Abstract
Foods and botanical supplements can interfere with the endocrine system through the presence of phytosteroids – chemicals that interact with steroids receptors. Phytoestrogens are well studied, but compounds such as kaempferol, apigenin, genistein, ginsenoside Rf, and glycyrrhetinic acid have been shown to interact with non-estrogen nuclear receptors. These compounds can have agonist, antagonist, or mixed agonist/antagonist activity depending on compound, receptor, cell line or tissue, and concentration. Some phytosteroids have also been shown to inhibit steroid metabolizing enzymes, resulting in biological effects through altered endogenous steroid concentrations. An interesting example, compound A (4-[1-chloro-2-(methylamino)ethyl]phenyl acetate hydrochloride (1:1)) is a promising selective glucocorticoid receptor modulator (SGRM) based on a phytosteroid isolated from Salsola tuberculatiformis Botschantzev. Given that $6.9 billion of herbal supplements are sold each year, is clear that further identification and characterization of phytosteroids is needed to ensure the safe and effective use of botanical supplements.
1. Introduction
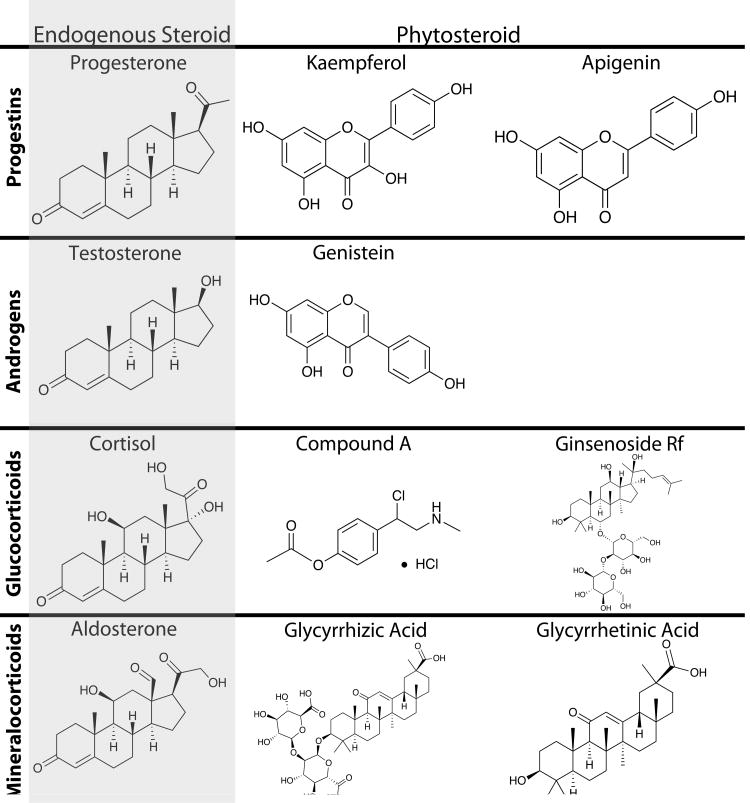
Phytosteroids are a class of specialized metabolites derived from plants that bind to steroid receptors in animals and can trigger or repress downstream receptor-mediated signaling events. Phytosteroids have diverse structures, sometimes very different from the endogenous steroid (Figure 1); yet they can act as agonists, antagonists, or frequently have mixed agonist/antagonist activity for steroid receptors (Lesovaya et al., 2015; Toh et al., 2012). In addition, some phytosteroids interact with multiple steroid receptors (Pihlajamaa et al., 2011) or interfere with steroid metabolizing enzymes (Blachley and Knochel, 1980), thus having complex effects on the endocrine and reproductive systems.
Figure 1.
Structure of the major endogenous steroid in each class, the phytosteroid(s) best supported by scientific literature, and compound A (4-[1-Chloro-2-(methylamino)ethyl]phenyl acetate hydrochloride (1:1)) a synthetic compound based on a phytosteroid.
2. Herbal and Dietary Effect on Fertility
Botanicals have been used to treat disease and control reproduction for centuries (Noumi and Tchakonang, 2001; Yun, 2001), a phenomenon that continues, as evidenced by the growing market for dietary herbal supplements (Smith et al., 2016). Phytosteroids have the potential to interfere with multiple aspects of the endocrine system, with early examples involving reduced fertility (Bennetts et al., 1946; De Lange, 1961). The first phytosteroids were reported when it was found that sheep grazing on subterranean clover in western Australia suffered from reduced fertility (Bennetts et al., 1946). Since then, molecules that activate the estrogen receptor (phytoestrogens; typically isoflavones, lignans, and coumestans) have been identified as steroidogenic constituents responsible for reduced fertility (Adams, 1995; Hughes, 1988). In a study examining the effects of caffeine on fertility, high consumption of tea was associated with double the frequency of pregnancies, while caffeine or other caffeinated drinks had no effect (Caan et al., 1998). A double-blinded placebo-controlled study of the herbal supplement FertilityBlend® found that the herbal supplement increased the pregnancy rate likely through enhanced luteal phase progesterone concentration increases in women trying to conceive (Lm et al., 2005). However, since herbal supplements likely influence reproductive biology in multiple ways, more research is required to identify the mechanisms responsible and determine whether these effects are the result of steroid receptor signaling (Dennehy, 2006).
Identification of novel phytoestrogens and characterization of their biological activities continues to be an area of active research (Nie et al., 2015). A thorough literature search reveals that many other phytosteroids exist and bind the progesterone, androgen, and corticoid receptors. However, though their potential biological activities have been characterized to widely varying degrees.
3. Phytoprogestins
Progesterone is primarily produced by the ovarian corpus luteum (CL) during the luteal phase of the menstrual cycle and early pregnancy, with the placenta taking over as the primary source of progesterone by late pregnancy. Progesterone prepares the uterus for pregnancy and is essential in maintaining an existing pregnancy. Defects in progesterone secretion and action have been linked to reduced fertility (Lydon et al., 1996; Soliman et al., 1994). At the same time, progestins are routinely used in medicine to control female reproduction and treat gynecological malignancies. For example, the synthetic progestins levonorgestrel and drospirenone are key components of birth and are also used treat gynecological conditions such as endometriosis and uterine bleeding (Leo et al., 2016).
3.1 Plants Produce Progesterone
For many years, it was assumed that only animals produced ovarian steroids. However, progesterone has been repeatedly isolated from plant material. The first evidence of progesterone in plants was published in 1968, when progesterone was isolated from apple seeds using thin-layer chromatography (Gawienowski and Gibbs, 1968). Pauli et al. (2010) identified progesterone in English Walnut (Juglans regia). In wheat, progesterone concentration varied by variety, age, stage of growth, and drought status, suggesting a role in the growth and development of the crop (Janeczko et al., 2013). As further evidence that progesterone has a biological effect in plants, the same group found that the 3β-hydroxysteroid dehydrogenase (3βHSD) inhibitor, trilostane, reduced free progesterone concentrations in wheat seedlings, evidencing its efficacy in plants (Janeczko et al., 2015). Trilostane reduced ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activity in wheat seedlings grown in drought conditions, and exogenous progesterone reversed the effect (Janeczko et al., 2015). These investigations clearly demonstrated that plants produced progesterone, and suggested that progesterone may regulate plant growth. It is unclear whether plant derived progesterone exerts biological effects in the animals that consume it. In post-menopausal women, oral administration of 100 mg/day of progesterone was required to achieve biologically relevant serum concentrations (20-30 nM; Adlercreutz and Martin, 1980; Whitehead et al., 1980). Given the range, but relatively low, of concentrations found in plants (pg/g to ng/g), it is unlikely that orally ingested plant-based progesterone would reach serum concentrations sufficient to have biological effects in humans.
3.2 Identification of Novel Phytoprogestins
The first progestins used for birth control, norethynodrel and norethindrone, were synthesized from diosgenin, which was isolated from yams (Crabbé, 1979). However, diosgenin does not bind the progesterone receptor (PR; Zava et al., 1998) and therefore cannot be considered a phytoprogestin. There is evidence that other molecules in plants do interact with the progesterone receptor. An early case report suggested that a ginseng supplement induced estrogenic changes in vaginal epithelium (Punnonen and Lukola, 1980). The investigators showed that a crude methanol extract of ginseng competed with R5020 (a progestin) and estradiol for binding sites in the rat uterus (Punnonen and Lukola, 1980), indicating the presence of progestin and estrogen. Zava et al. (1998) screened 150 plant extracts for binding to T47D cells (a breast cancer cell line with high levels of PR) as a surrogate for PR binding, and identified 20 plants that potentially contained phytoprogestins. However, subsequent analysis using alkaline phosphatase activity as a measure of progesterone signaling found no extracts with PR agonist activity. Extracts from damiana, dong quai, yucca, and mistletoe reduced alkaline phosphatase activity in the presence of progesterone, suggesting the presence of PR antagonists, but these extracts were not further characterized (Zava et al., 1998).
Rosenberg et al. (1998) used secretion of prostate specific antigen (PSA) from T47D cells to screen 45 compounds for phytosteroid actions. The investigators identified apigenin, naringenin, and syringic acid as potential phytosteroids. To determine if these molecules acted through the PR or androgen receptor (AR), they tested the ability of an anti-androgen (RU56,187) or anti-progestin (RU486) to block their effects. RU486 reduced the activity of each compound by 80-95%, confirming them as progestins. The authors also screened 45 compounds in the presence of the progestin norgestimate to look for anti-progestin actions and identified 11 compounds that reduced activity 24-90%. However, the compounds were not further characterized.
Toh et al. (2012) found that the 75% ethanolic extract from red clover (Trifolium pretense) significantly increased PRE/luciferase activity in T47D cells. Screening a library of 26 compounds from red clover, common phytoestrogens reported in red clover (genistein, daidzein, biochanin A, and formononetin) had no effect, but kaempferol and apigenin (Figure 1) both significantly increased PRE/luciferase induction in T47D and human endometrial stromal cells (HESC). Apigenin had a biphasic effect, and increased PRE/luciferase activity at low concentrations but not at high concentrations. In addition, RU486 completely blocked the PRE/luciferase activity of both kaempferol and apigenin, confirming that they interacted with the PR. Most interestingly, both compounds acted as weak agonists in isolation, increasing PRE/luciferase activity 5-7 fold as compared to the 54-fold induction detected from 100 nM progesterone. However, in the presence of progesterone, both compounds acted as antagonists, and reduced progesterone-stimulated PRE/luciferase activity by >50% (Toh et al., 2012). In a follow up study, kaempferol failed to induce ERE/luciferase or PSA/luciferase activity, showing its specificity toward PR (Toh et al., 2014). In vivo genistein (a phytoestrogen) increased proliferation of the uterine epithelium as measured by Ki67 immunostaining, and kaempferol reduced the effect. In the uterine stroma, genistein increased PR expression, as expected for an estrogenic compound. Interestingly, kaempferol did not reduce PR levels, as might be expected for a progestin (Toh et al., 2014). Kaempferol also increased amphiregulin A, a well-established progesterone stimulated gene. Collectively, these results indicate that kaempferol is a phytoprogestin, with mixed agonist/antagonist activity.
3.3 Apigenin Exhibits Progestin and Anti-Progestin Effects
Apigenin has repeatedly been identified as a phytoprogestin (Rosenberg et al., 1998; Stroheker et al., 2004; Toh et al., 2012), and hence should exert biological effects in progesterone-sensitive systems, such the uterus or breast cancer. Indeed, several studies have found such biological activity. For example, oral gavage of apigenin decreased total levels of estrogen receptor α in the uterus (Breinholt et al., 2000), a well-known effect of progesterone (Evans et al., 1980; Hsueh et al., 1976). Treatment of MBA-MB-468 cells, a breast cancer cell line, with either progesterone or R5020, resulted in increased proliferation and phosphorylation of AKT (Dressing et al., 2012). In a separate study, apigenin was shown to reduce both proliferation and AKT phosphorylation of MBA-MB-468 cells (Harrison et al., 2014), essentially having effects opposite of progestins.
More functionally, Willemsen et al. (2003) produced cell lines to identify novel steroid receptors and identified apigenin as a weak PR agonist. In Ishikawa cells, apigenin antagonized progesterone stimulated SLUTE1 expression with an EC50 of 850 nM. Apigenin was shown to block medroxyprogesterone (MPA)-induced increase in VEGF production and secretion by T47D cell (Mafuvadze et al., 2010). In a 7,12-dimethylbenz[a]anthracene induced breast cancer model, MPA enhanced tumor growth, an effect that was abrogated by apigenin co-treatment (Mafuvadze et al., 2011). The same group also showed that that MPA increased tumor growth in a BT-474 xenograft model and apigenin again blocked the effect (Mafuvadze et al., 2012). Supporting the idea that the effects of apigenin were due to anti-progestin activity, RU486 also reduced tumor growth in these same in vivo models (Liang et al., 2007; Wiehle et al., 2007). Collectively, these data indicate that apigenin is a phytoprogestin that can both activate and antagonize PR, depending on concentration and the abundance of endogenous progesterone. Future research should aim to characterize the effects of apigenin in other progesterone-responsive tissues and clarify factors that influence its agonist and antagonist activities.
4. Phytoandrogens
In males, testosterone is required for reproductive processes such as sexual behavior and sperm production (Borst and Yarrow, 2015; Muller, 2016). Outside the reproductive system, testosterone has important actions such as maintaining muscle mass and bone density of men (Borst and Yarrow, 2015; Sinnesael et al., 2011). In women the major circulating androgen is dehydroepiandrosterone (DHEA), which has very low affinity for the AR (Chen et al., 2005). Clinically, DHEA has been shown to increase the success of in vitro fertilization (IVF) and lower the rate of miscarriage (Gleicher et al., 2009; Kara et al., 2014); however, the mechanism mediating DHEA's effects are unclear (Widstrom and Dillon, 2004).
Testosterone can initiate signaling by binding directly to the androgen receptor (AR), be converted the more potent dihydrotestosterone (DHT), or be converted to estrogen. Both DHT and the estrogenic products of testosterone have important physiological and pathological effects. For example, normal bone health and development of prostate cancer is clearly dependent on both DHT and estrogens produced from systemic testosterone (Nelles et al., 2011; Sinnesael et al., 2011; Tan et al., 2015). It is clear that any compounds that bind to the AR or interfere with testosterone metabolism will have important effects. Food and herbal supplements have been shown to increase testosterone concentration and/or increase sperm concentrations (Sreedhar Naik et al., 2016; Tahvilzadeh et al., 2016). However, it is unclear if these effects are mediated by the AR.
4.1 Identification of Novel Phytoandrogens
Screening 45 molecules in the presence of DHT, Rosenberg et al. (1998) identified 11 molecules that reduced PSA production in T47D cells, with inhibition ranging from 21-87%. Ong and Tan (2007) showed that crude ethanolic extracts from the cortical tissue of Eucommia ulmoides displayed androgenic and estrogenic activity based on luciferase assays in HeLa cells, and the ethanolic extract competed with DHT for binding to AR. But the extract did not have any effect on the PR or GR, indicating receptor specificity. Interestingly, the ethanolic extract displayed synergistic activity with testosterone. It approximately doubled luciferase activity, even in the presence of saturating concentrations of testosterone. In vivo 2,500 and 5,000 μg of testosterone maximally increased prostate weight in rats. The ethanolic extract was unable to increase prostate weight over control, but treatment with the extract combined with 5,000 μg testosterone increased prostate weight over testosterone alone. These results show a clear synergistic interaction between the cortical extract and testosterone; however, the mechanism underlying this interaction is unknown.
4.2 Phytoestrogens also Exert Phytoandrogenic Effects
There is increasing evidence that some phytoestrogens, particularly genistein (Figure 1), can act as phytoandrogens. Wang et al. (2010) used AutoDock to examine the ability of eight xeno- and phytoestrogens to bind to the AR. Interestingly, the phytoestrogens (genistein, daidzein, and flavone) were predicted to have high affinity for the androgen receptor, which exhibited affinity energies ranging from -8.3 to -8.5 kcal/mol (compared to −11.2 kcal/mol for DHT). Stroheker et al. (2004) used a MMTV/luciferase construct (activated by the AR, PR, GR, and MR; Paguio et al., 2010) with MDA-MB-453 cells to screen for compounds acting as phytoandrogens. In their study, genistein (10 μM) increased luciferase activity to 244% of control, and apigenin (10 μM) increased activity to 174% of control compared to DHT (0.4 nM) which increased activity to 819% of control (Stroheker et al., 2004). When tested in the presence of DHT, genistein, apigenin, and kaempferol all acted as antagonists. Concentrations of the three compounds between 1-100 nM decreased activity. Interestingly, all three compounds displayed a biphasic antagonist activity, as concentrations higher than 100 nM had the same activity as control (Stroheker et al., 2004). Pihlajamaa (2011) generated a transgenic mouse model in which luciferase is under the control of the Spl promoter, as Spl expression is exclusively controlled by the AR (Adler et al., 1993). In castrated male mice genistein increased luciferase activity in prostate and brain, two androgen response tissues. Genistein decreased luciferase activity in those same two tissues in intact mice, indicating genistein is a mixed agonist/antagonist for the AR. Finally, genistein had no effect in skeletal muscle and lungs, suggesting tissue specific AR activity (Pihlajamaa et al., 2011). The results suggest that biological effects of genistein may come from interactions with the AR as well as the ER.
In agreement with genistein being a phytoandrogen (with antagonist effects), there is evidence that genistein is protective against prostate cancer, which is well known to be androgen sensitive. For example, 100 and 250 mg/kg of genistein in the diet dramatically reduced metastasis of PC3-M cells in mice without affecting tumor volume (Lakshman et al., 2008). It is well established that men in Asian countries consume more soy products and have a lower risk of prostate cancer (Chen et al., 2014). A study of Japanese men in Los Angeles county found the risk of prostate cancer in immigrants increased to the levels of American born men, suggesting an environmental cause (Shimizu et al., 1991). A meta-analysis of soy consumption in humans found that soy consumption was associated with a 26% reduction in risk for prostate cancer (Yan and Spitznagel, 2009). These data indicate that genistein-containing foods are likely protective against prostate cancer. This effect could be due to genistein inhibiting androgen signaling; however, it should be noted that increasing evidence shows that prostate cancer is also dependent on estradiol and its metabolites (Nelles et al., 2011). Therefore, it is also possible that the protective effects of genistein are due to altered estradiol metabolism or likely a combination of altered estradiol and androgen signaling. Future research on genistein should consider both mechanisms of action.
5. Phytocorticoids
The adrenal gland produces three classes of steroids – androgens, glucocorticoids and mineralocorticoids (Stocco and Clark, 1996). The androgen DHEA is a weak agonist for the AR with controversial effects in normal physiology (see section 4. Phytoadrogens; Rutkowski et al., 2014). Cortisol, the primary glucocorticoid, is produced by the zona fasciculata of the adrenal gland in response to stress. The primary effects of cortisol are to increase serum glucose concentrations (through gluconeogenesis) and suppress immune function. A useful, albeit simplified view of glucocorticoid action is that the beneficial effects (principally immune suppression) are due to reduced expression of genes associated with the immune response (termed transrepressive effects). However, continued glucocorticoid usage results in a sustained increase in glucose concentrations and bone reabsorption, limiting the usefulness of glucocorticoids clinically. In general, these negative side effects are due to increased expression of genes involved in gluconeogenesis and bone metabolism (termed transactive). Hence, there is a need to develop selective glucocorticoid receptor modulators (SEGRMs) with only transrepressive effects (Schäcke et al., 2005; Sundahl et al., 2015).
The primary mineralocorticoid is aldosterone, which is produced by the zona glomerulosa of the adrenal cortex. Aldosterone activates the mineralocorticoid receptor (MR) in the nephron of the kidney, triggering the conservation of sodium ions and the excretion of potassium ions. Interestingly, cortisol has high affinity for the MR (Krozowski and Funder, 1983), and 11-β-hydroxysteroid dehydrogenase (11βHSD, which inactivates cortisol) is required to keep cortisol levels low enough in the kidney as to not activate the MR (Diederich et al., 2000; Edwards et al., 1988; Funder et al., 1988).
5.1 Ginseng Contains Phytocorticoids
Ginseng has been used as an herbal medicine for thousands of years. In fact, the genus name Panax means “cure all” in Greek (Yun, 2001). While ginseng contains many bioactive molecules (Choi, 2008), much research has focused on ginsenosides, which are found in trace amounts in ginseng. Ginsenoside Rf (Figure 1) has been shown bind to and activate the GR. Early evidence came from a study showing that ginsenoside Rf competed with corticosterone for binding sites in rat hippocampus tissue (Kloet et al., 1987). In cultured hepatocytes, ginsenoside Rf increased transcription of tyrosine aminotransferase, an effect blocked by RU486 (Kang et al., 1994). Ginsenoside Rf also binds to and activates glucocorticoid receptors (GR), though with an affinity 100-fold lower than dexamethasone (Lee et al., 1997). Kropotov et al. (2002) found that a liquid extract from Siberian ginseng reduced urinary excretion of calcium and phosphorous and blocked the weakening of vertebrae seen in a steroid-induced osteoporosis model.
Ginsenoside Rg1 may also be a glucocorticoid. Ginsenoside Rg1 binds to the GR with an IC50 of 128 nM, approximately 10-fold higher than that of dexamethasone. Computer modeling indicated that ginsenoside Rg1 bound in the ligand-binding domain (LBD) of GR (Leung et al., 2006). Functionally, ginsenoside Rg1 increased phosphorylation of GR, phosphorylation of AKT, and increased production of nitric oxide (NO) in HUVEC cells, effects all blocked by RU486 (Leung et al., 2006). Du et al. (2011) found that ginsenoside Rg1 blocked LPS stimulated cytokine production and decreased NF-κB activity in RAW264.7 cells and a siRNA for GR inhibited these effects. Ginsenoside Rg1 did not interfere with proliferation or differentiation of osteoblasts. Confirming these observations in vivo, ginsenoside Rg1 and dexamethasone both reduced inflammation, but only dexamethasone increased plasma glucose concentration and caused osteoporosis (Du et al., 2011). Thus, ginsenoside Rg1 is a potential SGRM that warrants further investigation.
5.2 Compound A is a Selective Glucocorticoid Receptor Modulator
In 1961, there was a report of Karakul sheep in Namibia that grazed on plants from the genus Salsola experienced prolonged gestations; these incidences were later determined to be due to the shrub Salsola tuberculatiformis Botschantzev (previously called Salsola tuberculata) (Basson et al., 1969; De Lange, 1961). Initial investigations found that active component(s) also extended the estrous cycle of rats and inhibited adrenal steroidogenesis (Swart et al., 1993; Van der Merwe et al., 1976). Using bioassay guided fractionation, the active compound was eventually determined to be an unstable aziridine compound which was stabilized in vivo through interactions with serum proteins (Louw et al., 1997). In addition, the investigators synthesized a more stable aziridine analog that they named “compound A” (4-[1-Chloro-2-(methylamino)ethyl]phenyl acetate hydrochloride (1:1)) (Figure 1; Lesovaya et al., 2015; Swart et al., 2003, 1993).
Compound A displayed in vitro activities consistent with being a promising SEGRM. It had impressive affinity for the GR (EC50 values: 25.9 nM for compound A and 6.4 nM for dexamethasone); however, compound A did not exhibit significant GR activity via reporter assays using p(GRE)2-50-luc or pMMTV-luc constructs. It was able to block dexamethasone activity. Similarly, compound A did not induce expression of the GR responsive genes in A549 or BWTG3 cells, but it did decrease expression of basal and TNF-induced IL-6 protein levels in TC10 cells, and reduced expression of NF-κB drive genes (De Bosscher et al., 2005). On a more global scale, compound A only increased the expression of 11% of genes that were increased by dexamathsone. In contrast, compound A decreased the expression of 66% of genes decreased by dexamethasone. Theses results suggest that compound A had transrepressive effects typical of glucocorticoids, but that it lacked many of the transactive effects (De Bosscher et al., 2014). Compound A also exhibited a weak affinity for the androgen receptor, but it did not compete with mibolerone (a synthetic androgen) for AR binding (Tanner et al., 2003). Instead, compound A blocked the ligand-stimulated interaction between the NH2- and terminal domain and LBD of AR, which is known to be required for AR activity (Doesburg et al., 1997). Compound A did not bind to the PR or ER (De Bosscher et al., 2005; Tanner et al., 2003).
Compound A has been shown to slow the onset of symptoms in numerous in vivo models of inflammatory-related diseases such as induced arthritis and experimental autoimmune encephalomyelitis (Rauner et al., 2013; van Loo et al., 2010). It is well know that prostate cancer is extremely androgen sensitive (Tan et al., 2015), and accumulating evidence suggests that glucocorticoid also play a role in this disease (Smith et al., 1985; Yemelyanov et al., 2007). Therefore, compound A may be of interest in prostate cancer treatment due to its transrepressive effects. Compound A reduced the proliferation and induced apoptosis of prostate cancer cell lines DU145 and PC3 in vitro, in a GR-dependent manner (Yemelyanov et al., 2008). Additionally, compound A slowed growth of PC3 and Granta-519 xenografts in vivo (Lesovaya et al., 2015) making it a promising therapeutic lead based on a phytosteroid (Lesovaya et al., 2015).
5.3 Glycyrrhetic Acid Exhibits Mineralocorticoid-Like Effects
There are numerous case studies in the scientific literature of people consuming large amounts of licorice-containing foods (usually Glycyrrhiza glabra; Omar et al., 2012) and developing hypokalemia and hypertension, similar to patients with mineralocorticoid excess. Typically, the patient is advised to reduce their licorice intake and symptoms dissipate within days to weeks (Blachley and Knochel, 1980; Card et al., 1953; Epstein et al., 1977). In fact, many patients with adrenal insufficiency (i.e. Addision's disease) have been successfully treated (sometimes self treated) with licorice or licorice extracts (Cotterill and Cunliffe, 1973; Methlie et al., 2011).
Initially, many investigators stated that symptoms from licorice overconsumption was due to glycyrrhetinic acid and glycyrrhizic acid (major components of licorice; Figure 1) activating the MR (Ulmann et al., 1975). Armanini et al. (1983) found that both glycyrrhetinic and glycyrrhizic acid bound to the GR and MR but not the PR and AR, supporting the idea that these compounds contributed to pseudo-hyperaldosteronism by activation of the MR. However, the affinity of glycyrrhetinic acid and glycyrrhizic acid for the MR and GR was four to five orders of magnitude lower than that of aldosterone and dexamethasone, respectively (Armanini et al., 1983). Further supporting that glycyrrhetinic acid can directly activate the MR, albeit weakly, a study on cultured mononuclear leukocytes showed that both aldosterone and glycyrrhetinic acid increased expression of p22phox and PAI-1 (markers of oxidative stress), and the effect of both compounds was blocked by canrenone, an MR antagonist. Notably, 3,000-fold more glycyrrhetinic acid was required to achieve the same effect as aldosterone (Calò et al., 2004). Finally, neither ammoniated glycyrrhizin, nor licorice, was able to induce mineralocorticoid-like effects in adrenalized rats (Girerd et al., 1960), further indicating that direct activation of the MR by glycyrrhetinic acid is not responsible for the observed pseudo-hyperaldosteronism.
Glycyrrhetinic acid inhibits renal 11βHSD, resulting in higher local concentrations of cortisol, which activates the MR and induces pseudohyperaldosteronism. As evidence, Monder et al. (1989) showed that glycyrrhetinic acid inhibited conversion of corticosterone to 11-dehydrocorticosterone in a dose-dependent manner, and oral treatment of rats with glycyrrhetinic acid increased the urinary cortisol/cortisone ratio, evidencing inhibition of 11βHSD. In humans, treatment with 500 mg/day of glycyrrhetinic acid for five days resulted in a higher cortisol to cortisone ratio in the urine, indicative of 11βHSD inhibition (Ferrari et al., 2001). These data suggest that glycyrrhizin is a phytosteroid that weakly binds to both the GR and MR, however the relevant biological effect (pseudohyperaldosteronism) is due to 11βHSD inhibition.
6. Conclusions and Future Directions
Sales of herbal supplements is at an all-time high ($6.9 billion) and continuing to increase (Smith et al., 2016). And yet, the active component(s) in many of these supplements is unclear. It has been widely demonstrated that molecules from botanicals can interact with the PR, AR, MR, and GR (i.e. non-estrogen phytosteroids). In some cases, chemically undefined fractions have been shown to modify steroid signaling, but more often specific compounds were shown to bind steroid receptors. The biological actions of these compounds range from very well characterized (e.g. compound A; Lesovaya et al., 2015) to poorly characterized (e.g. the phytoprogestin apigenin; Rosenberg et al., 1998; Toh et al., 2012). One aspect complicating our understanding of these supplements toward human health is that many plants contain multiple phytosteroids (e.g. red clover; Burdette et al., 2002; Toh et al., 2012), contain phytosteroids that interact with multiple steroid receptors (e.g. genistein; Pihlajamaa et al., 2011), or contain phytosteroids that also interfere steroid metabolism, resulting in altered local steroid concentrations (e.g. glycyrrhizinic acid; Ramli et al., 2012). Future research is needed to identify novel phytosteroids, determine the biological effects of known phytosteroids, and characterize interactions between phytosteroids that are frequently consumed together. These issues need to be considered in future research in order to ensure the safe and effective use of herbal supplements.
Highlights.
Botanicals contain phytosteroids that bind non-estrogen steroid receptors.
Kaempferol, apigenin, and genistein bind progesterone and androgen receptors.
Ginsenoside Rf and glycyrrhetinic acid interact with corticoid receptors.
Compound A is a novel selective glucocorticoid, based on a phytosteroid.
Characterization of phytosteroids will help elucidate their safe and effective use.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) and the National Center for Complementary & Integrative Health (NCCIH) through grant R01 AT008824-01A1 to JB and BM and a T32 fellowship (T32AT007533) supporting MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- Adler AJ, Scheller A, Robins DM. The stringency and magnitude of androgen-specific gene activation are combinatorial functions of receptor and nonreceptor binding site sequences. Mol Cell Biol. 1993;13:6326–6335. doi: 10.1128/mcb.13.10.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlercreutz H, Martin F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J Steroid Biochem. 1980;13:231–244. doi: 10.1016/0022-4731(80)90196-x. [DOI] [PubMed] [Google Scholar]
- Armanini D, Karbowiak I, Funder JW. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin Endocrinol (Oxf) 1983;19:609–612. doi: 10.1111/j.1365-2265.1983.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Basson PA, Morgenthal JC, Bilbrough RB, Marais JL, Kruger SP, van der Merwe JL. “Grootlamsiekte”, a specific syndrome of prolonged gestation in sheep caused by a shrub Salsola tuberculata (Fenzl ex Moq) Schinz var. tomentosa C. A. Smith ex Aellen. Onderstepoort J Vet Res. 1969;36:59–103. [PubMed] [Google Scholar]
- Bennetts HW, Uuderwood Ej, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- Blachley JD, Knochel JP. Tobacco chewer's hypokalemia: licorice revisited. N Engl J Med. 1980;302:784–785. doi: 10.1056/NEJM198004033021405. [DOI] [PubMed] [Google Scholar]
- Borst SE, Yarrow JF. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am J Physiol Endocrinol Metab. 2015;308:E1035–1042. doi: 10.1152/ajpendo.00111.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinholt V, Hossaini A, Svendsen GW, Brouwer C, Nielsen SE. Estrogenic activity of flavonoids in mice. The importance of estrogen receptor distribution, metabolism and bioavailability. Food Chem Toxicol. 2000;38:555–564. doi: 10.1016/s0278-6915(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Liu J, Lantvit D, Lim E, Booth N, Bhat KPL, Hedayat S, Breemen RBV, Constantinou AI, Pezzuto JM, Farnsworth NR, Bolton JL. Trifolium pratense (Red Clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr. 2002;132:27–30. doi: 10.1093/jn/132.1.27. [DOI] [PubMed] [Google Scholar]
- Caan B, Quesenberry CP, Coates AO. Differences in fertility associated with caffeinated beverage consumption. Am J Public Health. 1998;88:270–274. doi: 10.2105/ajph.88.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò LA, Zaghetto F, Pagnin E, Davis PA, De Mozzi P, Sartorato P, Martire G, Fiore C, Armanini D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004;89:1973–1976. doi: 10.1210/jc.2003-031545. [DOI] [PubMed] [Google Scholar]
- Card WI, Strong JA, Tompsett SL, Mitchell W, Taylor NRW, Wilson JMG. Effects of Liquorice and its derivatives on salt and water metabolism. The Lancet. 1953;261:663–668. doi: 10.1016/s0140-6736(53)91801-7. [DOI] [PubMed] [Google Scholar]
- Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- Chen R, Ren S, Yiu MK, Fai NC, Cheng WS, Ian LH, Naito S, Matsuda T, Kehinde E, Kural A, Chiu JY, Umbas R, Wei Q, Shi X, Zhou L, Huang J, Huang Y, Xie L, Ma L, Yin C, Xu D, Xu K, Ye Z, Liu C, Ye D, Gao X, Fu Q, Hou J, Yuan J, He D, Pan T, Ding Q, Jin F, Shi B, Wang G, Liu X, Wang D, Shen Z, Kong X, Xu W, Deng Y, Xia H, Cohen AN, Gao X, Xu C, Sun Y. Prostate cancer in Asia: a collaborative report. Asian J Urol. 2014;1:15–29. doi: 10.1016/j.ajur.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Cotterill JA, Cunliffe WJ. Self-medication with liquorice in a patient with Addison's disease. The Lancet. 1973;301:294–295. doi: 10.1016/s0140-6736(73)91541-9. [DOI] [PubMed] [Google Scholar]
- Crabbé P. Some aspects of steroid research based on natural products from plant origin. Bull Sociétés Chim Belg. 1979;88:345–358. [Google Scholar]
- De Bosscher K, Beck IM, Dejager L, Bougarne N, Gaigneaux A, Chateauvieux S, Ratman D, Bracke M, Tavernier J, Vanden Berghe W, Libert C, Diederich M, Haegeman G. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cell Mol Life Sci CMLS. 2014;71:143–163. doi: 10.1007/s00018-013-1367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Berghe WV, Beck IME, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci U S A. 2005;102:15827–15832. doi: 10.1073/pnas.0505554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange M. Prolonged gestation in karakul ewes in South West Arica. 1961:590–592. [Google Scholar]
- Dennehy CE. The use of herbs and dietary supplements in gynecology: an evidence-based review. J Midwifery Womens Health. 2006;51:402–409. doi: 10.1016/j.jmwh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Diederich S, Quinkler M, Burkhardt P, Grossmann C, Bähr V, Oelkers W. 11Beta-hydroxysteroid-dehydrogenase isoforms: tissue distribution and implications for clinical medicine. Eur J Clin Invest. 2000;30(3):21–27. doi: 10.1046/j.1365-2362.2000.0300s3021.x. [DOI] [PubMed] [Google Scholar]
- Doesburg P, Kuil CW, Berrevoets CA, Steketee K, Faber PW, Mulder E, Brinkmann AO, Trapman J. Functional in vivo interaction between the amino-terminal, transactivation domain and the ligand binding domain of the androgen receptor. Biochemistry (Mosc) 1997;36:1052–1064. doi: 10.1021/bi961775g. [DOI] [PubMed] [Google Scholar]
- Dressing GE, Alyea R, Pang Y, Thomas P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm Cancer. 2012;3:101–112. doi: 10.1007/s12672-012-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Cheng B, Zhu X, Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol Baltim Md 1950. 2011;187:942–950. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
- Edwards CRW, Burt D, Mcintyre MA, De Kloet ER, Stewart PM, Brett L, Sutanto WS, Monder C. Localisation of 11β-hydroxysteroid dehydrosteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. The Lancet, Originally published as Volume 2, Issue 8618. 1988;332:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- Epstein MT, Espiner EA, Donald RA, Hughes H. Liquorice toxicity and the renin-angiotensin-aldosterone axis in man. Br Med J. 1977;1:209–210. doi: 10.1136/bmj.1.6055.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RW, Chen TJ, Hendry WJ, Leavitt WW. Progesterone regulation of estrogen receptor in the hamster uterus during the estrous cycle. Endocrinology. 1980;107:383–390. doi: 10.1210/endo-107-2-383. [DOI] [PubMed] [Google Scholar]
- Ferrari P, Sansonnens A, Dick B, Frey FJ. In vivo 11β-HSD-2 activity variability, salt-sensitivity, and effect of licorice. Hypertension. 2001;38:1330–1336. doi: 10.1161/hy1101.096112. [DOI] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith I. Mineralocorticoid action: target tissue specificity Is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Gawienowski AM, Gibbs CC. Identification of cholesterol and progesterone in apple seeds. Steroids. 1968;12:545–550. doi: 10.1016/s0039-128x(68)80117-5. [DOI] [PubMed] [Google Scholar]
- Girerd RJ, Rassaert CL, Dipasquale G, Kroc RL. Endocrine involvement in licorice hypertension. Am J Physiol. 1960;198:718–720. doi: 10.1152/ajplegacy.1960.198.4.718. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case control study. Reprod Biol Endocrinol. 2009;7:108. doi: 10.1186/1477-7827-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ME, Power Coombs MR, Delaney LM, Hoskin DW. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Exp Mol Pathol. 2014;97:211–217. doi: 10.1016/j.yexmp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Hsueh AJW, Peck EJ, Clark JH. Control of uterine estrogen receptor levels by progesterone. Endocrinology. 1976;98:438–444. doi: 10.1210/endo-98-2-438. [DOI] [PubMed] [Google Scholar]
- Hughes CL. Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ Health Perspect. 1988;78:171–174. doi: 10.1289/ehp.8878171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeczko A, Oklestkova J, Novak O, Śniegowska-Świerk K, Snaczke Z, Pociecha E. Disturbances in production of progesterone and their implications in plant studies. Steroids. 2015;96:153–163. doi: 10.1016/j.steroids.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Janeczko A, Oklešťková J, Siwek A, Dziurka M, Pociecha E, Kocurek M, Novák O. Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. J Steroid Biochem Mol Biol. 2013;138:384–394. doi: 10.1016/j.jsbmb.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Kang SY, Lee KY, Lee SK. Ginsenoside-Rg1 regulates the induction of tyrosine aminotransferase gene transcription in rat hepatocyte cultures. Biochem Biophys Res Commun. 1994;205:1696–1701. doi: 10.1006/bbrc.1994.2863. [DOI] [PubMed] [Google Scholar]
- Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Does dehydroepiandrosterone supplementation really affect IVF-ICSI outcome in women with poor ovarian reserve? Eur J Obstet Gynecol Reprod Biol. 2014;173:63–65. doi: 10.1016/j.ejogrb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Kloet ERD, Reul JMHM, Bosch FRVD, Tonnaer JaDM, Saito H. Ginsenoside RG1 and corticosteroid receptors in rat brain. Endocrinol Jpn. 1987;34:213–220. doi: 10.1507/endocrj1954.34.213. [DOI] [PubMed] [Google Scholar]
- Kropotov AV, Kolodnyak OL, Koldaev VM. Effects of Siberian Ginseng extract and ipriflavone on the development of glucocorticoid-induced osteoporosis. Bull Exp Biol Med. 2002;133:252–254. doi: 10.1023/a:1015834717178. [DOI] [PubMed] [Google Scholar]
- Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci U S A. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- Lee Y, Chung E, Youl Lee K, Hee Lee Y, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Leo VD, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: pharmacology tailored to women's health. Hum Reprod Update. 2016;22:634–646. doi: 10.1093/humupd/dmw016. [DOI] [PubMed] [Google Scholar]
- Lesovaya E, Yemelyanov A, Swart AC, Swart P, Haegeman G, Budunova I. Discovery of Compound A – a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget. 2015 doi: 10.18632/oncotarget.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KW, Cheng YK, Mak NK, Chan KKC, David Fan Tp, Wong RNS. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67:9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- Lm W, Ml P, As T. Double-blind, placebo-controlled study of Fertilityblend: a nutritional supplement for improving fertility in women. Clin Exp Obstet Gynecol. 2005;33:205–208. [PubMed] [Google Scholar]
- Louw A, Swart P, de Kock SS, van der Merwe KJ. Mechanism for the stabilization in vivo of the aziridine precursor 2-(4-acetoxyphenyl)-2-chloro-N-methyl-ethylammonium chloride by serum proteins. Biochem Pharmacol. 1997;53:189–197. doi: 10.1016/s0006-2952(96)00661-2. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Mafuvadze B, Benakanakere I, Hyder SM. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause. 2010;17:1055–1063. doi: 10.1097/gme.0b013e3181dd052f. [DOI] [PubMed] [Google Scholar]
- Mafuvadze B, Benakanakere I, López Pérez FR, Besch-Williford C, Ellersieck MR, Hyder SM. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev Res Phila Pa. 2011;4:1316–1324. doi: 10.1158/1940-6207.CAPR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafuvadze B, Liang Y, Besch-Williford C, Zhang X, Hyder SM. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm Cancer. 2012;3:160–171. doi: 10.1007/s12672-012-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methlie P, Husebye EES, Hustad S, Lien EA, Løvås K. Grapefruit juice and licorice increase cortisol availability in patients with Addison's disease. Eur J Endocrinol. 2011;165:761–769. doi: 10.1530/EJE-11-0518. [DOI] [PubMed] [Google Scholar]
- Monder C, Stewart PM, Lakshmi V, Valentino R, Burt D, Edwards CRW. Licorice inhibits corticosteroid 11β-dehydrogenase of rat kidney and liver: in vivo and in vitro studies. Endocrinology. 1989;125:1046–1053. doi: 10.1210/endo-125-2-1046. [DOI] [PubMed] [Google Scholar]
- Muller MN. Testosterone and reproductive effort in male primates. Horm Behav. 2016 doi: 10.1016/j.yhbeh.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6:437–451. doi: 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Q, Xing M, Hu J, Hu X, Nie S, Xie M. Metabolism and health effects of phyto-estrogens. Crit Rev Food Sci Nutr. 2015;0:00–00. doi: 10.1080/10408398.2015.1077194. [DOI] [PubMed] [Google Scholar]
- Noumi E, Tchakonang NY. Plants used as abortifacients in the Sangmelima region of Southern Cameroon. J Ethnopharmacol. 2001;76:263–268. doi: 10.1016/s0378-8741(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Omar HR, Komarova I, El-Ghonemi M, Fathy A, Rashad R, Abdelmalak HD, Yerramadha MR, Ali Y, Helal E, Camporesi EM. Licorice abuse: time to send a warning message. Ther Adv Endocrinol Metab. 2012;3:125–138. doi: 10.1177/2042018812454322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong VY, Tan BK. Novel phytoandrogens and lipidic augmenters from Eucommia ulmoides. BMC Complement Altern Med. 2007;7:3. doi: 10.1186/1472-6882-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paguio A, Stecha P, Wood KV, Fan F. Improved dual-luciferase reporter assays for nuclear receptors. Curr Chem Genomics. 2010;4:43–49. doi: 10.2174/1875397301004010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli GF, Friesen JB, Gödecke T, Farnsworth NR, Glodny B. Occurrence of progesterone and related animal steroids in two higher plants. J Nat Prod. 2010;73:338–345. doi: 10.1021/np9007415. [DOI] [PubMed] [Google Scholar]
- Pihlajamaa P, Zhang FP, Saarinen L, Mikkonen L, Hautaniemi S, Jänne OA. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology. 2011;152:4395–4405. doi: 10.1210/en.2011-0221. [DOI] [PubMed] [Google Scholar]
- Punnonen R, Lukola A. Oestrogen-like effect of ginseng. Br Med J. 1980;281:1110. doi: 10.1136/bmj.281.6248.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramli ESM, Suhaimi F, Asri SFM, Ahmad F, Soelaiman IN. Glycyrrhizic acid (GCA) as 11β-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2012;31:262–273. doi: 10.1007/s00774-012-0413-x. [DOI] [PubMed] [Google Scholar]
- Rauner M, Thiele S, Sinningen K, Winzer M, Salbach-Hirsch J, Gloe I, Peschke K, Haegeman G, Tuckermann JP, Hofbauer LC. Effects of the selective glucocorticoid receptor modulator compound A on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology. 2013;154:3719–3728. doi: 10.1210/en.2012-2221. [DOI] [PubMed] [Google Scholar]
- Rosenberg RS, Grass L, Jenkins DJ, Kendall CW, Diamandis EP. Modulation of androgen and progesterone receptors by phytochemicals in breast cancer cell lines. Biochem Biophys Res Commun. 1998;248:935–939. doi: 10.1006/bbrc.1998.8977. [DOI] [PubMed] [Google Scholar]
- Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014;74:1195–1207. doi: 10.1007/s40265-014-0259-8. [DOI] [PubMed] [Google Scholar]
- Schäcke H, Rehwinkel H, Asadullah K. Dissociated glucocorticoid receptor ligands: compounds with an improved therapeutic index. Curr Opin Investig Drugs Lond Engl 2000. 2005;6:503–507. [PubMed] [Google Scholar]
- Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnesael M, Boonen S, Claessens F, Gielen E, Vanderschueren D. Testosterone and the male Skeleton: a dual mode of action. J Osteoporos 2011. 2011:e240328. doi: 10.4061/2011/240328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Syms AJ, Nag A, Lerner S, Norris JS. Mechanism of the glucocorticoid regulation of growth of the androgen-sensitive prostate-derived R3327H-G8-A1 tumor cell line. J Biol Chem. 1985;260:12454–12463. [PubMed] [Google Scholar]
- Smith T, Kawa K, Eckl V, Johnson J. Sales of herbal dietary supplements in US increased 7.5% in 2015 consumers spent $6.92 billion on herbal supplements in 2015, marking the 12th consecutive year of growth. HerbaGram. 2016:67–73. [Google Scholar]
- Soliman S, Salim Daya MB, Collins J, Hughes EG. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil Steril. 1994;61:1068–1076. doi: 10.1016/s0015-0282(16)56758-2. [DOI] [PubMed] [Google Scholar]
- Sreedhar Naik B, Dangi NB, Sapkota HP, Wagle N, Nagarjuna S, Sankaranand R, Anantha kumari B. Phytochemical screening and evaluation of anti-fertility activity of Dactyloctenium aegyptium in male albino rats. Asian Pac J Reprod. 2016;5:51–57. [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in dteroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stroheker T, Picard K, Lhuguenot JC, Canivenc-Lavier MC, Chagnon MC. Steroid activities comparison of natural and food wrap compounds in human breast cancer cell lines. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2004;42:887–897. doi: 10.1016/j.fct.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacol Ther. 2015;152:28–41. doi: 10.1016/j.pharmthera.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Swart P, Swart AC, Louw A, van der Merwe KJ. Biological activities of the shrub Salsola tuberculatiformis Botsch.: contraceptive or stress alleviator? BioEssays News Rev Mol Cell Dev Biol. 2003;25:612–619. doi: 10.1002/bies.10285. [DOI] [PubMed] [Google Scholar]
- Swart P, van der Merwe KJ, Swart AC, Todres PC, Hofmeyr JH. Inhibition of cytochrome P-450(11)beta by some naturally occurring acetophenones and plant extracts from the shrub Salsola tuberculatiformis. Planta Med. 1993;59:139–143. doi: 10.1055/s-2006-959629. [DOI] [PubMed] [Google Scholar]
- Tahvilzadeh M, Hajimahmoodi M, Toliyat T, Karimi M, Rahimi R. An evidence-based approach to medicinal plants for the treatment of sperm abnormalities in traditional Persian medicine. Andrologia. 2016;48:860–879. doi: 10.1111/and.12676. [DOI] [PubMed] [Google Scholar]
- Tan ME, Li J, Xu HE, Melcher K, Yong E. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3–23. doi: 10.1038/aps.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner TM, Verrijdt G, Rombauts W, Louw A, Hapgood JP, Claessens F. Anti-androgenic properties of Compound A, an analog of a non-steroidal plant compound. Mol Cell Endocrinol. 2003;201:155–164. doi: 10.1016/s0303-7207(02)00411-2. [DOI] [PubMed] [Google Scholar]
- Toh MF, Mendonca E, Eddie SL, Endsley MP, Lantvit DD, Petukhov PA, Burdette JE. Kaempferol exhibits progestogenic effects in ovariectomized rats. J Steroids Horm Sci. 2014;5:136. doi: 10.4172/2157-7536.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh MF, Sohn J, Chen SN, Yao P, Bolton JL, Burdette JE. Biological characterization of non-steroidal progestins from botanicals used for women's health. Steroids. 2012;77:765–773. doi: 10.1016/j.steroids.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann A, Menard J, Corvol P. Binding of glycyrrhetinic acid to kidney mineralocorticoid and glucocorticoid receptors. Endocrinology. 1975;97:46–51. doi: 10.1210/endo-97-1-46. [DOI] [PubMed] [Google Scholar]
- Van der Merwe KJ, Hofmeyr JHS, Swart P, Parkin DP, Rossowu J, Hartshome J, Van Rensburg SJ, Morgenthal JC, Basson PA. Natural products affecting the gestation period of sheep and their mode of action. Afr J Sci. 1976;72:184. [Google Scholar]
- van Loo G, Sze M, Bougarne N, Praet J, Mc Guire C, Ullrich A, Haegeman G, Prinz M, Beyaert R, De Bosscher K. Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Mol Endocrinol Baltim Md. 2010;24:310–322. doi: 10.1210/me.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li J, Gao Y, Xu Y, Pan Y, Tsuji I, Sun ZJ, Li XM. Xeno-oestrogens and phyto-oestrogens are alternative ligands for the androgen receptor. Asian J Androl. 2010;12:535–547. doi: 10.1038/aja.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MI, Townsend PT, Gill DK, Collins WP, Campbell S. Absorption and metabolism of oral progesterone. Br Med J. 1980;280:825–827. doi: 10.1136/bmj.280.6217.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widstrom RL, Dillon JS. Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med. 2004;22:289–298. doi: 10.1055/s-2004-861546. [DOI] [PubMed] [Google Scholar]
- Wiehle RD, Christov K, Mehta R. Anti-progestins suppress the growth of established tumors induced by 7,12-dimethylbenz(a)anthracene: comparison between RU486 and a new 21-substituted-19-nor-progestin. Oncol Rep. 2007;18:167–174. [PubMed] [Google Scholar]
- Willemsen P, Scippo ML, Kausel G, Figueroa J, Maghuin-Rogister G, Martial JA, Muller M. Use of reporter cell lines for detection of endocrine-disrupter activity. Anal Bioanal Chem. 2003;378:655–663. doi: 10.1007/s00216-003-2217-2. [DOI] [PubMed] [Google Scholar]
- Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- Yemelyanov A, Czwornog J, Chebotaev D, Karseladze A, Kulevitch E, Yang X, Budunova I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26:1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- Yemelyanov A, Czwornog J, Gera L, Joshi S, Chatterton RT, Budunova I. Novel steroid receptor phyto-modulator compound A inhibits growth and survival of prostate cancer cells. Cancer Res. 2008;68:4763–4773. doi: 10.1158/0008-5472.CAN-07-6104. [DOI] [PubMed] [Google Scholar]
- Yun TK. Brief introduction of Panax ginseng C.A. Meyer J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zava DT, Dollbaum CM, Blen M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1998;217:369–378. doi: 10.3181/00379727-217-44247. [DOI] [PubMed] [Google Scholar]