Abstract
Studies on the role of hormones in male reproductive aging have traditionally focused on testosterone, but estradiol also plays important roles in the control of masculine physiology and behavior. Our goal was to examine the effects of E2 on the expression of genes selected for E2-sensitivity, involvement in behavioral neuroendocrine functions, and impairments with aging. Mature adult (MAT, 5 mo.) and aged (AG, 18 mo.) Sprague-Dawley male rats were castrated, implanted with either vehicle or estradiol (E2) subcutaneous capsules, and euthanized one month later. Bilateral punches were taken from the bed nucleus of the stria terminalis (BnST), posterodorsal medial amygdala (MePD) and the preoptic area (POA). RNA was extracted, and expression of 48 genes analyzed by qPCR using Taqman low-density arrays. Results showed that effects of age and E2 were age- and region-specific. In the POA, 5 genes were increased with E2 compared to vehicle, and there were no age effects. By contrast the BnST showed primarily age-related changes, with 6 genes decreasing with age. The MePD had 5 genes that were higher in aged than mature males, and 17 genes with significant interactions between age and E2. Gene families identified in the MePD included nuclear hormone receptors, neurotransmitters and neuropeptides and their receptors. Ten serum hormones were assayed in these same males, with results revealing both age- and E2-effects, in several cases quite profound. These results support the idea that the male brain continues to be highly sensitive to estradiol even with aging, but the nature of the response can be substantially different in mature and aging animals.
Keywords: Aging, Male, Estrogen, Preoptic Area, Medial Amygdala, BnST
1. Introduction
The hypothalamus is the body’s interface between the brain and the peripheral endocrine systems. Hypothalamic nuclei and their inputs and outputs from other limbic brain regions [e.g., bed nucleus of the stria terminalis (BnST) and amygdala] are involved in the regulation of reproduction, mood, and memory, in part through actions of gonadal steroid hormones on their receptors in the brain (Arimoto et al., 2013, Hogervorst et al., 2005, Rosario et al., 2010, Smith et al., 1992, Walf et al., 2009). The principal estrogen, estradiol (E2), has been extensively studied for age-related changes in neurobiological actions in females, but to a much lesser extent in males (Foster, 2012, Harburger et al., 2009, Raber, 2008, Russell et al., 2012, Walf et al., 2009). Furthermore, the literature in male rodents and humans on whether and how serum E2 changes with aging is inconsistent; various groups have reported increases (Fujita et al., 1990, Herath et al., 2001, Jasuja et al., 2013, Lakshman et al., 2010, Luine et al., 2007), decreases (Khosla et al., 2001, Leifke et al., 2000, van den Beld et al., 2000, Wu and Gore, 2009, Yeap et al., 2012), or no change (Goya et al., 1990, Gruenewald et al., 1994, Yeap et al., 2014) with advanced age. Furthermore, research on age-related changes in estradiol’s actions on male neuroendocrine systems is limited; this is an important area of study considering the profound effects that E2 plays on the aging female brain (Rao et al., 2013, Yin et al., 2015) and the importance of E2 in male neurobehavioral functions.
The preoptic area (POA), posterodorsal medial amygdala (MePD), and BnST, brain areas important in reproductive function in males, have high concentrations of estrogen receptors (ERs) (Mitra et al., 2003, Perez et al., 2003, Shughrue et al., 1997). Changes in ER expression with age, or in estrogenic regulation of genes in these areas, may underlie age-related deficits in sexual behavior and reproductive physiology in both sexes (Izumo et al., 2012, Navarro et al., 2013, Putnam et al., 2005, Yamaguchi and Yuri, 2012). When bound to the nuclear ERα or ERβ, E2 regulates genes and proteins associated with steroid hormone signaling, neurotransmission, and neuroendocrine functions. During aging, receptor density and binding affinity for the ERs, and ERα protein expression, change in a sex-, region-, and age-specific manner (Haji et al., 1981, Roselli et al., 1993, Thakur and Sharma, 2007, Wu and Gore, 2010, Wu et al., 2009, Zhao and Brinton, 2007). Furthermore, E2 may also act upon membrane ERs such as GPER; a recent study showed increased density of GPER-positive cells in two hypothalamic-preoptic regions in aging compared to young adult female rhesus monkeys (Naugle et al., 2014).
How estradiol may exert its influence more broadly, beyond direct effects on ERs in hypothalamic and limbic brain regions, and the influence of aging, is not well-understood. To investigate this, we profiled expression of 48 genes in the POA, MePD and BnST of young adult and aging male rats that were castrated and given E2 or vehicle. Our hypothesis was that the aging brain would lose responsiveness to E2, and that each region of interest would have unique E2- and age-related neuromolecular phenotypes. We selected genes from broad categories including steroid hormone receptors, the central control of energy balance, neuropeptides involved in reproduction and social behaviors, and neurotransmitters such as dopamine, serotonin, and glutamate. These targets have previously been shown to be regulated by age and estradiol, the latter largely from the female literature, but not studied together in the context of estrogen and aging in males (Chung et al., 1991, Diano et al., 1997, Guerra-Araiza et al., 2008, McQueen et al., 1999, Putnam et al., 2005).
2. Material and methods
2.1. Animals
Forty Sprague-Dawley male rats (Harlan, Indianapolis, IN; 3 or 12 months old at arrival) were purchased and housed in pairs in large plastic cages, in a climate-controlled room, on a 14:10 hr light–dark cycle (lights off at 1000 h). All procedures were done in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Food and water were freely available. The older males were retired breeders. To give the younger males sexual experience, these animals were each given a 90-minute mating session with a receptive female every other day for 6 sessions. On a final 7th session, males were tested to ensure that they met criteria of two ejaculations in one hour. All of the males met these criteria and were included in the study. Castration surgery was conducted under isoflurane anesthesia when rats were 3 months or 16 months of age. One month later, at 4 or 17 months, males were implanted with 12 mm Silastic capsules (1.98 I.D. × 3.18 O.D.) containing either estradiol (5% in cholesterol), or cholesterol (100% cholesterol). One month after hormone implantation, when rats were mature (MAT, 5 months) or aged (AG, 18 months), animals were rapidly decapitated, blood samples were collected, and their brains were removed and cut into 1 mm coronal sections on a chilled brain matrix before freezing on slides for storage at −80 degrees. Bilateral punches of the POA, BnST and MePD were later taken using a 0.98 mm Palkovits punch and transferred to frozen microfuge tubes.
2.2. RNA expression
RNA was extracted from frozen POA, BnST, and MePD punches using an Allprep DNA/RNA mini-kit (QIAGEN, Valencia, California), according to the manufacturer’s protocols. This and other molecular work was conducted as per standard laboratory protocols (Walker et al., 2013, Yin et al., 2015). RNA (200 ng) was converted to single-stranded cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and stored at −20°C. RNA samples were eluted with nuclease-free water and treated with 1 U of TURBO deoxyribonuclease (Applied Biosystems Inc, Foster City, California) to rid samples of genomic DNA before ethanol precipitation. Resuspended samples were diluted to a concentration of 50 ng/μL. The purity, integrity and concentration of all samples were verified by running them on a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, California) and a nanodrop. All samples had RINs of 8 or higher.
Samples (n = 8-10 per group) from the BnST, MePD, and POA were run on customized rat Taqman low-density array (TLDA) Microfluidic 48-gene real-time PCR cards (Applied Biosystems) as described (Walker et al., 2013) with specific gene assays chosen based on a priori hypotheses and publications on their importance in neuroendocrine function and reproductive aging (46 genes of interest and two normalizing genes; Supplemental Table 1).
Real-time PCR was performed using Taqman universal mastermix (Applied Biosystems) on an ABI ViiA7 machine with the following run parameters: 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Relative expression of each gene was determined using the comparative cycle threshold method (Pfaffl, 2001, Schmittgen and Livak, 2008). Each sample was normalized to Gapdh expression and then calibrated to the median δ-cycle threshold of the lowest-expressing group within each gene.
2.3. Serum Hormones
After collection blood was centrifuged at 2300 × g for 5 min. Serum was collected and stored at −80°C until analysis. Hormone assay protocols were identical to those published previously (Yin et al., 2015). Estradiol was analyzed in duplicate samples (200 μl) in a single assay, using an estradiol RIA kit (Cat. No. DSL-4800, Beckman Coulter, Webster, TX); assay sensitivity was 6 pg/mL and intra-assay CV was 1.2%. Concentrations of peripheral protein/peptide hormones were measured in duplicate 10 μl serum samples in a single Milliplex rat pituitary magnetic bead assay (RPTMAG-86K, Millipore, Billerica, MA). Intra-assay CV for each hormone was: luteinizing hormone (LH) 0.85%, follicle-stimulating hormone (FSH) 5.3%, thyroid stimulating hormone (TSH) 4.3%, growth hormone (GH) 3.2%, prolactin (PRL) 1.5%, and brain-derived neurotrophic factor (BDNF) 2.1%. A rat steroid Magnetic Bead assay (STTHMAG-21K; Millipore) was used to measure serum progesterone (P4), triiodothyronine (T3) and thyroxine (T4), with intra-assay CVs: P4 8.1%, T3 11.4%, and T4 7.6%. The Magnetic Bead assays were conducted in the laboratory of Dr. Andrew Wolfe, Johns Hopkins University School of Medicine, and the E2 RIA in the Gore Lab at the University of Texas at Austin.
2.4. Data Analyses
Gene expression data were normalized to Gapdh within each animal, expressed relative to the MAT vehicle group. For both mRNA and hormone results, a Grubb’s test for outliers was conducted, and up to two outliers per group were removed if indicated. A two-way analysis of variance was performed to test for main effects of age, hormone treatment, and interactions, followed by Tukey HSD post-hoc analysis, the latter correcting for multiple comparisons. When data failed tests for normality and homogeneity, they were square root transformed and analyzed with an ANOVA. If transformation did not resolve normality issues, data were analyzed with a non-parametric Kruskal-Wallis test (KW). Because this test does not enable the determination of an interaction effect, we conducted pairwise comparisons between groups using the Conover method (Conover and Iman, 1979), and corrected for multiple comparisons with the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) using the R package PMCMR (Pohlert, 2014). For all significant results, effect sizes were calculated as eta-squared (η2) using R (3.3.2). Eta-squared represents the proportion of variance accounted for by the factor that is tested. An η2 greater than 0.06 is considered medium and an η2 greater than 0.14 is considered large (Gillette et al., 2016).
Gene expression heatmaps were created to visualize relationships across experimental groups. In order to cluster genes based on expression, a Pearson pairwise correlation matrix was generated for all genes across all treatment groups within each brain region (mPOA, BnST, MePD). A distance matrix was then created by subtracting one from the absolute value of each pairwise correlation, and complete linkage hierarchical clustering was performed based on this distance matrix. A hierarchical dendrogram was then generated for each heatmap based on the clustering results. To visualize gene expression differences between groups on the heatmap, gene expression values within a group were normalized as z-scores. Treatment groups were then hierarchically clustered based on Euclidian distance between these values. Heatmap construction (heatmap.2 in gplots) and clustering were performed in R (3.1.2). An effect was considered significant at p < 0.05.
3. Results
3.1. Gene expression in the POA
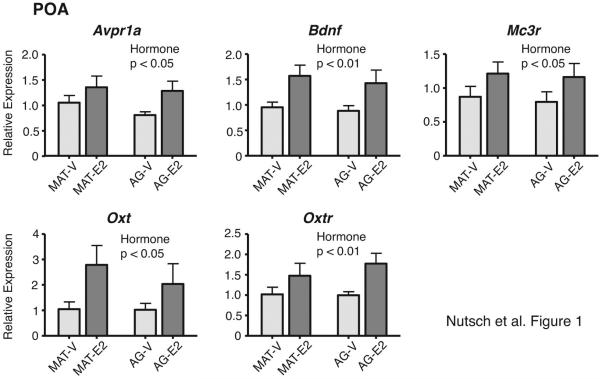
Of the 46 genes of interest, five had significant main effects of hormone treatment; in all cases, expression was higher in E2 than vehicle-treated males (Figure 1). These 5 genes were: Avpr1a [F(1,29) = 4.45, η2 = 0.13, p < 0.05], Bdnf [F(1,30) = 10.23, η2 = 0.25, p < 0.01], Mc3r [F(1,29) = 4.28, η2 = 0.13, p < 0.05], Oxt [F(1,29) = 5.27, η2 = 0.15, p < 0.05], and Oxtr [F(1,28) = 8.04, η2 = 0.22, p < 0.01]. There were no significant main effects of age, or any significant hormone by age interactions, for genes in the POA. Results for the residual genes not significantly affected in the POA are provided in Supplemental Table 2.
Figure 1.
Genes in the POA for which there were significant main effects of hormone treatment are shown. Expression of all was higher in E2 than Vehicle male rats. There were no interactions or age effects. P-values for significant main effects of hormone treatment are specified for each panel. Data are shown as mean + SEM. Abbreviations here and in other figures are: MAT-V, mature-vehicle; MAT-E2, mature-estradiol; AG-V, aged-vehicle; AG-E2, aged-estradiol.
3.2. Gene expression in the BnST
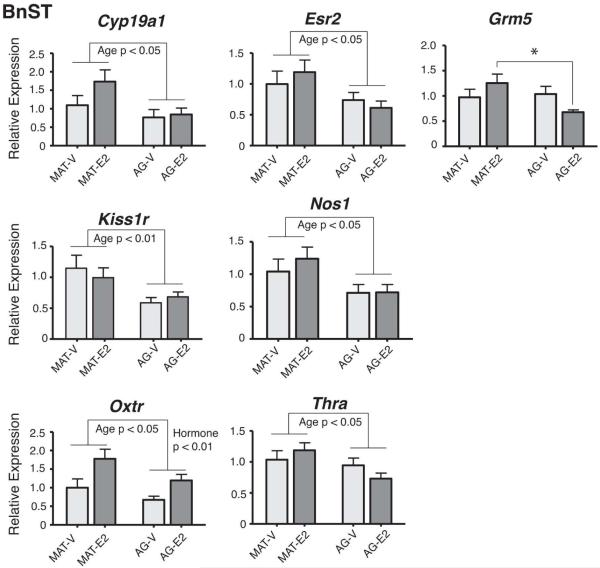
In the BnST, significant main effects of age were found for 6 genes, with expression lower in aged than mature males in all cases (Figure 2). These genes were: Cyp19a1 [F(1,27) = 5.52, η2 = 0.17, p < 0.05], Esr2 [F(1,27) = 6.06, η2 = 0.18, p < 0.05], Kiss1r [F(1,29) = 9.03, η2 = 0.25, p < 0.01], Nos1 [F(1,27) = 6.86, η2 = 0.20, p < 0.05], Oxtr [F(1,26) = 4.85, η2 = 0.16, p < 0.05], and Thra [F(1,27) = 5.08, η2 = 0.16, p < 0.05]. Oxtr also had a significant main effect of hormone [F(1,26) = 9.96, η2 = 0.28, p < 0.01], with expression higher in E2 than vehicle males. Grm5 had a significant interaction of E2 and age [F(1,29) = 4.79, η2 = 0.15, p < 0.05], attributable to the mature-E2 males having higher expression than the aged-E2 males (p < 0.05). Detailed results for residual genes not significantly affected in the BnST are provided in Supplemental Table 3.
Figure 2.
Expression of genes in the BnST that were significantly affected by age, hormone, or an interaction are shown. Significant main effects of age were found for Cyp19a1, Esr2, Kiss1r, Nos1, Oxtr, and Thra, all lower in aged than mature male rats. Oxtr also had a main hormone effect. Grm5 had a significant interaction of age and hormone, attributable to the mature-E2 rats having higher expression than the aged-E2 rats (*, p < 0.05). P-values for significant main effects are indicated for each panel. Data are shown as mean + SEM.
3.3. Gene expression in the MePD
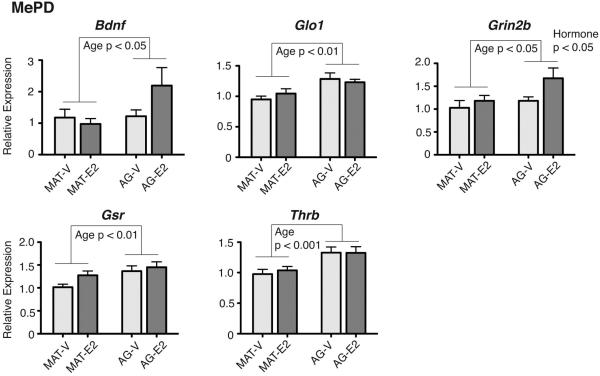
Of the three regions studied, the MePD had the largest number of changes. Five genes had significant main effects of age (Figure 3), with expression higher in aged than mature males. These genes were: Bdnf [F(1,33) = 3.99, η2 = 0.11, p < 0.05], Glo1 [F(1,31) = 11.37, η2 = 0.27, p < 0.01], Gsr [F(1,32) = 8.58, η2 = 0.21, p < 0.01], Thrb [F(1,33) = 14.62, η2 = 0.31, p < 0.001], and Grin2b [F(1,33) = 4.59, η2 = 0.12, p < 0.05]. Grin2b also had a significant effect of hormone treatment, with expression higher in E2 than Vehicle males [F(1,33) = 4.58, η2 = 0.12, p < 0.05].
Figure 3.
Genes with significant main effects of age, but no interactions, are shown for the MePD. Five genes had increased expression in aged compared to mature animals: Bdnf, Glo1, Grin2b, Gsr, and Thrb. Grin2b also had a significant main hormone effect, higher in E2 than Vehicle males. P-values for significant main effects are shown for each panel. Data are shown as mean + SEM.
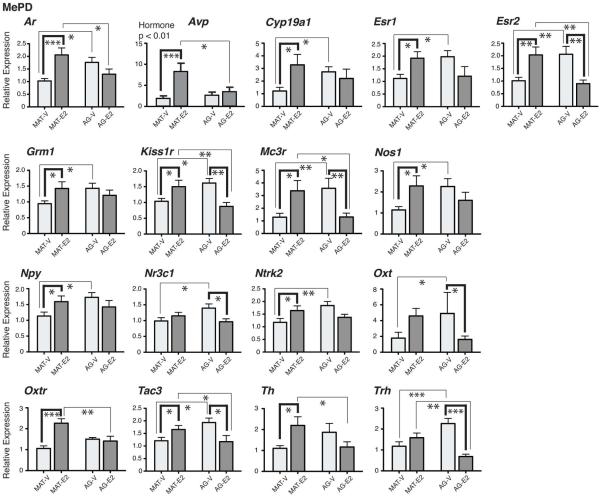
Seventeen genes had significant age by hormone interactions in the MePD, as shown in Figure 4. These genes were: Ar [F(1,33) = 12.9, η2 = 0.28, p < 0.001], Avp [F(1,33) = 5.51, η2 = 0.14, p < 0.05], Cyp19a1 [F(1,33) = 5.66, η2 = 0.15, p < 0.05], Esr1 [F(1,33) = 8.95, η2 = 0.21, p < 0.005], Esr2 [F(1,32) = 18.71, η2 = 0.37, p < 0.001], Grm1 [F(1,33) = 4.72, η2 = 0.13, p < 0.05], Kiss1r [F(1,32) = 15.68, η2 = 0.31, p < 0.001], Mc3r [F(1,33) = 14.64, η2 = 0.31, p < 0.001], Nos1 [F(1,33) = 6.75, η2 = 0.17, p < 0.05], Npy [F(1,33) = 5.29, η2 = 0.14, p < 0.05], Nr3c1 [F(1,33) = 6.92, η2 = 0.17, p < 0.05], Ntrk2 [F(1,33) = 8.29, η2 = 0.20, p < 0.01], Oxt [F(1,32) = 8.96, η2 = 0.22, p < 0.005], Oxtr [F(1,33) = 14.29, η2 = 0.30, p < 0.001], Tac3 [F(1,33) = 12.14, η2 = 0.27, p < 0.001], Th [F(1,32) = 8.11, η2 = 0.20, p < 0.01], and Trh [F(1,33) = 22.44, η2 = 0.41, p < 0.001]. Avp also had a significant main effect of hormone, higher in E2 than vehicle males: [F(1,33) = 9.71, η2 = 0.23, p < 0.01]. Post-hoc statistics for the 17 genes with significant interaction effects are presented in Figure 4. Several of genes with significant interactions were up-regulated by E2 in the mature males and down-regulated or unchanged by E2 in the aged males. Results for residual genes not significantly affected in the MePD are provided in Supplemental Table 4.
Figure 4.
Genes with significant interactions between age and hormone treatment are shown for the MePD. Significant effects of hormone within an age group are indicated by bold brackets. Significant effects of age within a hormone group are indicated by thin brackets. For Avp, there was also a significant main effect of hormone, higher in E2 than vehicle males. P-values for post-hoc analyses of the interaction effects are indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data are shown as mean + SEM.
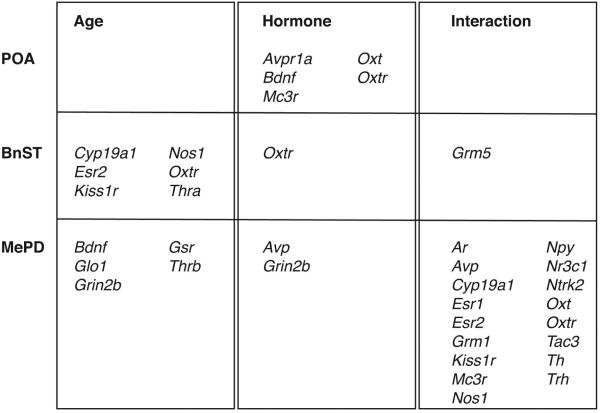
A summary and comparison of the changes in the POA, BnST and MePD is presented in Figure 5. Interestingly, each region had unique expression patterns, with POA genes affected only by hormone (increased by E2); the BnST primarily by age (decreased with age); and the MePD by age (increased with age) or interactions of age and hormone. The only gene identified in all 3 regions was Oxtr, although the directionality of effects differed between the regions.
Fig 5.
Summary of genes in the POA, BnST and MePD with significant main effects of age (left), hormone (middle), or a significant interaction (right).
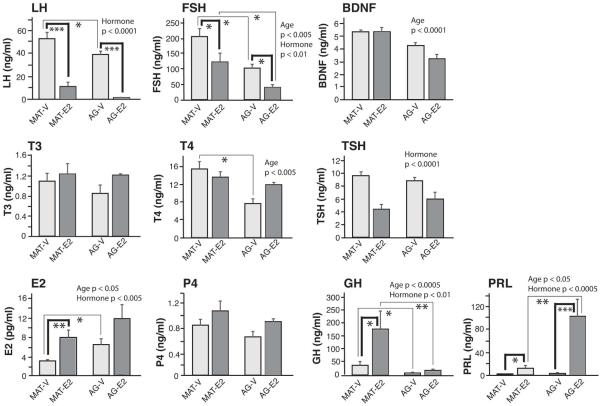
3.4. Effects of estradiol and aging on serum hormone concentrations
Ten serum hormones were assayed (Figure 6), with post-hoc statistics shown in the graphs, and main effects presented both here and in the figure. Serum E2 was measured to validate the efficacy of the hormone treatment regime. A significant main effect of hormone was found, with serum E2 concentrations higher in the E2 replacement groups compared to vehicle, as expected [KW, η2 = 0.28, p < 0.005]. There was also a main effect of age, with estradiol concentrations elevated in aged compared to mature animals [KW, η2 = 0.14, p < 0.05]. Serum progesterone concentrations were unaffected by age or hormone. Regarding the gonadotropins, LH concentrations were down-regulated by E2 [KW, η2 = 0.72, p < 0.0001]. Serum FSH concentrations were also lower in E2 than vehicle males [KW, η2 = 0.22, p < 0.01], and were decreased in aged compared to mature males [KW, η2 = 0.24, p < 0.005]. BDNF had a main effect of age, and was lower in the aged group [F(1, 32) = 32.86, η2 = 0.51, p < 0.0001].
Fig 6.
Serum hormone concentrations are shown. P-values for main effects of age or hormone are indicated on the graphs. For interactions, significant effects of hormone within an age group are demarcated by bold brackets, and significant effects of age within a hormone group are indicated by thin brackets. Interaction p-values are indicated as: *, p < 0.05, **p < 0.01, ***p < 001. Data are shown as mean + SEM.
Of the thyroid hormones, serum TSH had a main treatment effect, and was lower in E2 than vehicle rats [F(1,32) = 29.43, η2 = 0.48, p < 0.0001]. For T4, there was a significant effect of age [F(1,33) = 12.45, η2 = 0.27, p < 0.005], with concentrations lower in aged than mature males. There was also a significant interaction [F(1,33) = 5.18, η2 = 0.14, p < 0.05], attributable to a difference between MAT-V and AG-V rats (p < 0.05). Serum T3 was unaffected by age or hormone.
GH concentrations decreased with age [KW, η2 = 0.36, p < 0.0005], and were increased by E2 [KW, η2 = 0.22, p < 0.01]. Serum PRL had a main effect of age, greater in aged than mature males [KW, η2 = 0.12, p < 0.05], and a main hormone effect, higher in E2 than vehicle males [KW, η2 = 0.35, p < 0.0005].
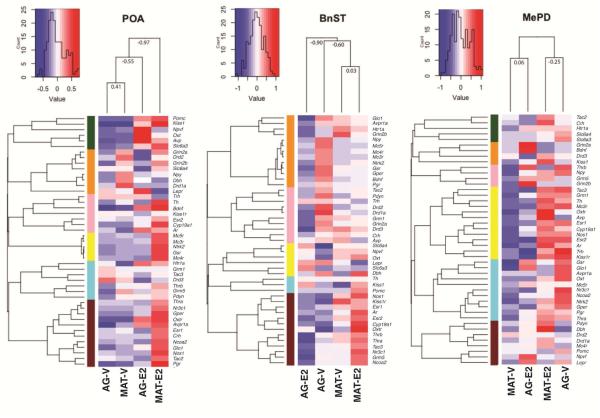
3.5. Heatmaps
To ascertain relationships among groups of genes within each region, and to determine clustering of animals by age and treatment, we conducted a hierarchical cluster analysis and generated heatmaps (Figure 7). An unbiased group order was generated based on similarities of gene groups and the ordering of the treatment groups; these differed for the three regions in accordance with differential effects of age, hormones, and their interactions. Five to six groups were generated within each region based on top-down hierarchical splitting, so that each group would contain at least 5% of the total genes (Yin et al., 2015). The results are consistent with data for each region: namely, in the POA, the two vehicle groups and the two E2 groups cluster by hormone treatment, irrespective of age; in the BnST the clustering is by age but not hormone treatment; and in the MePD, the opposite effects of hormone within each age dominate the clustering. These unique gene clustering patterns within the color modules for each region may be informative for future studies.
Fig 7.
Heatmaps of gene expression in the POA, BnST, and MePD are shown. Five to six groups were generated within each region based on top-down hierarchical splitting, indicated by color codes, so that each group would contain at least 5% of the total genes.
4. Discussion
This study sought to evaluate the effects of estradiol, aging, and their interactions in male rats, focusing on gene expression in the POA, BnST, and MePD, and peripheral serum hormone levels. The brain regions were selected because of their high concentration of steroid hormone receptors, and their high expression of aromatase, the enzyme that converts testosterone into estradiol (Mitra et al., 2003, Perez et al., 2003, Stanic et al., 2014, Takahashi et al., 2006). In addition, as part of the brain’s social decision-making network, these regions are highly conserved across species (O'Connell and Hofmann, 2012) and play critical roles in a variety of reproductive, social, olfactory, and affective behaviors and underlying physiology. For example, the POA contains the GnRH and kisspeptin neurons that are obligatory to reproductive function (d'Anglemont de Tassigny and Colledge, 2010, Xue et al., 2014), and kisspeptin is necessary for the mediation of steroid hormone feedback actions on the hypothalamus (Clarke et al., 2015, Smith, 2013). The BnST, along with the MePD, is involved in olfaction and social recognition (Ervin et al., 2015). It is also sexually dimorphic, with males having a larger BnST than females (Bian et al., 2014). The POA, BnST and MePD are all modulated by estradiol, mediated primarily by ERα. Of these, the POA and BnST have larger roles in social preference, mating and territorial aggression, while the MePD is linked to social recognition (Ervin et al., 2015, Russell et al., 2012, Sano et al., 2013). Other studies have linked the MePD with aggressive behaviors (Sano et al., 2016, Unger et al., 2015).
Estradiol but not age increases expression of five neuroendocrine genes in the POA
One of our most surprising findings was that in the POA, only 5 of the 46 genes (Avpr1a, Bdnf, Mc3r, Oxt, Oxtr) showed significant changes, with expression increased in the E2 treatment groups. Three of these genes that encode oxytocin, oxytocin receptor, and vasopressin receptor, are critical to the control of social behavior, although most of that literature has focused on the paraventricular nucleus and supraoptic nucleus (Hrabovszky et al., 2004, Winslow and Insel, 2004). Relatively little is known about E2 regulation of these nonapeptide signaling systems in the POA in males. In females, E2 tended to upregulate oxytocin mRNA expression in the POA, but this was not significant (Chung et al., 1991). Work from our lab on an ovariectomized female rat model found fewer effects of age and E2 on nonapeptide gene expression in the POA (Yin et al., 2015), suggesting a greater sensitivity of the male than the female brain. Oxytocin in the POA facilitates sexual behavior in males, and this undergoes age-related impairments (Gil et al., 2011). Furthermore, oxytocin colocalizes with the p450 aromatase enzyme in the male POA (El-Emam Dief et al., 2013), suggesting the capacity of these cells to convert testosterone to estradiol.
Regarding BDNF, to our knowledge age- and estrogen-induced changes in the POA have not been reported in males, but other brain regions have been examined. Katoh-Semba et al. (Katoh-Semba et al., 1998) reported age-related increases of BDNF in the hippocampus and cortex of male mice. There is also strong evidence for estradiol regulation of BDNF in the female brain, including in hypothalamic regions (Yin et al., 2015, Zhu et al., 2013). While sex differences in expression of BNDF were reported in hippocampus (Franklin and Perrot-Sinal, 2006), this was not found in the hypothalamus of mice (Ren-Patterson et al., 2006).
Also surprising in the POA was the lack of effect of age or E2 on expression of ERα and ERβ. In intact males, previous studies have found that neither the number of ERα-immunopositive cells or mRNA expression in the medial preoptic nucleus (MPN) changed with age (Madeira et al., 2000, Wu et al., 2009). In males that were castrated and given either testosterone or vehicle replacement during the castration surgery, there was no difference in numbers of ERα immunoreactive cells with age, but there was a significant decrease with testosterone (Wu and Gore, 2010). That study differs from the current one in the hormone treatment (testosterone vs. E2), post-castration interval and duration, and level of analysis (ERα protein vs. gene expression), but despite these differences, its results suggest the possibility that the ERα may be more sensitive to androgen than estrogen regulation in the male preoptic area. Further work is needed to better resolve the experimental differences.
The BnST is predominately affected by age
The BnST, unlike the POA, showed mainly age-related changes, with most identified genes expressed at lower levels in aged than young males. The BnST is involved in social recognition, stress responses, duration of fear states, and mating behavior, and projects more directly to areas important in activation of the HPA axis than areas in the amygdala (Lebow and Chen, 2016). Though no studies were found for the BnST, in the PVN E2 acts through ERβ to increase NOS, thereby increasing NO production (Gingerich and Krukoff, 2005, Gingerich and Krukoff, 2006). Our results add to this literature by showing that both Nos1 and Esr2 decrease with age in the BnST. Decreased oxytocin receptor expression and binding in the BnST have been reported to be associated with decreased aggression. Rats who were first time fathers exhibited facilitated parental care compared to virgins, and lower oxytocin receptor expression in the BnST, but not the MeA or POA (Perea-Rodriguez et al., 2015). On the other hand, when introduced to a novel male intruder, rats classified as excessively aggressive also showed the highest levels of oxytocin receptor binding in the BnST (Calcagnoli et al., 2014). Although the phenotypic outcomes of these studies were different, results indicate that both age and E2 regulate Oxtr in the BnST.
Other affected genes in the BnST included Cyp19a1 (aromatase), the age-related decrease in which may affect local estradiol synthesis if aromatase activity is similarly affected as its genes. The kisspeptin receptor, encoded by Kiss1r, was also identified. The kisspeptin system has been studied in the amygdala in the context of estrogen signaling in males (Stephens et al., 2016) and in sexual behavior (Gresham et al., 2016). Another gene, Thra, to our knowledge has not bee studied in the BnST of males, but knockout mice exhibit increased anxiety, as well as facilitated sexual behavior, with decreased mount and intromission latencies (Vasudevan et al., 2013). Thra has also been shown to interfere with ER-mediated transcription of other genes, such as Oxtr (Vasudevan et al., 2001). Finally, we found an effect of E2 on expression Grm5, a metabotropic glutamate receptor 5 (mGluR5). MGluR5 antagonists have anxiolytic effects, and induce induce c-Fos expression in the BnST (Inta et al., 2012, Palucha and Pilc, 2007). In that study, young mGluR5 knockout mice showed anti-depressive like effects of antagonist treatment, but aged mGluR5 knockouts showed increased anxiety (Inta et al., 2013). Taken together, these studies suggest that the mGluR5 changes in its behavioral and hormone-responsiveness with aging.
Genes in the MePD were affected by interactions of age and E2 treatment
Seventeen genes in the MePD, falling into several functional classes including nuclear hormone receptors, neurotransmitters and neuropeptides and their receptors, had significant interaction effects. The MePD is involved in both olfaction and social behavior, and receive projections from the medial amygdala (MeA), the latter conveying chemosensory information (Maras and Petrulis, 2006, Maras and Petrulis, 2010) but the former having higher density of steroid hormone receptors (Maras and Petrulis, 2010). The MePD itself makes reciprocal projections with areas involved in reproductive behavior, including the POA and BnST (Morrell et al., 1984, Pardo-Bellver et al., 2012). Either castration or lesions of the MePD resulted in decreased preference for female odors by males (Maras and Petrulis, 2006, Xiao et al., 2015). Interestingly, oxytocin receptor binding densities in the medial amygdala correlated positively with social interest in males, but negatively in females, and males also showed higher overall levels of oxytocin receptor binding than females (Dumais et al., 2013).
It is notable that many affected genes in the MePD showed a pattern by which E2 increased expression in mature animals, but had no effect or decreased expression in older animals. For those genes that were up-regulated by E2 in mature males and were unaffected in aged males, this result is consistent with a loss of E2-responsiveness with aging. By contrast, genes that were up-regulated by E2 in mature males and down-regulated by E2 in aging males, continue to be responsive to this hormone, and the expression pattern may represent a compensatory mechanism caused by E2 treatment with aging. For these latter genes, castrated males had higher gene expression with aging, and E2 treatment in the aging animals “normalized” expression to the mature castrate baseline.
There were also 5 genes undergoing age-related increases: Bdnf, Glo1, Grin2b, Gsr, and Thrb. Since these genes come from different families we cannot infer any generalities, but it is notable that the directionality of this response is the opposite from that in the BnST, in which all age effects were a decrease with age. This result underscores the importance of assessing effects of aging in a brain region-specific manner.
Serum hormones change as a result of age and estradiol
A hallmark of aging is changes in concentrations of the release patterns of peripheral hormones (Gore, 1998). Our results add to the literature of effects of castration and E2 treatment during aging in male rats. Interestingly, while E2 treatment was effective in increasing serum E2 concentrations, aged males, whether given vehicle or E2, had higher serum E2 concentrations than their younger counterparts. These aged males are significantly heavier and have much larger fat depots, so this difference may be due to peripheral aromatization of adrenal hormones. The interpretation of our gene expression results therefore needs to be made in this context. Notably, the serum E2 concentrations achieved by hormone replacement were lower than those reported by our labs in intact male Sprague-Dawley rats of similar ages (Wu and Gore, 2009, Wu et al., 2009) so we were not in the supraphysiological range – in fact, we were in the subphysiological range compared to intact male rats. This underscores the exquisite sensitivity of the male brain, that even low concentrations of E2 have profound gene expression effects.
Our results in castrated male rats showed a profound decrease in serum LH in response to E2, consistent with E2’s negative feedback actions. Our data extend previous work by showing that this response is maintained even in our aging male rats, similar to what has been reported for aging men (Veldhuis and Dufau, 1993) and female rats (Yin et al., 2015). Previous studies in intact male rats and men have reported decreases in LH during aging (Bonavera et al., 1997, Veldhuis et al., 2005, Walker et al., 2013). While there was not a main effect of age in our study, serum LH concentrations were lower in AG-V than MAT-V rats, consistent with the intact literature.
The serum PRL results were very interesting, as they showed a profound age-related increase, together with an up-regulation by E2 that was much greater in the old than the young male rats. The age-related increase in prolactin has been reported previously in intact male rats (Estes and Simpkins, 1980), but to our knowledge, this change in sensitivity to E2 with age is a novel finding of our study, and differs from our aging female rats whose LH response to E2 treatment was comparable in young and aging animals (Yin et al., 2015).
Concentrations of several protein hormones were decreased with age; this finding for FSH, GH, and BDNF is largely consistent with the literature in rats and men (Dobado-Berrios et al., 1996, Driscoll et al., 2012, Heaton, 2003, Lommatzsch et al., 2005, Raven et al., 2006). For FSH, we also found a down-regulation with E2 treatment, the latter expected based on negative feedback effects of E2 on gonadotropins in humans of both sexes (Greenblatt et al., 1976, Raven et al., 2006) as well as in female rats (Yin et al., 2015).
Finally, the thyroid hormone axis hormones also showed regulation by age and hormone. Serum T4 was lower in aged than mature males, a result that was not found for T3. By contrast, TSH was decreased by E2 compared to vehicle, but there was no aging effect. These results are quite different to what we reported in ovariectomized females, in which TSH was increased by E2 (the opposite of our castrated males in the current study), and T4 was decreased by E2 at all ages in the females but there was no effect of age (Yin et al., 2015). Further work in males on the regulation of the hypothalamic-pituitary-thyroid axis by E2 is merited.
Summary, conclusions and limitations
Based on our results in castrated male rats, it is clear that the efficacy of hormone treatments on gene expression and hormone concentrations is profoundly influenced by chronological age. In fact, for many genes, the aging brain continues to be very responsive to E2 treatment, albeit differentially from the young adult brain. Because our study was limited to an array of 48 genes in 3 brain regions, future work ought to extend findings to more gene targets, e.g. by RNA sequencing methods, and to more brain regions. Moreover, while our goal for the present study was to disentangle effects of E2 from those of other hormones on gene expression in brain nuclei, it would be interesting to compare these results in castrated males with gonadally-intact counterparts, or animals castrated and given other hormone treatments such as DHT or a combination of estrogen and androgen.
It is notable that the mature and aging rats did not have identical sexual experience and our results should be interpreted accordingly. We tried to take this into account as much as possible by trying to estimate the amount of experience a retired breeder would have, and to allow our mature animals to mate to ejaculation in 8 trials. Nevertheless, the timing between the last experience in the two age groups is different and was not possible to match perfectly based on the limited availability of aged rats from the vendor. We addressed this in a previous study in which we compared the effects of sexual experience and age on expression of ERα and AR (Wu and Gore, 2009) and found no effect of time lag after the last mating experience.
Despite these limitations, our study demonstrates conclusively that the E2 regulation of gene expression is region-specific and changes during aging in male rats. In particular, our molecular results in the BnST and MePD suggest that these are important regions for future research on functional outcomes. The gene expression results may also provide information for identifying targets for further analysis, to determine if the protein products change in a similar manner, and to ascertain consequences on other behavioral or physiological endpoints.
Supplementary Material
E2, aging, and their interactions altered gene expression in a region-specific manner.
In the preoptic area of male rats, E2 increased expression of 5 genes.
The BnST showed age-related changes in 7 genes, 6 decreasing with aging.
In the amygdala, 22 genes were altered, most due to E2-age interactions.
The aging male brain is highly sensitive to E2, albeit differently from the young brain.
Acknowledgments
Grant support: NIH PO1 AG16765 (ACG), NIH RO1 DA032789 (JD), NIH P30 DK079637 (AW), NIH F32 ES023291 (MRB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimoto JM, Wong A, Rozovsky I, Lin SW, Morgan TE, Finch CE. Age increase of estrogen receptor-alpha (ERalpha) in cortical astrocytes impairs neurotrophic support in male and female rats. Endocrinology. 2013;154:2101–2113. doi: 10.1210/en.2012-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bian C, Zhu H, Zhao Y, Cai W, Zhang J. Intriguing roles of hippocampus-synthesized 17beta-estradiol in the modulation of hippocampal synaptic plasticity. J Mol Neurosci. 2014;54:271–281. doi: 10.1007/s12031-014-0285-8. [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superlano L, Sinha-Hikim AP, Wang C. In the male brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18:359–365. [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Beiderbeck DI, Althaus M, Koolhaas JM, Neumann ID. Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav Brain Res. 2014;261:315–322. doi: 10.1016/j.bbr.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Chung SK, McCabe JT, Pfaff DW. Estrogen influences on oxytocin mRNA expression in preoptic and anterior hypothalamic regions studied by in situ hybridization. J Comp Neurol. 1991;307:281–295. doi: 10.1002/cne.903070209. [DOI] [PubMed] [Google Scholar]
- Clarke H, Dhillo WS, Jayasena CN. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol Metab (Seoul) 2015;30:124–141. doi: 10.3803/EnM.2015.30.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Technical Report LA-7677-MS. Los Alamos Scientific Laboratory; 1979. On multiple-comparisons procedures. [Google Scholar]
- d'Anglemont de Tassigny X, Colledge WH The role of kisspeptin signaling in reproduction. Physiology (Bethesda) 2010;25:207–217. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- Dobado-Berrios PM, Ruiz-Navarro A, Almaden Y, Malagon MM, Garrido JC, Ramirez-Gutierrez JL, Gracia-Navarro F. Heterogeneity of growth hormone (GH)-producing cells in aging male rats: ultrastructure and GH gene expression in somatotrope subpopulations. Mol Cell Endocrinol. 1996;118:181–191. doi: 10.1016/0303-7207(96)03781-1. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- El-Emam Dief A, Caldwell JD, Jirikowski GF. Colocalization of p450 aromatase and oxytocin immunostaining in the rat hypothalamus. Horm Metab Res. 2013;45:273–276. doi: 10.1055/s-0032-1327680. [DOI] [PubMed] [Google Scholar]
- Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Estes KS, Simpkins JW. Age-related alterations in catecholamine concentrations in discrete preoptic area and hypothalamic regions in the male rat. Brain Res. 1980;194:556–560. doi: 10.1016/0006-8993(80)91241-x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Fujita S, Chiba M, Ohta M, Kitani K, Suzuki T. Alteration of plasma sex hormone levels associated with old age and its effect on hepatic drug metabolism in rats. J Pharmacol Exp Ther. 1990;253:369–374. [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM. Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat. Horm Behav. 2011;59:435–443. doi: 10.1016/j.yhbeh.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav. 2016;87:8–15. doi: 10.1016/j.yhbeh.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich S, Krukoff TL. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology. 2005;146:2933–2941. doi: 10.1210/en.2004-1375. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Krukoff TL. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension. 2006;48:1130–1136. doi: 10.1161/01.HYP.0000248754.67128.ff. [DOI] [PubMed] [Google Scholar]
- Gore AC. Circadian rhythms during aging. In: Hof PR, Mobbs CV, editors. The Endocrinology of Aging. Karger Press; Basel: 1998. pp. 127–165. [Google Scholar]
- Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech Ageing Dev. 1990;56:77–88. doi: 10.1016/0047-6374(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Greenblatt RB, Oettinger M, Bohler CS. Estrogen-androgen levels in aging men and women: therapeutic considerations. J Am Geriatr Soc. 1976;24:173–178. doi: 10.1111/j.1532-5415.1976.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Gresham R, Li S, Adekunbi DA, Hu M, Li XF, O'Byrne KT. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci Lett. 2016;627:13–17. doi: 10.1016/j.neulet.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol. 1994;49:B42–B50. doi: 10.1093/geronj/49.2.b42. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Miranda-Martinez A, Neri-Gomez T, Camacho-Arroyo I. Sex steroids effects on the content of GAD, TH, GABA(A), and glutamate receptors in the olfactory bulb of the male rat. Neurochem Res. 2008;33:1568–1573. doi: 10.1007/s11064-008-9665-1. [DOI] [PubMed] [Google Scholar]
- Haji M, Kato KI, Nawata H, Ibayashi H. Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res. 1981;204:373–386. doi: 10.1016/0006-8993(81)90596-5. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JP. Hormone treatments and preventive strategies in the aging male: whom and when to treat? Rev Urol. 2003;5(Suppl 1):S16–21. [PMC free article] [PubMed] [Google Scholar]
- Herath CB, Watanabe G, Wanzhu J, Noguchi J, Akiyama K, Kuramoto K, Groome NP, Taya K. Elevated levels of inhibin-A and immunoreactive inhibin in aged male Wistar rats with testicular Leydig cell tumor. J Androl. 2001;22:838–846. [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Moffat SD. Increasing testosterone levels and effects on cognitive functions in elderly men and women: a review. Curr Drug Targets CNS Neurol Disord. 2005;4:531–540. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Inta D, Filipovic D, Lima-Ojeda JM, Dormann C, Pfeiffer N, Gasparini F, Gass P. The mGlu5 receptor antagonist MPEP activates specific stress-related brain regions and lacks neurotoxic effects of the NMDA receptor antagonist MK-801: significance for the use as anxiolytic/antidepressant drug. Neuropharmacology. 2012;62:2034–2039. doi: 10.1016/j.neuropharm.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Inta D, Vogt MA, Luoni A, Filipovic D, Lima-Ojeda JM, Pfeiffer N, Gasparini F, Riva MA, Gass P. Significant increase in anxiety during aging in mGlu5 receptor knockout mice. Behav Brain Res. 2013;241:27–31. doi: 10.1016/j.bbr.2012.11.042. [DOI] [PubMed] [Google Scholar]
- Izumo N, Ishibashi Y, Ohba M, Morikawa T, Manabe T. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res. 2012;227:1–6. doi: 10.1016/j.bbr.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Jasuja GK, Travison TG, Davda M, Murabito JM, Basaria S, Zhang A, Kushnir MM, Rockwood AL, Meikle W, Pencina MJ, Coviello A, Rose AJ, D'Agostino R, Vasan RS, Bhasin S. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2013;68:733–740. doi: 10.1093/gerona/gls216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci Res. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, Bhasin S. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010;95:3955–3964. doi: 10.1210/jc.2010-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Paula-Barbosa MM. Hypertrophy of the ageing rat medial preoptic nucleus. J Neurocytol. 2000;29:173–197. doi: 10.1023/a:1026598906739. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010;170:610–622. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63:241–247. doi: 10.1016/s0169-328x(98)00281-2. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor b in the mouse brain: Comparison with estrogen receptor a. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Schwanzel-Fukuda M, Fahrbach SE, Pfaff DW. Axonal projections and peptide content of steroid hormone concentrating neurons. Peptides. 1984;5(Suppl 1):227–239. doi: 10.1016/0196-9781(84)90281-x. [DOI] [PubMed] [Google Scholar]
- Naugle MM, Nguyen LT, Merceron TK, Filardo E, Janssen WG, Morrison JH, Rapp PR, Gore AC. G-protein coupled estrogen receptor, estrogen receptor alpha, and progesterone receptor immunohistochemistry in the hypothalamus of aging female rhesus macaques given long-term estradiol treatment. Journal of experimental zoology. Part A, Ecological genetics and physiology. 2014;321:399–414. doi: 10.1002/jez.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Ordonez C, Martinez E, Perez C, Alonso A, Gonzalez C, Tolivia J. Aging and substitutive hormonal therapy influence in regional and subcellular distribution of ERalpha in female rat brain. Age (Dordr) 2013;35:821–837. doi: 10.1007/s11357-012-9415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. 2012;6:33. doi: 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Rodriguez JP, Takahashi EY, Amador TM, Hao RC, Saltzman W, Trainor BC. Effects of reproductive experience on central expression of progesterone, oestrogen alpha, oxytocin and vasopressin receptor mRNA in male California mice (Peromyscus californicus) J Neuroendocrinol. 2015;27:245–252. doi: 10.1111/jne.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlert T. The pairwise multiple comparison of mean ranks package (PMCMR) 2014 R Package http://cran.r-project.org/package=PMCMR.
- Putnam SK, Sato S, Riolo JV, Hull EM. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm Behav. 2005;47:513–522. doi: 10.1016/j.yhbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Raber J. AR, apoE, and cognitive function. Horm Behav. 2008;53:706–715. doi: 10.1016/j.yhbeh.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao YS, Mott NN, Wang Y, Chung WC, Pak TR. MicroRNAs in the aging female brain: a putative mechanism for age-specific estrogen effects. Endocrinology. 2013;154:2795–2806. doi: 10.1210/en.2013-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven G, de Jong FH, Kaufman JM, de Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab. 2006;91:3324–3328. doi: 10.1210/jc.2006-0462. [DOI] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-dependent modulation of brain monoamines and anxiety-like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cell Mol Neurobiol. 2006;26:755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Thornton JE, Chambers KC. Age-related deficits in brain estrogen receptors and sexual behavior of male rats. Behav Neurosci. 1993;107:202–209. doi: 10.1037//0735-7044.107.1.202. [DOI] [PubMed] [Google Scholar]
- Russell NV, Ogaga-Mgbonyebi EV, Habteab B, Dunigan AI, Tesfay MA, Clancy AN. Sexual responses of the male rat medial preoptic area and medial amygdala to estrogen II: site specific effects of selective estrogenic drugs. Horm Behav. 2012;62:58–66. doi: 10.1016/j.yhbeh.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Sano K, Nakata M, Musatov S, Morishita M, Sakamoto T, Tsukahara S, Ogawa S. Pubertal activation of estrogen receptor alpha in the medial amygdala is essential for the full expression of male social behavior in mice. Proc Natl Acad Sci U S A. 2016;113:7632–7637. doi: 10.1073/pnas.1524907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Tsuda MC, Musatov S, Sakamoto T, Ogawa S. Differential effects of site-specific knockdown of estrogen receptor alpha in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur J Neurosci. 2013;37:1308–1319. doi: 10.1111/ejn.12131. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013;784:275–295. doi: 10.1007/978-1-4614-6199-9_13. [DOI] [PubMed] [Google Scholar]
- Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PLoS One. 2014;9:e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen Stimulation of Kiss1 Expression in the Medial Amygdala Involves Estrogen Receptor-alpha But Not Estrogen Receptor-beta. Endocrinology. 2016;157:4021–4031. doi: 10.1210/en.2016-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Bergstrom M, Frandberg P, Vesstrom EL, Watanabe Y, Langstrom B. Imaging of aromatase distribution in rat and rhesus monkey brains with [11C]vorozole. Nucl Med Biol. 2006;33:599–605. doi: 10.1016/j.nucmedbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Sharma PK. Transcription of estrogen receptor alpha and beta in mouse cerebral cortex: effect of age, sex, 17beta-estradiol and testosterone. Neurochem Int. 2007;50:314–321. doi: 10.1016/j.neuint.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Unger EK, Burke KJ, Jr., Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10:453–462. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Davidkova G, Zhu YS, Koibuchi N, Chin WW, Pfaff D. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology. 2001;74:309–324. doi: 10.1159/000054698. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Morgan M, Pfaff D, Ogawa S. Distinct behavioral phenotypes in male mice lacking the thyroid hormone receptor alpha1 or beta isoforms. Horm Behav. 2013;63:742–751. doi: 10.1016/j.yhbeh.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Dufau ML. Steroidal regulation of biologically active luteinizing hormone secretion in men and women. Hum Reprod. 1993;8(Suppl 2):84–96. doi: 10.1093/humrep/8.suppl_2.84. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab. 2005;90:302–309. doi: 10.1210/jc.2004-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Nociceptive and anxiety-like behavior in reproductively competent and reproductively senescent middle-aged rats. Gend Med. 2009;6(Suppl 2):235–246. doi: 10.1016/j.genm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wu D, Gore AC. Sexual experience changes sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged male rats. Horm Behav. 2009;56:299–308. doi: 10.1016/j.yhbeh.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Gore AC. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58:306–316. doi: 10.1016/j.yhbeh.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. The Journal of comparative neurology. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Chiba A, Sakuma Y, Kondo Y. Transient reversal of olfactory preference following castration in male rats: Implication for estrogen receptor involvement. Physiol Behav. 2015;152:161–167. doi: 10.1016/j.physbeh.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Xue H, Gai X, Sun W, Li C, Liu Q. Morphological changes of gonadotropin-releasing hormone neurons in the rat preoptic area across puberty. Neural Regen Res. 2014;9:1303–1312. doi: 10.4103/1673-5374.137578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Yuri K. Changes in oestrogen receptor-beta mRNA expression in male rat brain with age. J Neuroendocrinol. 2012;24:310–318. doi: 10.1111/j.1365-2826.2011.02231.x. [DOI] [PubMed] [Google Scholar]
- Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE, Flicker L. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–4039. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- Yeap BB, Knuiman MW, Divitini ML, Handelsman DJ, Beilby JP, Beilin J, McQuillan B, Hung J. Differential associations of testosterone, dihydrotestosterone and oestradiol with physical, metabolic and health-related factors in community-dwelling men aged 17-97 years from the Busselton Health Survey. Clin Endocrinol (Oxf) 2014;81:100–108. doi: 10.1111/cen.12407. [DOI] [PubMed] [Google Scholar]
- Yin W, Maguire SM, Pham B, Garcia AN, Dang NV, Liang J, Wolfe A, Hofmann HA, Gore AC. Testing the Critical Window Hypothesis of Timing and Duration of Estradiol Treatment on Hypothalamic Gene Networks in Reproductively Mature and Aging Female Rats. Endocrinology. 2015;156:2918–2933. doi: 10.1210/en.2015-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Liu X, Senthil Kumar SP, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm Behav. 2013;63:533–542. doi: 10.1016/j.yhbeh.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.