Abstract
Objective
To assess construct validity of the Prosthetic Limb Users Survey of Mobility (PLUS-M), a self-report mobility measure for people with lower limb amputation (LLA).
Design
Cross-sectional study.
Setting
Private prosthetic clinics (n=37).
Participants
Current lower limb prosthesis users (n=199, mean age=55.4±14.3 years, 71.4% male) were assessed before receiving a replacement prosthesis, prosthetic socket, and/or prosthetic knee.
Intervention
Not applicable.
Main Outcome Measure(s)
Convergent construct validity was examined using correlations between participants’ PLUS-M T-scores and measures of physical function, mobility, and balance, including the Amputee Mobility Predictor (AMP), Timed Up and Go (TUG), Patient Reported Outcomes Measurement Information System-Physical Function (PROMIS-PF), Prosthesis Evaluation Questionnaire-Mobility Subscale (PEQ-MS), and Activities-Specific Balance Confidence Scale (ABC). Known-groups construct validity was evaluated by comparing differences in PLUS-M T-scores among participants grouped by Medicare Functional Classification Level (MFCL).
Results
PLUS-M T-scores demonstrated a moderate positive relationship with AMP scores (rho=0.54, p<0.001) and a moderate negative relationship with TUG times (rho=- 0.56, p<0.001). The PLUS-M also showed a strong positive relationship with PEQ-MS scores (rho=0.78, p<0.001), ABC scores (rho=0.81, p<0.001), and PROMIS-PF T-scores (rho=0.81, p<0.001). Significant differences (p<0.05) in PLUS-M T-scores were found among groups of people classified by different MFCLs.
Conclusion
Study results support validity of the PLUS-M as a self-report measure of prosthetic mobility. Correlations between PLUS-M and measures of physical function, mobility, and balance indicate convergent construct validity. Similarly, significant differences in PLUS-M T-scores across MFCL groups provide evidence of known-groups construct validity. In summary, evidence indicates that PLUS-M has good construct validity among people with LLA.
Keywords: Amputation, artificial limbs, motor activity, movement, outcomes assessment (health care), self-report
Introduction
Functional mobility is a fundamental goal for people who have experienced lower limb amputation (LLA). The ability to change one’s body position or location is often required to perform desired home, community, recreational, and vocational activities.1 For people with LLA, mobility also is recognized as a key determinant of quality-of-life.2–6 Because mobility is often compromised in people with LLA,1,7 many rehabilitation interventions (e.g., prostheses, gait training) are intended to restore or facilitate individuals’ ability to move effectively from one place to another. Mobility is also the primary factor by which insurance beneficiaries’ eligibility for prosthetic components is determined.8 Measurement of mobility outcomes is therefore central to selecting, optimizing, and evaluating the effectiveness of prosthetic interventions for people with LLA.
Self-report instruments are well suited to measuring health outcomes like mobility because they provide unique information about individuals’ experiences, perceptions, and opinions.9 Their use in clinical practice is recommended for screening patients, monitoring health outcomes, facilitating communication with patients and clinicians, informing treatment decisions, and evaluating effectiveness of care.10–12 Data obtained with self-report instruments is also increasingly used to inform healthcare policies and payment for services.13,14 Although several self-report instruments for measuring mobility outcomes in people with LLA exist,15,16 they are used rarely in clinical practice.17 Questionable relevance of items, long administration times, and difficulties in score interpretation may explain clinicians’ reluctance to use existing instruments.18,19 Limited psychometric testing in the targeted clinical population and lack of normative data may also contribute to clinicians’’ reluctance to use existing instruments with prosthetic patients.16
Self-report instruments created using contemporary standards and modern psychometric methods (e.g., item response theory (IRT)) are advocated because they address many of the practical issues and psychometric limitations that limit use of traditional health surveys.20–22 For example, involvement of rehabilitation stakeholders during development helps to maximize value of the instrument and items to patients and clinicians.9 Instruments that use standardized T-scores centered on population means are easily interpreted.23 Short forms and computerized adaptive tests developed from these item banks minimize administration burden, yet maintain the robust psychometric qualities of the parent bank.20,21 Given these characteristics, such instruments seem ideally suited to measuring mobility of people with LLA.
The Prosthetic Limb Users Survey of Mobility (PLUS-M™)24 was developed using the rigorous methods proposed by Reeve and colleagues23 to fulfill the need for a brief, accurate, and clinically meaningful self-report mobility instrument specific to people with LLA. Development of a new instrument using contemporary standards was intended to address barriers to routine use, including relevance of items, administration burden, and ease of interpretation. Prior development efforts and psychometric testing, including focus groups of prosthetic limb users,25 cognitive interviews with target respondents,26 and evaluation of mode-of-administration equivalency and test-retest reliability27 are described elsewhere. Briefly, PLUS-M Version 1 includes 44 mobility items that have been calibrated to an IRT model using mobility data from more than 1000 people with LLA. Two short forms (i.e., 12- and 7-item) and simple scoring tables are available for use in clinical care and research involving people with LLA. Normative data from a large, national sample (1019 people with LLA) are also available to facilitate interpretation of respondent scores.28 While PLUS-M appears well suited to clinical use, and addresses key limitations present in existing self-report mobility instruments designed for people with LLA (e.g., questionable clinical relevance, lengthy administration times, and challenges with interpreting scores), validation in an independent sample is required.
The purpose of this study was therefore to evaluate the construct validity of PLUS-M as a measure of self-reported mobility for people with LLA. Convergent construct validity was evaluated by comparing PLUS-M T-scores to both performance-based and self-report measures of mobility and related constructs that have been used previously with people with LLA. Known-groups construct validity was evaluated by comparing PLUS-M T-scores across groups of people classified by functional levels (i.e., limited community ambulator, unlimited community ambulator, active adult).29,30 The authors hypothesized that PLUS-M T-scores would be highly correlated (r>0.7) with self-report instruments designed to evaluate mobility-related constructs (e.g., balance, physical function) and moderately correlated (|r|>0.5) with physical performance measures of mobility.31 Further, the authors hypothesized that PLUS-M T-scores would significantly differ among people classified into different functional levels. Confirmation of these hypotheses would provide evidence that PLUS-M is appropriate for measuring mobility in prosthesis users.
Methods
Study Design
A multi-site, cross-sectional study was conducted at 37 prosthetic clinics between December 2012 and February 2014. Mobility outcomes were collected as part of a longitudinal study to evaluate effects of prosthetic interventions; data presented here were collected pre-intervention. Clinics were selected based on their willingness to assist with recruitment and administer instruments as directed. All clinics were required to have space and equipment necessary to administer the selected instruments.
Training
Sixty-five prosthetists were trained to administer the mobility instruments used in this study.17 In-person training was provided at each clinic using didactic (e.g., presentation and demonstration) and interactive (e.g., role play and practice with volunteers with LLA) instructional methods.
Participants
Individuals with unilateral LLA were targeted for recruitment. Selection criteria were applied to reflect the population for which PLUS-M was developed (i.e., adult prosthesis users with unilateral LLA). Inclusion criteria were 1) 18 years of age or older; 2) amputation between the hip and ankle; 3) amputation due to trauma, dysvascular complications, tumor, or infection; 4) use of a prosthesis to ambulate for at least four months; and 5) ability to read, write, and understand English. Individuals were excluded from participation if they had another amputation (e.g., arm or contralateral leg). A recruitment target of 250 was set for longitudinal study analyses; for instrument validation, samples of 100 or more are recommended.32
Procedures
Measures were administered to participants at a typical clinical appointment. The order of administration (i.e., self-report–performance-based or performance-based–self-report) was not controlled, but self-report instruments were administered in the same sequence. All procedures were conducted while participants wore their current prosthesis.
Self-report outcomes were collected using an online survey administered on a tablet computer.a Surveys were administered using the Assessment Centerb33 Performance-based outcomes (i.e., AMP scores and TUG times) were collected using WebQc electronic survey software. The survey provided prosthetists with written and graphical administration instructions, as well as standardized verbal instructions.
Study procedures were approved by a University of Washington institutional review board. All participants provided informed consent.
Measurements
Participants’ level of amputation, cause of amputation, and functional level (i.e., Medicare Functional Classification Level (MFCL) or K-Level29,30) were obtained from medical records. Sociodemographic characteristics (i.e., sex, race, ethnicity, educational level, employment status, Veteran status, and income), assistive device, and presence of comorbid conditions (e.g., cancer, diabetes, stroke)34 was obtained via a self-report. Participants’ physical function, mobility, and balance were obtained using standardized self-report and performance-based instruments. In absence of criterion measures of LLA mobility, these instruments (Table 1) were selected as those best for establishing PLUS-M’s construct validity.
Table 1.
Overview of instruments used in the study
| Instrument | Type of instrument | Number of items | Response options | Score | Approximate administration time |
|---|---|---|---|---|---|
| Prosthetic Limb Users Survey of Mobility (PLUS-M) | Self-report | 12 | 5 = without any difficulty 4 = with a little difficulty 3 = with some difficulty 2 = with much difficulty 1 = unable to do |
T-score | 1–2 minutes |
| Patient Reported Outcome Measurement Information System – Physical Function (PROMIS-PF) | Self-report | 4 | 5 = without any difficulty 4 = with a little difficulty 3 = with some difficulty 2 = with much difficulty 1 = unable to do |
T-score | <1 minute |
| Prosthesis Evaluation Questionnaire Mobility Subscale (PEQ-MS) | Self-report | 12 | 0 = unable or hardly able at all 1 = high difficulty 2 = moderate difficulty 3 = little difficulty 4 = no problems or almost fully able |
Average score | 1–2 minutes |
| Activities Specific Balance Confidence Scale (ABC) | Self-report | 16 | 4 = complete confidence 3 = high confidence 2 = moderate confidence 1 = low confidence 0 = no confidence |
Average score | 2–3 minutes |
| Timed Up and Go (TUG) | Performance-based | 1 | N/A | Time | 4–5 minutes |
| Amputee Mobility Predictor (AMP) | Performance-based | 20 | Ordinal response options vary by task | Summary score | 20 minutes |
Self-Report Measures
The Prosthetic Limb Users Survey of Mobility (PLUS-M) is a measure of perceived mobility that was developed by the investigators for lower limb prosthesis users. The PLUS-M (v1.2) 12-Item Short Form was administered in this study.35 The PLUS-M 12-Item Short Form provides T-scores that range from 21.8 to 71.4; higher scores indicate better mobility. T-scores obtained with the 12-item short form are highly correlated to those based on all 44 items in the PLUS-M item bank (R2 = 0.96).
The Patient Reported Outcome Measure Information System (PROMIS) is a system of instruments for measuring health outcomes across patient populations.36 The PROMIS Physical Function v1.0 instrument (PROMIS-PF) measures respondents’ perceived ability to perform functional activities. PROMIS-PF has excellent reliability and good convergent construct validity, as evidenced with strong correlations to legacy measures of physical function (e.g., Health Assessment Questionnaire [HAQ]37 and the Short Form 36 Physical Functioning subscale [PF-10]38,39 in large scale testing.40,41 PROMIS-PF has been used to characterize perceived physical function across a range of clinical populations, including people with LLA.42 The 4-item PROMIS-PF was administered.43 The 4-item PROMIS-PF provides T-scores that range from 22.9 to 56.9; higher scores indicate better physical function.
The Prosthesis Evaluation Questionnaire–Mobility Subscale (PEQ-MS) is a measure of perceived ability to perform a range of ambulation and transfer tasks with a lower limb prosthesis.44 The PEQ-MS combines the Ambulation and Transfer subscales from the PEQ.45 The PEQ-MS has been reported to exhibit high internal consistency, excellent test-retest reliability, and good convergent construct validity in people with LLA. The PEQ-MS was administered as recommended by Franchignoni et al..46 The PEQ-MS is scored from 0 to 4; higher scores indicate higher perceived ability.
The Activities-specific Balance Confidence (ABC) Scale is a 16-item measure of self-efficacy designed to identify balance confidence issues in aged adults.47,48 The ABC scale has demonstrated high internal consistency and good test-retest reliability, convergent construct validity, and known-groups construct validity in people with LLA.49 The ABC was administered using the 5-point ordinal scale and scoring methods recommended by Sakakibara et al..50 The ABC is scored from 0 to 4; higher scores indicate greater balance confidence.
Performance-Based Measures
The Timed Up and Go (TUG) is designed to evaluate ability to perform basic mobility tasks, such as sit-to-stand, walking, turning, and stand-to-sit. It was originally intended to assess fall risk in aged adults,51 but has excellent inter- and intra-rater reliability in people with LLA.52 It has good construct validity in people with transtibial amputation, as indicated by a strong negative correlation with timed walking distance.53 It has also been shown to discriminate between fallers and non-fallers with LLA.54 The TUG is scored using the time to complete the test; lower times indicate better performance.
The Amputee Mobility Predictor (AMP) is a clinician-assessed measure designed to evaluate the ambulatory potential of people with LLA. The AMP includes 20 tasks of increasing difficulty, ranging from sitting balance to negotiating environmental barriers (e.g., obstacles and stairs). The AMP has excellent intra- and inter-rater reliability, known-groups construct validity, and convergent construct validity in people with LLA. The AMP was administered and scored according to the developers’ instructions. The AMP is scored from 0 to 47; higher scores indicate better prosthetic mobility.55
Statistical Analysis
Statistical analyses were performed using SAS 9.3d. Score distributions were evaluated for normality using the Shapiro-Wilk test,56 and visual inspection of histograms and Quantile-Quantile (QQ) plots. Although PLUS-M T-scores (W=0.994, p=0.54) and PEQ-MS scores (W=0.987, p=0.07) were normally distributed, ABC, TUG, and AMP scores were not (p>0.004). Non-parametric tests were therefore used to assess convergent and known-groups construct validity. Convergent construct validity was examined via comparison of participants’ PLUS-M scores and results obtained from the aforementioned measures of physical function, mobility, and/or balance. Spearman’s rank correlation coefficients were derived to determine the strength and direction of the relationship between participants’ PLUS-M T-scores, and associated TUG times and AMP, PEQ-MS, ABC, and PROMIS-PF scores. Correlation coefficients were evaluated with respect to thresholds established by Dancey and Reidy (i.e., |r|>0.7 and |r|>0.5 is evidence of a strong and moderate correlation, respectively).57 Strong-to-moderate correlations (i.e., rho > 0.5) were considered evidence of convergent construct validity.
Known-groups construct validity was examined by comparing PLUS-M T-scores across participants grouped according to their clinician-reported functional level (i.e., MFCL-2, MFCL-3, and MFCL-4).29,30 Differences in T-scores between groups were assessed using the Kruskal Wallis and Wilcoxon-Mann-Whitney tests. Significant differences in PLUS-M T-scores between clinical groups were considered evidence of known-groups construct validity. The level of significance for all tests (α = 0.05) was adjusted using the Holm-Bonferroni method58 to account for multiple comparisons.
Results
Two hundred sixty-one people were recruited, and one hundred ninety-nine participated in the study (Table 2). Reasons for non-participation included unable-to-contact (n=16), ineligibility (n=25), voluntary withdrawal (n=11), and death (n=1). Nine participants had missing data and were not included in this analysis. The average age of participants was 55.4 years (SD=14.8 years, range 19.3 to 88.7 years). Participants experienced their amputation, on average, 10.9 years prior (SD=13.2 years, range 3 months to 70 years). They reported using their prosthesis for an average of 11.9 hours per day (SD=4.3 hours, range 1 to 24 hours). Participants were largely male (71.4%) and non-Hispanic white (74.9%). Thirty-eight (19.1%) reported being Veterans from the U.S. Armed Forces.
Table 2.
Participant demographics and personal characteristics
| Patient Demographics | MFCL-2 (N=24) | MFCL-3 (N=151) | MFCL-4 (N=24) | Total (N=199) | ||||
|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | % | N | |
| Gender | ||||||||
| Female | 45.8% | 11 | 28.5% | 43 | 12.5% | 3 | 28.6% | 57 |
| Male | 54.2% | 13 | 71.5% | 108 | 87.5% | 21 | 71.4% | 142 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 79.2% | 19 | 74.2% | 112 | 75.0% | 18 | 74.9% | 149 |
| Non-Hispanic black | 4.2% | 1 | 13.2% | 20 | 12.5% | 3 | 12.1% | 24 |
| Hispanic | 12.5% | 3 | 5.3% | 8 | 4.2% | 1 | 6.0% | 12 |
| Other | 0.0% | 0 | 6.0% | 9 | 8.3% | 2 | 5.5% | 11 |
| Not reported | 4.2% | 1 | 1.3% | 2 | 0.0% | 0 | 1.5% | 3 |
| Education | ||||||||
| High school graduate or less | 37.5% | 9 | 29.1% | 44 | 33.3% | 8 | 30.7% | 61 |
| Some college or tech school | 50.0% | 12 | 43.7% | 66 | 33.3% | 8 | 43.2% | 86 |
| College graduate | 12.5% | 3 | 18.5% | 28 | 20.8% | 5 | 18.1% | 36 |
| Advanced degree | 0.0% | 0 | 7.9% | 12 | 12.5% | 3 | 7.5% | 15 |
| Not reported | 0.0% | 0 | 0.7% | 1 | 0.0% | 0 | 0.5% | 1 |
| Employment status | ||||||||
| Employed | 8.3% | 2 | 27.2% | 41 | 45.8% | 11 | 27.1% | 54 |
| Homemaker | 4.2% | 1 | 3.3% | 5 | 8.3% | 2 | 4.0% | 8 |
| Student | 0.0% | 0 | 2.6% | 4 | 8.3% | 2 | 3.0% | 6 |
| Retired | 54.2% | 13 | 27.8% | 42 | 0.0% | 0 | 27.6% | 55 |
| On disability | 33.3% | 8 | 35.1% | 53 | 29.2% | 7 | 34.2% | 68 |
| Unemployed | 0.0% | 0 | 4.0% | 6 | 8.3% | 2 | 4.0% | 8 |
| Veteran status | ||||||||
| Active service | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 |
| Veteran | 12.5% | 3 | 21.2% | 32 | 12.5% | 3 | 19.1% | 38 |
| Individual income | ||||||||
| <$25,000 | 58.3% | 14 | 44.4% | 67 | 50.0% | 12 | 46.7% | 93 |
| $25,000–$39,999 | 29.2% | 7 | 18.5% | 28 | 25.0% | 6 | 20.6% | 41 |
| $40,000–$54,999 | 8.3% | 2 | 14.6% | 22 | 4.2% | 1 | 12.6% | 25 |
| $55,000–$69,999 | 0.0% | 0 | 4.0% | 6 | 0.0% | 0 | 3.0% | 6 |
| $70,000–$84,999 | 0.0% | 0 | 6.0% | 9 | 12.5% | 3 | 6.0% | 12 |
| $85,000–$99,999 | 0.0% | 0 | 3.3% | 5 | 4.2% | 1 | 3.0% | 6 |
| $100,000+ | 0.0% | 0 | 6.0% | 9 | 4.2% | 1 | 5.0% | 10 |
| Not reported | 4.2% | 1 | 3.3% | 5 | 0.0% | 0 | 3.0% | 6 |
Participant medical characteristics (Table 3) indicated that there were similar proportions of participants with traumatic and dysvascular amputation (41.2% and 43.7%, respectively). The majority of participants had a transtibial amputation (75.9%). The two most common co-morbidities were diabetes mellitus (42.1%) and arthritis (41.1%). Participant history (Table 4) showed that those classified as MFCL-4 were significantly younger (p<0.001) at the time of testing and amputation (40 years and 32 years, respectively) than those classified as MFCL-2 (60 and 53 years, respectively) or 3 (57 and 45 years, respectively). Participants classified as MFCL-2 used their prostheses significantly fewer (p<0.005) hours per day (10 hours) than those classified as MFCL-3 or 4 (12 and 14 hours, respectively).
Table 3.
Participant medical characteristics
| Medical History | MFCL-2 (N=24) | MFCL-3 (N=151) | MFCL-4 (N=24) | Total (N=199) | ||||
|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | % | N | |
| Level of amputation | ||||||||
| Ankle disarticulation | 0.0% | 0 | 1.3% | 2 | 8.3% | 2 | 2.0% | 4 |
| Transtibial | 87.5% | 21 | 74.8% | 113 | 70.8% | 17 | 75.9% | 151 |
| Knee disarticulation | 0.0% | 0 | 4.0% | 6 | 4.2% | 1 | 3.5% | 7 |
| Transfemoral | 8.3% | 2 | 19.9% | 30 | 16.7% | 4 | 18.1% | 36 |
| Other | 4.2% | 1 | 0.0% | 0 | 0.0% | 0 | 0.5% | 1 |
| Amputation etiology | ||||||||
| Trauma | 16.7% | 4 | 39.6% | 59 | 79.2% | 19 | 41.2% | 82 |
| Dysvascular | 79.2% | 19 | 45.0% | 68 | 0.0% | 0 | 43.7% | 87 |
| Tumor | 0.0% | 0 | 6.6% | 10 | 4.2% | 1 | 5.5% | 11 |
| Infection | 4.2% | 1 | 7.3% | 11 | 12.5% | 3 | 7.5% | 15 |
| Other/unknown | 0.0% | 0 | 2.0% | 3 | 4.2% | 1 | 2.0% | 4 |
| Comorbid conditions1 | ||||||||
| Asthma | 4.2% | 1 | 12.8% | 19 | 12.5% | 3 | 11.7% | 23 |
| Arthritis | 54.2% | 13 | 42.3% | 63 | 20.8% | 5 | 41.1% | 81 |
| Cancer | 4.2% | 1 | 6.0% | 9 | 0.0% | 0 | 5.1% | 10 |
| Diabetes | 79.2% | 19 | 41.6% | 62 | 8.3% | 2 | 42.1% | 83 |
| Digestive problems | 8.3% | 2 | 8.7% | 13 | 4.2% | 1 | 8.1% | 16 |
| Heart trouble | 41.7% | 10 | 27.5% | 41 | 8.3% | 2 | 26.9% | 53 |
| HIV / AIDS | 0.0% | 0 | 2.0% | 3 | 0.0% | 0 | 1.5% | 3 |
| Kidney disease | 41.7% | 10 | 13.4% | 20 | 4.2% | 1 | 15.7% | 31 |
| Liver problems | 0.0% | 0 | 2.7% | 4 | 0.0% | 0 | 2.0% | 4 |
| Stroke | 8.3% | 2 | 6.0% | 9 | 0.0% | 0 | 5.6% | 11 |
| Assistive Device Use1,2 | ||||||||
| 1 cane | 41.7% | 10 | 21.5% | 32 | 0.0% | 0 | 21.3% | 42 |
| 2 canes | 4.2% | 1 | 2.7% | 4 | 0.0% | 0 | 2.5% | 5 |
| 1 crutch | 8.3% | 2 | 2.7% | 4 | 4.2% | 1 | 3.6% | 7 |
| 2 crutches (with prosthesis) | 4.2% | 1 | 1.3% | 2 | 0.0% | 0 | 1.5% | 3 |
| Walker (with prosthesis) | 33.3% | 8 | 8.7% | 13 | 0.0% | 0 | 10.7% | 21 |
| Manual wheelchair | 25.0% | 6 | 11.4% | 17 | 8.3% | 2 | 12.7% | 25 |
| Scooter/powered wheelchair | 20.8% | 5 | 4.0% | 6 | 0.0% | 0 | 5.6% | 11 |
May check all that apply; total n=197 (MFCL-2: n=24, MFCL-3: n=149, MFCL-4: n=24)
Used assistive device daily (selected either <2 hours/day or >2 hours/day)
Table 4.
Participant temporal characteristics by functional level subgroups
| Outcomes | MFCL-2 (N=24) | MFCL-3 (N=151) | MFCL-4 (N=24) | Total (N=199) | Differences between MFCL groups (p<0.05) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | ||
| Age at evaluation (years) | 60.0 (13.4) | 27.3–76.5 | 57.1 (12.4) | 22.3–84.6 | 39.7 (16.5) | 19.3–88.7 | 55.4 (14.3) | 19.3–88.7 | 2–4, 3–4 |
| Age at amputation (years) | 53.4 (16.8) | 5.3–74.6 | 45.0 (18.5) | 0–81.1 | 31.7 (16.5) | 0.1–81.5 | 44.4 (18.8) | 0–81.5 | 2–3, 2–4, 3–4 |
| Time since amputation (years) | 6.6 (13.2) | 0.5–66.8 | 12.1 (13.7) | 0.3–70.3 | 8.0 (8.1) | 0.6–29.7 | 10.9 (13.2) | 0.3–70.3 | |
| Typical prosthesis use (hrs/day) | 9.5 (4.4) | 1.0–18.0 | 12.1 (4.2) | 1.0–24.0 | 13.5 (3.2) | 4.0–19.0 | 11.9 (4.3) | 1.0–24.0 | 2–3, 2–4 |
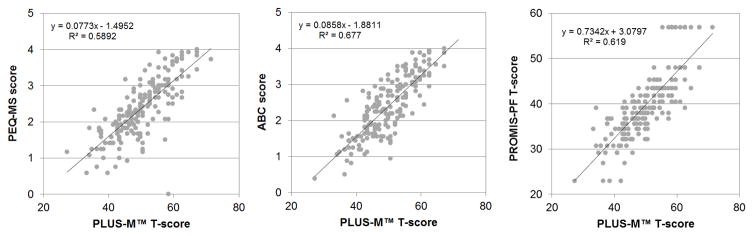
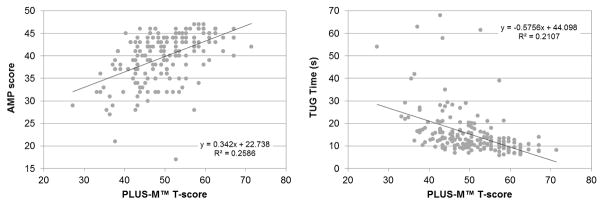
Participants’ mean PLUS-M T-score was 50.3 (SD=8.0) with a minimum and maximum T-score of 27.2 and 71.1, respectively (Table 5). No evidence of floor or ceiling effects were observed for PLUS-M (i.e., only two participants scored the maximum T-score). PLUS-M T-scores demonstrated a strong positive correlation (Figure 1) with PEQ-MS (rho=0.78, p<0.001), ABC (rho=0.81, p<0.001), and PROMIS physical function (rho = 0.81, p < 0.001). Similarly, PLUS-M T-scores showed moderate positive and negative correlations (Figure 2) with AMP scores (rho=0.54, p<0.001) and TUG times (rho=-0.56, p<0.001), respectively.
Table 5.
Mean instrument times and scores by functional level subgroups
| Outcomes | MFCL-2 (N=24) | MFCL-3 (N=151) | MFCL-4 (N=24) | Total (N=199) | Differences between MFCL groups (p<0.05) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | ||
| Physical performance measures | |||||||||
| AMP | 32.1 (6.2) | 17.0–43.0 | 40.4 (4.3) | 28.0–47.0 | 44.6 (1.9) | 39.0–47.0 | 39.9 (5.4) | 17.0–47.0 | 2–3, 2–4, 3–4 |
| TUG (seconds) | 26.3 (15.6) | 10.2–62.9 | 14.5 (8.8) | 6.2–68.0 | 9.5 (3.4) | 5.9–20.6 | 15.3 (10.3) | 5.9–68.0 | 2–3, 2–4, 3–4 |
| Self-report measures | |||||||||
| PLUS-M 12-item short form | 45.2 (9.0) | 27.2–64.5 | 50.5 (7.7) | 34.1–71.4 | 53.8 (6.6) | 41.5–62.5 | 50.3 (8.0) | 27.2–71.4 | 2–3, 2–4, 3–4 |
| PEQ-MS | 2.1 (0.9) | 0.6–3.9 | 2.4 (0.8) | 0–4.0 | 2.8 (0.6) | 1.7–3.8 | 2.4 (0.8) | 0–4.0 | 2–4, 3–4 |
| ABC | 2.0 (0.8) | 0.4–3.6 | 2.4 (0.8) | 0.8–4.0 | 2.8 (0.7) | 1.4–4.0 | 2.4 (0.8) | 0.4–4.0 | 2–3, 2–4, 3–4 |
| PROMIS Physical function | 35.4 (7.5) | 22.9–56.9 | 40.3 (7.2) | 22.9–56.9 | 42.0 (6.0) | 33.3–56.9 | 39.9 (7.3) | 22.9–56.9 | 2–3, 2–4 |
Figure 1.
Correlations between participants’ (n=199) PLUS-M T-scores and PEQ-MS scores (left), ABC scores (center), and PROMIS-PF T-scores (right)
Figure 2.
Correlations between participants’ (n=199) PLUS-M T-scores and AMP scores (left) and TUG times (right)
Mean PLUS-M T-scores increased with MFCL, and ranged from 45.2 (SD=9.0) for MFCL-2 participants to 53.8 (SD=6.6) for MFCL-4 participants. Significant differences in PLUS-M T-scores were found between participants classified as MFCL-2, 3, or 4 (p<0.002). The ABC, TUG, and AMP also showed significant differences in scores by MFCL (p<0.002). As with PLUS-M T-scores, mean scores were also in the expected directions.
Discussion
The objective of this study was to assess the construct validity of the PLUS-M, a self-report measure of mobility for prosthetic limb users. Study results provide convincing evidence of both convergent and known-groups construct validity, and support use of PLUS-M in people with unilateral LLA.
In this study, evidence of convergent construct validity was established through comparison of scores obtained with the 12-item PLUS-M short form and instruments that measure mobility-related constructs. In accordance with the proposed hypotheses, PLUS-M T-scores were highly correlated (rho>0.78) with scores from self-report measures of mobility, balance, and physical function. Correlations between PLUS-M T-scores and scores on the PEQ-MS, ABC, and PROMIS-PF were similar to those between other LLA-specific questionnaires reported in the literature. For example, correlations between the LCI and Rivermead Mobility Index,59 and the PEQ Ambulation scale and the SF-36 Physical Function scale45 were 0.74 and 0.61, respectively. As expected,31 moderate correlations (|rho|>0.54) were observed between PLUS-M T-scores and performance measures. Correlations between self-report and performance-based measures are typically lower than correlations between self-report measures for a variety of reasons, including differences in the way the construct is measured (e.g., time to complete a task as compared to level of difficulty in completing it) and the breadth of the concept (i.e., functional tasks performed in a clinic as compared to a range of activities performed in the respondent’s natural environment).60 The direction and magnitude of correlations observed in this study were also comparable to those obtained in prior studies. For example, correlations between the LCI and Functional Independence Measure,59 PEQ-MS and TUG,44 and Houghton Scale and 2 minute walk test,61 have been reported as 0.61, −0.50, and 0.49 respectively. The directions and relative strengths of correlations between instruments observed in this study indicates that PLUS-M has good convergent construct validity and is appropriate for measuring mobility in people with LLA.
Known-groups construct validity was established through evaluation of participants classified into MFCL groups. As hypothesized, PLUS-M T-scores increased with functional level (i.e., participants with MFCL-4 and MFCL-2 had the highest and lowest PLUS-M T-scores, respectively), and were significantly different across participants grouped by MFCL. AMP scores by MFCL in this study (i.e., 32.1, 40.4, and 44.6 for MFCL-2, 3, and 4, respectively) were nearly identical to those reported previously (i.e., 34.7, 40.5, and 44.7 for 149 similarly classified participants). Small differences in scores in the MFCL-2 group may be due to a smaller sample here (n=24), compared to the prior study (n=43).55 Collectively, these findings indicate evidence of known-groups construct validity for the PLUS-M instrument.
Study Limitations
A large number of prosthetists collected data for this study. Although prosthetists were trained by the investigators to administer performance measures,17 and self-report surveys were collected via a standardized tablet provided to each clinic, variations in clinical settings and/or administration methods may have been present. The AMP and TUG were purposefully selected as validation measures for this study because each has evidence of good inter-rater reliability with people with LLA (i.e., r = 0.99 and 0.96, respectively). Thus, administration variations were expected to minimally affect the results. Further, prosthetists were given access to videos that showed instrument set-up and administration, and were asked to attend conference calls and webinars, where administration procedures were reviewed.
The sample size in this study (n=199) was large, compared to similar instrument validation efforts among people with LLA. However, a majority (n=151) of study participants were classified as unlimited community ambulators (i.e., MFCL-3). The true proportion of people with LLA by MFCL is unknown, but it is expected that the majority of prosthesis users are unlimited community ambulators.62 The fewer number of participants classified as limited community ambulators and active adults/athletes (i.e., MFCL-2 and 4, respectively) in this study (n=24 in each group) may limit generalization of the evidence presented here.
Conclusion
This study found that PLUS-M exhibits good convergent and known-groups construct validity in people with LLA who are receiving prosthetic care. Observed correlations between the PLUS-M 12-item short form and both self-report and performance- based measures indicate that PLUS-M well measures the construct of mobility. Similarly, observed differences in PLUS-M T-scores by groups of patients classified into different mobility categories lends additional evidence that PLUS-M is capable of measuring clinically-meaningful differences in mobility. Results collectively support use of PLUS-M for evaluating prosthetic mobility and indicate that PLUS-M short forms are suitable for use in clinical care.
Acknowledgments
Financial support: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD065340. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Acronyms
- ABC
Activities-specific balance confidence scale
- AMP
Amputee mobility predictor
- LCI
Locomotor capabilities index
- LLA
lower limb amputation
- MFCL
Medicare functional classification level
- IH
National Institutes of Health
- PEQ-MS
Prosthesis evaluation questionnaire – mobility subscale
- PLUS-M
Prosthetic limb users survey of mobility
- PROMIS
Patient-reported outcomes measurement information system
- PROMIS-PF
PROMIS Physical Function TUG, Timed up and go
Footnotes
Apple iPad, Cupertino, CA
Assessment Center, Northwestern University, Chicago, IL
WebQ;,University of Washington, Seattle, WA
SAS Institute, Inc., Cary, NC
Presentation of material: Gaunaurd I, Gailey R, Salem R, Hafner B. Construct Validity of the Prosthetic Limb Users Survey of Mobility (PLUS-M). 15th World Congress of the International Society of Prosthetics and Orthotics, Lyon, France, June 22–25, 2015 (podium presentation).
Financial Disclosure: The authors declare no competing interest.
Clinical Trials Registration: NCT01750372
Reprints: Reprints are not available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fortington LV, Rommers GM, Geertzen JH, Postema K, Dijkstra PU. Mobility in elderly people with a lower limb amputation: a systematic review. Journal of the American Medical Directors Association. 2012;13(4):319–25. doi: 10.1016/j.jamda.2010.12.097. [DOI] [PubMed] [Google Scholar]
- 2.Suckow BD, Goodney PP, Nolan BW, Veeraswamy RK, Gallagher P, Cronenwett JL, Kraiss LW. Domains that Determine Quality of Life in Vascular Amputees. Ann Vasc Surg. 2015;29(4):722–30. doi: 10.1016/j.avsg.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norvell DC, Turner AP, Williams RM, Hakimi KN, Czerniecki JM. Defining successful mobility after lower extremity amputation for complications of peripheral vascular disease and diabetes. J Vasc Surg. 2011;54(2):412–9. doi: 10.1016/j.jvs.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies B, Datta D. Mobility outcome following unilateral lower limb amputation. Prosthet Orthot Int. 2003;27(3):186–90. doi: 10.1080/03093640308726681. [DOI] [PubMed] [Google Scholar]
- 5.Pell JP, Donnan PT, Fowkes FG, Ruckley CV. Quality of life following lower limb amputation for peripheral arterial disease. Eur J Vasc Surg. 1993;7(4):448–51. doi: 10.1016/s0950-821x(05)80265-8. [DOI] [PubMed] [Google Scholar]
- 6.Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231–43. doi: 10.1080/03093640802024955. [DOI] [PubMed] [Google Scholar]
- 7.Penn-Barwell JG. Outcomes in lower limb amputation following trauma: A systematic review and meta-analysis. Injury. 2011;42(12):1474–9. doi: 10.1016/j.injury.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Laferrier JZ, Gailey R. Advances in lower-limb prosthetic technology. Phys Med Rehabil Clin N Am. 2010;21(1):87–110. doi: 10.1016/j.pmr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Amtmann D, Cook KF, Johnson KL, Cella D. The PROMIS initiative: involvement of rehabilitation stakeholders in development and examples of applications in rehabilitation research. Arch Phys Med Rehabil. 2011;92(10 Suppl):S12–9. doi: 10.1016/j.apmr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract. 1999;5(4):401–16. doi: 10.1046/j.1365-2753.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 11.Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, Revicki DA, Symonds T, Parada A, Alonso J. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17(2):179–93. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res. 2009;18(1):115–23. doi: 10.1007/s11136-008-9430-6. [DOI] [PubMed] [Google Scholar]
- 13.Black N, Burke L, Forrest CB, Ravens Sieberer UH, Ahmed S, Valderas JM, Bartlett SJ, Alonso J. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2015 doi: 10.1007/s11136-015-1168-3. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger M, Grob R, Shaller D. Using Patient-Reported Information to Improve Clinical Practice. Health Serv Res. 2015 doi: 10.1111/1475-6773.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condie E, Scott H, Treweek S. Lower limb prosthetic outcome measures: a review of the literature 1995 to 2005. J Prosthet Orthot. 2006;18(1):P13–45. [Google Scholar]
- 16.Heinemann AW, Connelly L, Ehrlich-Jones L, Fatone S. Outcome instruments for prosthetics: clinical applications. Phys Med Rehabil Clin N Am. 2014;25(1):179–98. doi: 10.1016/j.pmr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Gaunaurd I, Spaulding SE, Amtmann D, Salem R, Gailey R, Morgan SJ, Hafner BJ. Use of and confidence in administering outcome measures among clinical prosthetists: Results from a national survey and mixed-methods training program. Prosthet Orthot Int. 2015;39(4):314–21. doi: 10.1177/0309364614532865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafner BJ, Spualding SE, Salem R, Morgan SJ, Gaunaurd IA, Gailey RS. Prosthetists’ perceptions and use of outcome measures in clinical practice: long-term effects of focused continuing education. Prosthet Orthot Int. 2016 Sep 16; doi: 10.1177/0309364616664152. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jette DU, Halbert J, Iverson C, Miceli E, Shah P. Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys Ther. 2009;89(2):125–35. doi: 10.2522/ptj.20080234. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133–41. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 21.Jette AM, Haley SM. Contemporary measurement techniques for rehabilitation outcomes assessment. J Rehabil Med. 2005;37(6):339–45. doi: 10.1080/16501970500302793. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Morales LS, Reise SP. Item response theory and health outcomes measurement in the 21st century. Med Care. 2000;38(9 Suppl):Ii28–42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 Suppl 1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 24.Amtmann D, Abrahamson D, Morgan S, Salem R, Askew R, Gailey R, Gaunaurd I, Kajlich A, Hafner B. The PLUS-M: item bank of mobility for prosthetic limb users. Qual Life Res. 2014;23:39–40. [Google Scholar]
- 25.Hafner BJ, Morgan SJ, Abrahamson DC, Amtmann D. Characterizing mobility from the prosthetic limb user’s perspective: use of focus groups to guide development of the Prosthetic Limb Users Survey of Mobility. Prosthet Orthot Int. 2015 May 5; doi: 10.1177/0309364615579315. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan SJ, Amtmann D, Abrahamson DC, Kajlich AJ, Hafner BJ. Use of cognitive interviews in the development of the PLUS-M item bank. Qual Life Res. 2014;23(6):1767–75. doi: 10.1007/s11136-013-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafner BJ, Morgan SJ, Askew RA. Psychometric evaluation of self-report outcome measures for prosthetic applications. J Rehabil Res Dev. 2016 doi: 10.1682/JRRD.2015.12.0228. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prosthetic Limb Users Survey of Mobility (PLUS-M™) Version 1.2 Short Forms Users Guide. 2014 [June 24, 2016.]. Available from: http://www.plus-m.org.
- 29.Palmetto Government Benefits Administrators. DMERC Medicare Advisory. Baltimore: Centers for Medicare and Medicaid Services; Dec, 1994. Lower limb prostheses; pp. 99–105. [Google Scholar]
- 30.Noridian Healthcare Solutions. Local coverage determination (LCD) for lower limb prostheses (L33787) American Medical Association; 2016. [Accessed June 24, 2016]. Available at: https://med.noridianmedicare.com/documents/2230715/2240923/Lower+Limb+Prostheses.pdf. [Google Scholar]
- 31.Coman L, Richardson J. Relationship between self-report and performance measures of function: a systematic review. Can J Aging. 2006;25(3):253–70. doi: 10.1353/cja.2007.0001. [DOI] [PubMed] [Google Scholar]
- 32.Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2012;21(4):651–7. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment Center. Qual Life Res. 2010;19(5):677–85. doi: 10.1007/s11136-010-9634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhry S, Jin L, Meltzer D. Use of a Self-Report-Generated Charlson Comorbidity Index for Predicting Mortality. Med Care. 2005;43(6):607–15. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 35.Potter K, Fulk GD, Salem Y, Sullivan J. Outcome measures in neurological physical therapy practice: part I. Making sound decisions. Journal of neurologic physical therapy : JNPT. 2011;35(2):57–64. doi: 10.1097/NPT.0b013e318219a51a. [DOI] [PubMed] [Google Scholar]
- 36.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcomes in arthritis. Arthritis Rheum. 1980;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 38.Haley SM, McHorney CA, Ware JE. Evaluation of the MOS SF-36 physical functioning scale (PF-10): I. Unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol. 1994;47(6):671–84. doi: 10.1016/0895-4356(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 39.McHorney CA, Haley SM, Ware JE., Jr Evaluation of the MOS SF-36 Physical Functioning Scale (PF-10): II. Comparison of relative precision using Likert and Rasch scoring methods. J Clin Epidemiol. 1997;50(4):451–61. doi: 10.1016/s0895-4356(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 40.Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE., Jr The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67(5):516–26. doi: 10.1016/j.jclinepi.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and PROMIS: assessment of physical function. J Rheumatol. 2014;41(1):153–8. doi: 10.3899/jrheum.130813. [DOI] [PubMed] [Google Scholar]
- 42.Amtmann D, Morgan SJ, Kim J, Hafner BJ. Health-related profiles of people with lower limb loss. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patient Reported Outcomes Measurement Information System. [Accessed June 24, 2016];PROMIS scoring guide: Version 1.0 short forms, profile short forms, computerized adaptive testing. 2011 Available from: http://www.assessmentcenter.net.
- 44.Miller WC, Deathe AB, Speechley M. Lower extremity prosthetic mobility: a comparison of 3 self-report scales. Arch Phys Med Rehabil. 2001;82(10):1432–40. doi: 10.1053/apmr.2001.25987. [DOI] [PubMed] [Google Scholar]
- 45.Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931–8. doi: 10.1016/s0003-9993(98)90090-9. [DOI] [PubMed] [Google Scholar]
- 46.Franchignoni F, Giordano A, Ferriero G, Orlandini D, Amoresano A, Perucca L. Measuring mobility in people with lower limb amputation: Rasch analysis of the mobility section of the prosthesis evaluation questionnaire. J Rehabil Med. 2007;39(2):138–44. doi: 10.2340/16501977-0033. [DOI] [PubMed] [Google Scholar]
- 47.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 48.Myers AM, Powell LE, Maki BE, Holliday PJ, Brawley LR, Sherk W. Psychological indicators of balance confidence: relationship to actual and perceived abilities. J Gerontol A Biol Sci Med Sci. 1996;51(1):M37–43. doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 49.Miller WC, Deathe AB, Speechley M. Psychometric properties of the Activities-specific Balance Confidence Scale among individuals with a lower-limb amputation. Arch Phys Med Rehabil. 2003;84(5):656–61. doi: 10.1016/s0003-9993(02)04807-4. [DOI] [PubMed] [Google Scholar]
- 50.Sakakibara BM, Miller WC, Backman CL. Rasch analyses of the Activities-specific Balance Confidence Scale with individuals 50 years and older with lower-limb amputations. Arch Phys Med Rehabil. 2011;92(8):1257–63. doi: 10.1016/j.apmr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 52.Schoppen T, Boonstra A, Groothoff JW, de Vries J, Goeken LN, Eisma WH. The Timed “up and go” test: reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil. 1999;80(7):825–8. doi: 10.1016/s0003-9993(99)90234-4. [DOI] [PubMed] [Google Scholar]
- 53.Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008;89(12):2354–9. doi: 10.1016/j.apmr.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Dite W, Connor HJ, Curtis HC. Clinical identification of multiple fall risk early after unilateral transtibial amputation. Arch Phys Med Rehabil. 2007;88(1):109–14. doi: 10.1016/j.apmr.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Gailey RS, Roach KE, Applegate EB, Cho B, Cunniffe B, Licht S, Maguire M, Nash MS. The amputee mobility predictor: an instrument to assess determinants of the lower-limb amputee's ability to ambulate. Arch Phys Med Rehabil. 2002;83(5):613–27. doi: 10.1053/apmr.2002.32309. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3–4):591–611. [Google Scholar]
- 57.Dancey CP, Reidy J. Statistics Without Maths for Psychology Using SPSS for Windows. Prentice Hall, Pearson Education; Harlow, England: 2002. [Google Scholar]
- 58.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- 59.Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743–8. doi: 10.1016/j.apmr.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Stratford PW, Kennedy D, Pagura SM, Gollish JD. The relationship between self-report and performance-related measures: questioning the content validity of timed tests. Arthritis Rheum. 2003;49(4):535–40. doi: 10.1002/art.11196. [DOI] [PubMed] [Google Scholar]
- 61.Brooks D, Parsons J, Hunter JP, Devlin M, Walker J. The 2-minute walk test as a measure of functional improvement in persons with lower limb amputation. Arch Phys Med Rehabil. 2001;82(10):1478–83. doi: 10.1053/apmr.2001.25153. [DOI] [PubMed] [Google Scholar]
- 62.Kaluf B. Evaluation of Mobility in Persons with Limb Loss Using the Amputee Mobility Predictor and the Prosthesis Evaluation Questionnaire–Mobility Subscale: A Six-Month Retrospective Chart Review. J Prostet Orthot. 2014;26(2):70–6. [Google Scholar]