, Wallingford JB. High-magnification in vivo imaging of Xenopus embryos for cell and developmental biology. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5427. pdb prot5427. [DOI] [PubMed] [Google Scholar]
- Kieserman EK, Wallingford JB. In vivo imaging reveals a role for Cdc42 in spindle positioning and planar orientation of cell divisions during vertebrate neural tube closure. J Cell Sci. 2009;122:2481–2490. doi: 10.1242/jcs.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Hazar M, Vijayraghavan DS, Song J, Jackson TR, Joshi SD, Messner WC, Davidson LA, LeDuc PR. Mechanochemical actuators of embryonic epithelial contractility. Proc Natl Acad Sci U S A. 2014;111:14366–14371. doi: 10.1073/pnas.1405209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW. HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol. 2006;16:743–754. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kotak S, Gonczy P. Mechanisms of spindle positioning: cortical force generators in the limelight. Current Opinion in Cell Biology. 2013;25:741–748. doi: 10.1016/j.ceb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends in Cell Biology. 2009;19:174–179. doi: 10.1016/j.tcb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Legoff L, Rouault H, Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- Levy DL, Heald R. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell. 2010;143:288–298. doi: 10.1016/j.cell.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL, Heald R. Mechanisms of intracellular scaling. Annu Rev Cell Dev Biol. 2012;28:113–135. doi: 10.1146/annurev-cellbio-092910-154158. [DOI] [PubMed] [Google Scholar]
- Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ, Heald R. Katanin contributes to interspecies spindle length scaling in Xenopus. Cell. 2011;147:1397–1407. doi: 10.1016/j.cell.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu O, David R, Ninomiya H, Winklbauer R. Large-scale mechanical properties of Xenopus embryonic epithelium. Proc Natl Acad Sci U S A. 2011;108:4000–4005. doi: 10.1073/pnas.1010331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- Mao YL, Tournier AL, Hoppe A, Kester L, Thompson BJ, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. Embo Journal. 2013;32:2790–2803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc N, Burgess D, Chang F. Influence of Cell Geometry on Division-Plane Positioning. Cell. 2011;144:414–426. doi: 10.1016/j.cell.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Perlman ZE, Burbank KS, Groen AC, Mitchison TJ. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J Cell Biol. 2004;167:813–818. doi: 10.1083/jcb.200407126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW. A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Trans Biomed Eng. 1994;41:45–50. doi: 10.1109/10.277270. [DOI] [PubMed] [Google Scholar]
- Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- Muller HA, Hausen P. Epithelial cell polarity in early Xenopus development. Dev Dyn. 1995;202:405–420. doi: 10.1002/aja.1002020410. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Horwitz AF. Talin: an emerging focal point of adhesion dynamics. Current Opinion in Cell Biology. 2004;16:94–98. doi: 10.1016/j.ceb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Nestor-Bergmann A, Goddard G, Woolner S. Force and the spindle: mechanical cues in mitotic spindle orientation. Semin Cell Dev Biol. 2014;34:133–139. doi: 10.1016/j.semcdb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LL, Albee AJ, Liu L, Tao W, Dobrzyn P, Lizarraga SB, Wiese C. The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly. Mol Biol Cell. 2005;16:2836–2847. doi: 10.1091/mbc.E04-10-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Nakamura M, Nishitani H, Nishimoto T. Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science. 1999;284:1356–1358. doi: 10.1126/science.284.5418.1356. [DOI] [PubMed] [Google Scholar]
- Pease JC, Tirnauer JS. Mitotic spindle misorientation in cancer--out of alignment and into the fire. J Cell Sci. 2011;124:1007–1016. doi: 10.1242/jcs.081406. [DOI] [PubMed] [Google Scholar]
- Petridou NI, Skourides PA. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat Commun. 2014;5:5240. doi: 10.1038/ncomms6240. [DOI] [PubMed] [Google Scholar]
- Pfister K, Shook DR, Chang C, Keller R, Skoglund P. Molecular model for force production and transmission during vertebrate gastrulation. Development. 2016;143:715–727. doi: 10.1242/dev.128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A, Yew PR. The Xenopus cell cycle: an overview. Mol Biotechnol. 2008;39:9–19. doi: 10.1007/s12033-008-9033-z. [DOI] [PubMed] [Google Scholar]
- Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Quesada-Hernandez E, Caneparo L, Schneider S, Winkler S, Liebling M, Fraser SE, Heisenberg CP. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol. 2010;20:1966–1972. doi: 10.1016/j.cub.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Nathke IS. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rolo A, Skoglund P, Keller R. Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol. 2009;327:327–338. doi: 10.1016/j.ydbio.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist JC, Larson ME, Hine KJ. Myosin-10 independently influences mitotic spindle structure and mitotic progression. Cytoskeleton (Hoboken) 2016;73:351–364. doi: 10.1002/cm.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Poleward microtubule flux mitotic spindles assembled in vitro. J Cell Biol. 1991;112:941–954. doi: 10.1083/jcb.112.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin L, Poulson ND, Foote HP, Lechler T. NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol Biol Cell. 2013;24:3651–3662. doi: 10.1091/mbc.E13-05-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Strauss B, Adams RJ, Papalopulu N. A default mechanism of spindle orientation based on cell shape is sufficient to generate cell fate diversity in polarised Xenopus blastomeres. Development. 2006;133:3883–3893. doi: 10.1242/dev.02578. [DOI] [PubMed] [Google Scholar]
- Streichan SJ, Hoerner CR, Schneidt T, Holzer D, Hufnagel L. Spatial constraints control cell proliferation in tissues. Proc Natl Acad Sci U S A. 2014;111:5586–5591. doi: 10.1073/pnas.1323016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler JM, Yamanaka H, Green JB. PAR-1 promotes primary neurogenesis and asymmetric cell divisions via control of spindle orientation. Development. 2010;137:2501–2505. doi: 10.1242/dev.049833. [DOI] [PubMed] [Google Scholar]
- Takagi C, Sakamaki K, Morita H, Hara Y, Suzuki M, Kinoshita N, Ueno N. Transgenic Xenopus laevis for live imaging in cell and developmental biology. Dev Growth Differ. 2013;55:422–433. doi: 10.1111/dgd.12042. [DOI] [PubMed] [Google Scholar]
- Tandon P, Conlon F, Furlow JD, Horb ME. Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery M, Jimenez-Dalmaroni A, Racine V, Bornens M, Julicher F. Experimental and theoretical study of mitotic spindle orientation. Nature. 2007;447:493–496. doi: 10.1038/nature05786. [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol. 2011;21:270–277. doi: 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dassow M, Strother JA, Davidson LA. Surprisingly simple mechanical behavior of a complex embryonic tissue. PLoS One. 2010;5:e15359. doi: 10.1371/journal.pone.0015359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- Wheeler GN, Brandli AW. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev Dyn. 2009;238:1287–1308. doi: 10.1002/dvdy.21967. [DOI] [PubMed] [Google Scholar]
- Woolner S, Miller AL, Bement WM. Imaging the cytoskeleton in live Xenopus laevis embryos. Methods Mol Biol. 2009;586:23–39. doi: 10.1007/978-1-60761-376-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S, Papalopulu N. Spindle position in symmetric cell divisions during epiboly is controlled by opposing and dynamic apicobasal forces. Dev Cell. 2012;22:775–787. doi: 10.1016/j.devcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TP, Harris AR, Lam M, Cheng Q, Bellis J, Dimitracopoulos A, Kabla AJ, Charras GT, Baum B. Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc Natl Acad Sci U S A. 2015;112:5726–5731. doi: 10.1073/pnas.1420585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong FZ, Ma WZ, Hiscock TW, Mosaliganti KR, Tentner AR, Brakke KA, Rannou N, Gelas A, Souhait L, Swinburne IA, Obholzer ND, Megason SG. Interplay of Cell Shape and Division Orientation Promotes Robust Morphogenesis of Developing Epithelia. Cell. 2014;159:415–427. doi: 10.1016/j.cell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Tsuboi T, Ishinabe N, Kitaguchi T, Michiue T. Wide and high resolution tension measurement using FRET in embryo. Sci Rep. 2016;6:28535. doi: 10.1038/srep28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Pal S, Maiti S, Davidson LA. Force production and mechanical accommodation during convergent extension. Development. 2015;142:692–701. doi: 10.1242/dev.116533. [DOI] [PMC free article] [PubMed] [Google Scholar]