Abstract
The Trypanosoma cruzi trypomastigote membrane provides a major protective role against mammalian host-derived defense mechanisms while allowing the parasite to interact with different cell types and trigger pathogenesis. This surface has been historically appreciated as a rather unstructured ‘coat’, mainly consisting of a continuous layer of glycolipids and heavily O-glycosylated mucins, occasionally intercalated with different developmentally-regulated molecules displaying adhesive and/or enzymatic properties. Recent findings, however, indicate that the trypomastigote membrane is made up of multiple, densely packed and discrete 10–150 nm lipid-driven domains bearing different protein composition; hence resembling a highly organized ‘patchwork quilt’ design. Here, we discuss different aspects underlying the biogenesis, assembly and dynamics of this cutting edge fashion outfit, as well as its functional implications.
Keywords: Membrane domains, membrane vesicle, mucin, sialic acids, trans-sialidase
Trypanosoma cruzi: Dressed for Success
Trypanosoma cruzi, the etiological agent of Chagas disease, traverses a complex life-cycle, which alternates between hematophagous triatomine vectors and a wide range of susceptible mammals, including humans. Transitions between hosts as well as from different niches within hosts pose major adaptation challenges (i.e. different temperature and nutrient availability), and are accordingly accompanied by extensive modifications in different aspects of the parasite biology including its primary metabolism, signaling properties, gene expression profiling, post-translational protein modification and overall trypanosome morphology [1, 2]. One key aspect underlying these differentiation processes, and determining their outcome, is a major remodeling of the parasite surface coat. Being a unicellular, parasitic organism, the T. cruzi coat fulfills a dual purpose: to provide protection against vector- and/or mammalian host-derived defense mechanisms, and to interact with and respond to different environmental cues [3, 4]. Similarly to other protozoa of medical and veterinary relevance, the T. cruzi surface coat is majorly composed of glycophosphatidylinositol (GPI) (see Glossary) -anchored glycoconjugates of varied natures, often of types absent in insects or mammals [5]. The advantages of GPI moieties over trans-membrane domains are not clear, though they may provide surface molecules of trypanosomatids with accurate sorting signals in the secretory and/or endocytic pathways [6–8]. Alternatively, or additionally, it has been proposed that GPI-anchored molecules may pack at higher densities on the surface of trypanosomatids for protective and/or specialized signal transduction purposes and, due to their ‘labile’ membrane anchorage, they may be conditionally secreted as a means to get rid of immune complexes and/or to exchange signals among cells [5].
The trypomastigote coat is apparently formed by two juxtaposed layers of heavily O-glycosylated mucins and, underneath, glycoinositolphospholipids (GIPLs) [9–11], with other less prevalent glycoproteins such as trans-sialidase (TS) [12], mucin-associated surface proteins (MASPs) [13], Gp85 surface glycoproteins [14], trypomastigote small surface antigen (TSSA) [15] and Tol-T antigens [16] speckled on this coat. All of these, except for the GIPLs, are encoded by highly polymorphic and developmentally-regulated gene families [17] bearing a similar predicted architecture, in which the outermost N-terminal adhesive domain likely protrudes from the ~15nm thick, mucin O-glycan canopy. This topology is ideally suited for engaging with different constituents of the host cell membrane and/or extracellular matrix such as fibronectin, laminin or galectins, thus promoting initial recognition, sensitization and signaling of the target cell before parasite internalization [4, 17, 18].
Over the last decades, different studies converged in putting forward the idea of the T. cruzi coat as a rather unstructured and homogeneous ‘glycocalix’ that covers the entire parasite surface, including the cell body, the flagellum and the flagellar pocket. Here, we discuss recent evidence indicating that instead of a continuum of glycoconjugates, the trypomastigote surface is a highly organized ‘patchwork quilt’-like structure, made up of multiple nano-scale membrane domains of different composition.
trans-Sialidase and Mucins: The Yin and Yang of the Trypomastigote Coat
T. cruzi has evolved a unique metabolic pathway by which sialic acid (SA) residues are directly transferred from host glycoconjugates to acceptors on its surface coat [19]. This transfer involves the cleavage of a terminal SA α2-3-linked to a galactopyranose (SA α2-3βGalp) in the donor macromolecule and the subsequent formation of the same linkage with a terminal βGalp group in the acceptor substrate [20]. TS, the enzyme which catalyzes this unique reaction, is a trans-glycosidase that does not use cytidine monophosphate-SA as the monosaccharide donor, it is located on the cell surface instead of the Golgi apparatus, and it is by far more efficient in transferring terminal SA residues between glycoconjugates than in hydrolyzing them [21]. Acquisition of SA residues onto the trypomastigote coat provides protection against direct parasite killing by lytic antibodies [10], and inhibits complement opsonization [22]. Moreover, the Ssp-3 SA-containing epitope has been demonstrated crucial for trypomastigote recognition and invasion of mammalian cells [23]. In addition to its essential role in SA scavenging, TS emerges as a major T. cruzi phenotypic determinant, involved in multiple phenomena underlying parasite immune evasion, virulence and pathogenesis (Box 1).
BOX 1. T. cruzi trans-Sialidase, a Multitasking Virulence Factor.
TS is encoded by a large gene family of ~1400 members scattered throughout the T. cruzi genome [12, 66], with only a few members displaying trans-sialylation capacity. These are characterized by the presence of a Tyr342 residue, directly involved in catalysis [67], and a repetitive and antigenic C-terminal SAPA domain that has been shown to improve TS pharmacokinetics [68]. TS is detected in the bloodstream solely during the acute phase of T. cruzi infections, both in animals and humans [69]. Circulating TS modifies the surface glycosylation pattern of different host cell types [70], thereby inducing erythropenia, thrombocytopenia [71] and histological alterations in spleen, ganglia and thymus [72]. In the thymus, in particular, TS triggers apoptosis of immature CD4+CD8+ thymocytes inside the nurse cell complexes [73, 74], which may lead to the transient thymic aplasia observed early after T. cruzi infection [75]. TS involvement as a neuroprotective factor is also proposed [76]. A strict correlation between the amount of TS shed into the bloodstream and the extent of pathogenesis induced by different T. cruzi strains was observed in experimental infections [77]. Interestingly, mice infected with less virulent strains elicit TS-neutralizing antibodies (TS-NtAbs) [77, 78], which contribute to limit the pathogenesis. The importance of TS-NtAbs was confirmed by showing that their passive transfer into mice ameliorates histological findings upon T. cruzi challenge [71, 73, 75]. Further studies revealed the presence of a complex mesh of cross-reactive epitopes in TS, likely devoted to delay the elicitation of TS-NtAbs [79].
Catalytically inactive TS (iTS) displaying an overall structural similarity to TS, except for the presence of a His342 instead of Tyr residue, were only found in the more virulent parasite lineages [80]. These iTS molecules retain lectin abilities, thus they may contribute to parasite-host interactions. Both TS and iTS manipulate the immune response through the induction of a regulatory type 2 (Th2) phenotype in lymphocytes that might harness the protective, although dangerous, type 1 (Th1) response [81]. TS is also associated with CD4+ lymphocyte co-stimulation, and rescue from programmed cell death, via CD43 interaction [82]; T lymphocyte activation through antigen presenting cells interaction by CD43 and CD40 independent pathways [83]; CD8+ lymphocytes inactivation by re-sialylation [84] and production of the pro-inflammatory cytokine IL-17 by B cells [85]. Overall, TS (and iTS) are centrally involved not only on parasite persistence, but also in the development of the Chagas disease-associated pathogenesis.
Following biochemical criteria, major SA acceptors on the parasite coat have been identified as mucins [24]. The polypeptide scaffolds for these molecules are encoded by a vast number of genes (~850 per haploid parasite genome), which were split into two gene families (TcMUC and TcSMUG) based on structural criteria [25, 26]. This dichotomy turned out to have a functional correlate, as TcMUC and TcSMUG are exclusively expressed by the mammalian- and insect-dwelling stages of the parasite, respectively [11, 27–29]. Mucins from bloodstream trypomastigotes (henceforth tGPI-mucins) are highly heterogeneous molecules due to the simultaneous expression of multiple TcMUC genes showing differences in length and/or sequence, as well as in the extent and/or structure of attached oligosaccharides [27]. O-glycans in tGPI-mucins start with the addition of a single αN-acetylglucosamine (αGlcNAc) unit, which may remain un-substituted or become elongated with different residues (mainly Galp) in multiple linkages and configurations by way of a complex array of parasite-encoded glycosyl-transferases [20]. At least part of the tGPI-mucins O-glycans bears terminal βGalp units, which may undergo trans-sialylation [23]. Notably, and at stark variance with insect-dwelling stages mucins, certain βGalp units at the non-reducing end of tGPI-mucins may be modified with αGalp residues within the secretory pathway, while en route to the parasite surface [30]. These α-galactosylations provide further diversification to tGPI-mucins and lead to the eventual display on the parasite surface of the αGal glycotope [31–33]. Importantly, intracellular α-galactosylation of tGPI-mucins ‘titter out’ terminal βGalp units, and thus putative SA acceptors on the parasite surface.
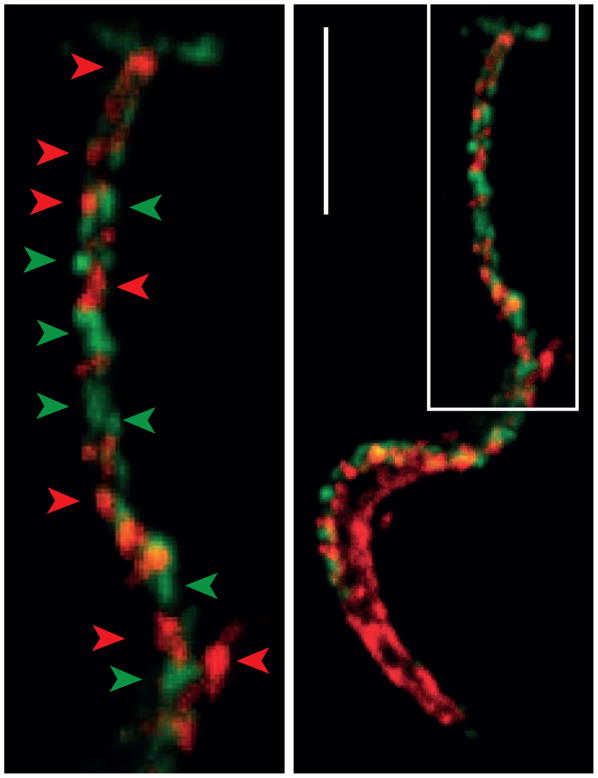
Overall, the critical role of SA for T. cruzi survival within the mammal and its content on the trypomastigote coat, which may add up to ~107 residues per parasite [10], hinted at the persistent interaction between TS and mucins on the parasite surface. Recent findings using in vivo labeling methods with SA analogs followed by microscopy techniques, however, indicate that mucins and TS are not intermingled but rather contained in different and highly stable, nanoscale membrane domains [34] (Figure 1). Moreover, TS- and mucin-rich domains are segregated on the parasite surface, thus posing major limitations, or even precluding, their direct interaction. Similar ‘patchy’ and mutually exclusive distribution, resembling protein nanoclusters defined in mammalian cells [35] is observed for other trypomastigote surface molecules (see below). Such a complex surface design implies the existence of tightly regulated mechanisms underpinning its biogenesis and maintenance and, more importantly, also suggests that this ‘patchwork quilt’ may be of structural and/or functional relevance.
Figure 1. trans-Sialidase and Mucins Localize to Distinct Nanodomains on the Trypanosoma cruzi Trypomastigote Coat.
Upper: T. cruzi trypomastigote domains studied by confocal microscopy. Lower: Magnification of the flagellum. Arrowheads denote different membrane domains (in green, sialic acid revealed with anti-Flag antibody and in red trans-Sialidase revealed by anti-shed acute phase antigen (SAPA) antibody). Bar: 5 μm.
Lipid Domains as Pillars of Membrane Organization
Lipid Rafts
Chemical reactions taking place in biological systems are usually optimized by confining them to restricted locations (i.e. inside organelles or multi-enzymatic complexes) and precise timings. The same principles apply to membrane processes. Membrane research in the last decades has replaced the classical paradigm of the fluid mosaic for a highly compartmentalized membrane model, in which lipids and membrane-associated proteins are organized in specific domains of varying sizes (within the nanometric scale) and composition [36, 37]. These membrane domains -that include lipid rafts- are composed by association mainly between sterols, polar lipids such as sphingolipids and membrane proteins. At variance with the surrounding membrane bilayer, they spontaneously form liquid-ordered metastable phases with variable and differential physico-chemical properties [38]. Although more abundant in the plasma membrane, lipid rafts have been also shown to occur in membrane-derived extracellular vesicles, where they are likely involved in vesicle fusion or fission events, and in organelles of the secretory system (i.e. the Golgi/trans-Golgi network) [37].
One of the most important properties associated with lipid rafts is their increased thickness and stability provided by the association between long-chain lipids and sterols in the interstice of the bilayer [39]. This differential thickness, in turn becomes an organizing principle for membrane protein sorting (i.e. GPI-anchored proteins vs. type I integral membrane proteins) [37]. Briefly, during their transit through the Golgi apparatus, where the sterol content increases, short-anchored proteins are retained and those with longer anchors progress towards the plasma membrane [40–42]. In this way, the cell is able to establish spontaneous compartmentalization during synthesis and maturation, taking advantage of the local lipidic composition. Most importantly, in addition of being key mechanistic contributors to cellular logistics, lipid domains on the cell surface are thought to constitute platforms capable of supporting differential membrane functions such as cell adhesion, cell migration, endo/exocytosis and/or signal transduction [39].
Lipid domains in T. cruzi
Lipid-driven domains, and particularly lipid rafts have been studied to some extent in protozoan parasites where they emerge as key factors involved in different aspects of their interaction with host(s) such as membrane vesicle (MV) trafficking and release, host cell recognition, motility and pathogenesis [43]. In T. cruzi, first indications of lipid raft-like structures came from freeze-fracture electron microscopy studies, in which parasites treated with filipin, a polyenic antibiotic which binds to 3-β-OH sterols displayed ~30 nm protuberances randomly distributed in both inner and outer plasma membrane leaflets [44]. More recently, using standard biochemical procedures, lipid rafts were identified and partially characterized in the plasma membrane [45, 46] and the contractile vacuole complex (CVC) of replicative insect-dwelling stages [47]. To get insights into trypomastigote lipid domains, we carried out standard lipid raft purification by discontinuous Optiprep gradients. Despite being both GPI-anchored molecules, TSs were majorly excluded from lipid raft domains whereas sialylated tGPI-mucins were mostly included, further supporting their mutually exclusive distribution on the parasite surface (Figure 1) [34]. Following unbiased mass spectrometry analyses of the lipid raft-enriched fractions, the surface distribution of lipid raft-associated proteins was analyzed by immuno-fluorescence. Adenylate kinase 1, kinetoplastid membrane protein 11 (KMP11), Tol-T antigens and the calpain-like cysteine peptidase, display a ‘patchy’ and very low co-localization rate with sialylated tGPI-mucins [34]. Hence, the overall scenario indicates that the surface coat of the trypomastigote is far from being a continuum of glycoconjugates. Instead, it may be more of a highly organized ‘patchwork quilt’-like structure, made up of multiple 10–150 nm membrane domains bearing different composition of proteins (one or very few proteins each) and, likely, also of lipids.
Independently of their type of predicted membrane anchorage, most of lipid raft-associated proteins were localized to the flagellum; with only adenylate kinase displaying a more uniform distribution [34]. Atomic force microscopy studies also revealed the enrichment of 10–90nm membrane domains along the trypomastigote flagellum, which are closely packed together [34]. These observations are in line with previous ultrastructural studies in T. cruzi [44] and also with recent lipidomic analyses indicating the preferential presence of raft-forming phospholipids in the flagelar membrane of Trypanosoma brucei [48, 49]. The molecular determinants that target certain proteins to the flagellar membrane of trypanosomatids are not properly understood. Enrichment of lipid rafts in the flagellum and the preference of certain post-translational modifications (i.e. acylation) for these domains could be one possible explanation. However, there are some evidences that other non-lipidic determinants are involved in flagellar localization of membrane proteins in trypanosomatids [46, 50–53]. Thus, although there is not enough information about how proteins are sorted to a specific place in the plasma membrane, it seems that acylation and protein determinants or the combination of both may be associated with this process.
Membrane domains were shown to run in parallel tracks along the T. cruzi trypomastigote flagellum [34], a distribution compatible with their possible association to the underlying flagellar cytoskeleton [54]. A rather similar distribution along the flagellum has been described for the GPI-anchored phospholipase C of T. brucei [55]. Although mainly lipid-driven, several factors have been proposed to assist in the formation and stabilization of membrane domains, including protein-lipid and protein-protein interactions. Regarding the latter, different constituents of the underlying cortical cytoskeleton emerge as particularly appealing interacting partners [35]. As revealed by high resolution FRET microscopy studies on the surface of mammalian cells, there is a non-random distribution of nanoclusters of GPI-anchored proteins, which is dependent on both cholesterol and the actin cortex [56]. The direct interaction of trans-membrane proteins and the underlying cytoskeleton may define a ‘picket-fence’ or grid design, involved in controlling the lateral diffusion of membrane proteins and lipids [56]
Together, these findings support a major role for lipid-based domains on the organization and compartmentalization of the T. cruzi trypomastigote membrane.
trans-Sialidase and Mucins: Drifting Apart
The most puzzling of our findings was that both tGPI-mucins and TS are spotted in distinct membrane domains, with inter-distances estimated in ~150nm [34]. According to topological predictions, the N-terminal, catalytic domain of a TS hovers at about 50nm from the lipid bilayer, by virtue of its C-terminal shed acute phase antigen (SAPA) extension (Box 1). Based on these estimates, plasma membrane-associated TS may be localized too far from most of tGPI-mucins to be able to sialylate them. The rationale for this counterintuitive disposition is far from obvious and so is its underlying mechanistic. One likely possibility is that TS and tGPI-mucins are in proximity before being sorted at some point in the secretory pathway, prior to their surface display. In this regard, we have shown that even when both TS and α-galactosylated tGPI-mucins go through the CVC, they follow a different intracellular path to reach the plasma membrane [47]. Whether this differential trafficking along the secretory system is due to variations in the GPI-anchor composition and, thereby to their different raftophilic properties (see above), to their differential interactions with accessory molecules and/or other post-translational modifications (i.e. O-glycosylation) remains to be determined [7, 57]. Another possibility is that tGPI-mucins and TS domains are actively segregated on the parasite surface upon a yet-to-be-determined molecular cue. Moreover, since our experimental setting using SA analogs allows only the tracking of sialylated proteins, the possibility of SA incorporation being the causal and determinant factor in TS-mucin detaching cannot be discarded. Such a case would constitute an elegant mechanism to maximize SA incorporation into the coat by preventing sialylated mucins to be used as SA donors in subsequent reactions.
Immunofluorescence assays indicate that membrane domains loaded with sialylated tGPI-mucins are not only segregated from TS and other surface glycoproteins, but also from those bearing α-galactosylated tGPI-mucins [34]. This fact strongly suggest that the intracellular acquired αGal and the extracellularly acquired SAα2-3βGalp glycotopes do not co-exist on a single molecule, despite TcMUC genes displaying multiple predicted O-glycan attachment sites, each of which may undergo different processing. In turn, this differential glycosylation seems as the most likely determinant for the sorting of both kinds of tGPI-mucins. It might be envisioned the presence of a lectin-like molecule in the Golgi/trans-Golgi able to mediate the sorting of α-galactosylated vs.β-galactosylated mucins. In support of this hypothesis, lectin activities are found associated with retention of improperly folded nascent proteins in the endoplasmic reticulum [58]. Alternatively, it may be considered that the peptide scaffolds for tGPI-mucins bearing terminal β-Galp or α-Galp residues are encoded by distinct sets of TcMUC genes. Moreover, it has been reported that α-galactosylation and terminal sialylation also occur on Gp85s [59] and MASPs [60], respectively thus conveying another layer of complexity to the system. Whatever the case, such a complex surface design implies the existence of tightly regulated mechanisms underpinning its biogenesis and upholding. More importantly, it also suggests that surface-associated tGPI-mucins become decorated with SA residues by means of an additional, not surface-associated TS source.
Vesicle-Shed trans-Sialidase: The Key Determinant of Sialic Acid Incorporation by Trypomastigotes
In recent years, proteomic analyses of the conditioned medium of trypanosomatids revealed the use of multiple non-classical secretion pathways by these organisms, involving the extensive release of MVs. In contrast, cleavage of GPI anchors by endogenous phospholipase(s), originally supposed to be very prevalent, seems to provide a minor contribution to the secretome of protozoan parasites [61, 62]. The relevance of MVs in protozoan biology, and particularly in their associated pathogenesis, is discussed in Box 2.
BOX 2. Vesicles in Protozoan Parasites, a Pathogenesis Delivery System.
The transference of biological material like mRNAs, microRNAs, tRNAs and proteins via MVs is conserved among organisms throughout the evolutionary tree [86], and has been described in both pathogenic and non-pathogenic situations [87]. These molecules are functional and can transduce signals in recipient cells [88]. In the case of unicellular pathogens, the release of vesicles is supposed to provide flexibility to respond to environmental cues, to promote cell-to-cell communication, and to manipulate the microenvironment (i.e. the infected cell and/or the host immune system) with the ultimate goal of balancing the rate of growth and transmission [86]. Protozoan parasites were also shown to secrete extra-cellular vesicles. For instance, communication via MVs between intra-erythrocytic forms promotes differentiation to sexual stages in Plasmodium falciparum. This synchronization is supposed to contribute to parasite survival in the human host and to maximize transmission efficacy to mosquitoes [89]. Several MVs were found to bud from the flagellar pocket or the plasma membrane of Leishmania parasites [62]. Co-injection of these purified MVs along with parasites worsen the infection outcome, increasing parasite loads, size lesion, and cytokine production [90]. In the same line, the active shedding of MVs by T. brucei rhodesiense provides a means to transfer virulent phenotypic determinants like serum resistance-associated protein (SRA) among parasites [91]. Transference of virulent phenotype via MVs and the modulation of the influx of neutrophils at the site of adherence was also reported in Trichomonas vaginalis, an extracellular protozoan parasite [92].
T. cruzi MVs display a complex cargo, and were shown to interact with and to induce changes in the gene expression profiling of mammalian cells [61, 93, 94]. They are also able to modulate infection increasing heart parasitism and, most likely via TS [95], to induce a mixed T helper cell response by down-regulating the pro-inflammatory response via IL-10 secretion [81, 96]. The ability to modulate innate and acquired immunity by MVs generated by different T. cruzi strains was also observed [96]. Overall, these data support that the secretion of MVs constitutes a major topic in the interplay between protozoan parasites and their hosts.
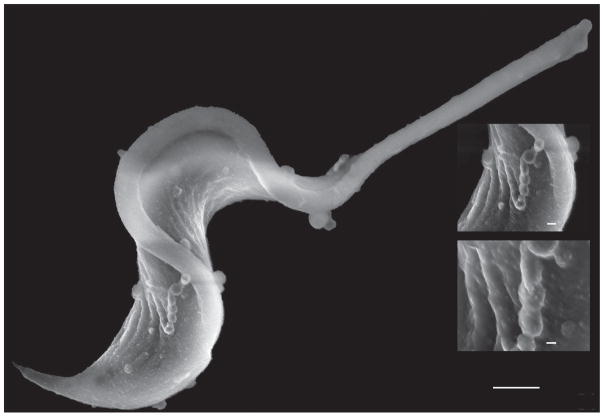
In T. cruzi there are just a few examples of phospholipases analyzed and the shedding of GPI-anchored membrane protein seems not to be their primary function [34]. Plasma membrane-derived MVs were found to be continuously shed by different T. cruzi developmental forms [63, 64]. Scanning electron microscopy studies also reveal profuse MV budding, sometimes forming bead-string-like structures, from the trypomastigote cell body and the flagellum (Figure 2). Following biochemical approaches, we clearly showed that TS and sialylated tGPI-mucins are secreted associated to MVs rather than in a ‘soluble’ form. Most importantly, MV-associated TS is able to transfer SA residues in trans (i.e. onto the coat of paraformaldehyde-fixed parasites) [34]. Together, these findings challenge the idea that the GPI-anchored proteins are secreted by a membrane phospholipase and re-fuel the long-lasting debate on which is the actual role of GPI-anchors in trypanosomatids. Most importantly, they allow us to infer that MV-associated TS, and not the membrane-associated TS, is the key determinant of SA incorporation onto the trypomastigote coat.
Figure 2. Shedding of Vesicles by Trypanosoma cruzi Trypomastigotes.
Scanning electron microscopy image of a T. cruzi trypomastigote, showing membrane vesicles budding from the flagellum and cell body.
How Do Trypomastigotes Become Sialylated in Their Natural Environment?
The trypomastigote has to become sialylated not only in order to be protected from lytic factors but also to acquire the ability to invade host cells. A major question is how does the trypomastigote become sialylated in its natural environment. Recent data suggests that mucins and TS are contained in membrane domains that do not overlap and therefore are majorly impaired to interact [34]. Moreover, several membrane proteins embedded in additional membrane domains are interspersed in between mucin and TS domains, therefore providing an additional steric hindrance for bound TS to access, and thereby trans-sialylate mucins [34].
The trypomastigote faces two very different natural environments: the infected solid tissue and the bloodstream. When a trypomastigote-ladden cell bursts in the parenchyma of the infected tissue, the trypomastigotes, together with the cytosolic content, which is already plenty with shed TS [65], is spilled into the confined intercellular space (Figure 3, Key Figure), where the SA donors become available. Experimental results indicate that minimum amounts of shed TS would be enough to rapidly sialylate mucins in the minute timescale [34]. Considering the immense sialylation potential of shed TS in this confined volume and the rapid SA uptake, the aforementioned restriction of the membrane bound TS to sialylate mucins seems irrelevant. In this situation, MV-associated TS takes over the process of SA incorporation onto the trypomastigote coat. Once in the open circulation, the mechanistic, kinetics and dynamics of trypomastigote sialylation follow completely different rules. Because the anchored enzyme has few possibilities to sialylate the surface and this duty mainly relies actually on the shed component, will the parasite decrease its sialyl residue acquisition? In the bloodstream, SA donors are available in large quantities from blood proteins and cells. However, shed enzyme might not be retained close to the parasite environment, as it gets diluted and transported by the bloodstream. The presence of TS in blood is detected either as enzyme activity itself or by changes in the surface glycosylation pattern of different host cell types (Box 1). It may be speculated that, although initially secreted embedded in MVs, the GPI-anchor of TS (and of other surface glycoproteins present in MVs) is cleaved by serum phospholipases, thereby solubilizing the enzyme. Once GPI-less and soluble in blood, the SAPA domain plays a central role into extend the pharmacokinetics of the enzyme, allowing it to exert its pathological effects far from the parasite liberation sites.
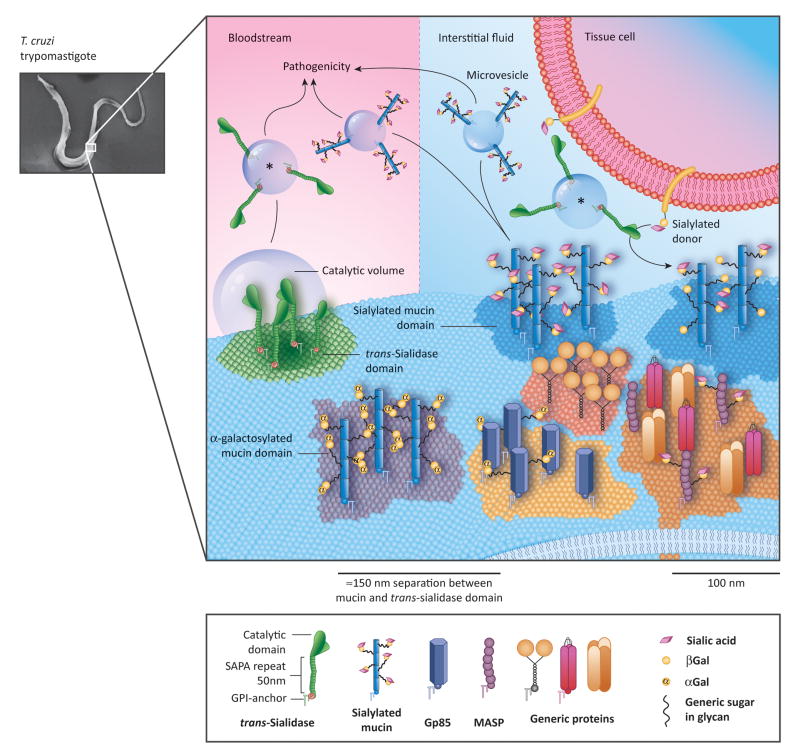
Figure 3 (Key Figure).
The ‘Patchy’ Surface of the Trypanosoma cruzi Trypomastigote.
Schematic representation of the trypomastigote surface. Several lipid domains (denoted as colored membrane patches) containing different proteins are drawn. trans-Sialidase (TS) molecules are included in non-raft lipid domains physically distant (~150 nm) to their mucins targets located in lipid raft domains making them unable to interact. Sialylation of target proteins thus relies on the shed TS (asterisk). The proposed maximal distance at which TS are effective, is drawn as a “catalytic volume” that is given by the longer distance that the TS catalytic region can reach averted form the surface by the shed acute phase antigen (SAPA) repetitive region (about 50 nm). Note the existence of separate domains where α-galactosylated and sialylated mucins are located. Proteins are mostly shed in membrane vesicles of varying sizes to the milieu instead of by the action of parasite phospholipases. In parasites allocated in solid tissues (top right), membrane vesicles containing TS are associated with surface sialylation scavenging sialic acid residues from the host tissues and fluids. Vesicles shed by parasites in blood (top left) are disseminated by the bloodstream and modify the host cell sialylation pattern, leading to pathogenesis (see Box 1) or induce inflammatory reactions. Abbreviations: αGal, α-Galactose; βGal, β-Galactose; Gp85, surface glycoproteins of ~85 kDa; MASP, mucin-associated surface proteins.
But going back to the trypomastigote, is the diluted amount of shed TS enough to keep parasites sialylated along their journey? An additional question arises, how long do trypomastigotes actually persist the in blood? We estimated that the trypomastigote renews its sialylated mucin coat entirely within a half-life of about 45 min under in vitro conditions [34]. This seems to be enough time for the bloodstream forms of the parasite to disseminate the infection and invade host cells. Because the shed enzyme is dispersed from the parasite by the bloodstream, it might be postulated that the parasite surface will become heavily re-sialylated in the confined space of the capillaries, where the parasites may begin invading solid tissues.
Concluding Remarks
Despite persistent controversies, the existence of nanoscale lipid domains displaying particular lipid composition and populated by protein nanoclusters appears to be very prevalent in nature. Due to their biochemical and biophysical properties, these domains play a central role in intracellular protein trafficking as well as in plasma membrane organization and compartmentalization. The recent identification and characterization of such structures on the T. cruzi trypomastigote shed new light onto the basic membrane biology of this fundamental though rather neglected parasite developmental form. Most importantly, it also raises new and challenging questions regarding the biogenesis, assembly, dynamics and function of the surface coat of this important pathogen that deserve to be investigated (see Outstanding Questions).
OUTSTANDING QUESTIONS BOX.
Are there membrane domains mainly associated with trypomastigote virulence or attachment? MVs contain virulence factors such as TS, mucins and MASPs. However, it remains to be clarified whether these domains are shed in different MVs or if they coalesce before budding and if the GPI-anchored proteins they contain are released by serum phospholipases.
For how long do the MVs persist in the bloodstream?
How does the parasite regulate the proportion of αGal vs. SA glycosylation? And what is their biological meaning? Sialylated and α-galactosylated proteins do not colocalize. Even when terminal β-Galp might accept both SA or α-Gal residues, it seems that only one type is incorporated in each molecule, and that molecules that bear the same glycosylation in time coalesce in a single membrane domain. If they are products from a defined group of genes for each differential glycosylation or whether the parasite can either sialylate or α-galactosylate the same protein at different moments remains to be disclosed.
How static or dynamic are membrane domains in T. cruzi? How are they separately maintained? Which is their connection with the cytoskeleton? It seems that the organization of the parasite in such a harlequin costume requires a huge energy investment, not only to generate it but also to maintain it. In fact, negatively charged sialylated mucins are located close together in single domains -without signs of repulsion - instead of distributed along the surface, intercalated with other markers, as expected.
If mucins are out of reach for the surface-anchored TS, and the shed TS is diluted in blood which is the actual sialylation mechanism of the bloodstream parasite form? As discussed in the main text, this aspect remains speculative. Due to the high TS efficiency in sialylate acceptors and its continuous shedding, parasite samples obtained from blood are always sialylated but the circulating parasites might be not.
TRENDS BOX.
The surface of Trypanosoma cruzi trypomastigotes provides a protective role against mammalian host-derived defense mechanisms and allows the parasite to interact with different cell types and trigger pathogenesis.
Initial studies suggested that the surface of T. cruzi was rather unstructured and homogeneous.
Recent data suggest that the surface of T. cruzi trypomastigotes is populated by multiple nanoscale membrane domains with different protein and lipid composition, defining a highly organized ‘patchwork quilt’-like structure.
Acknowledgments
We apologize to people whose work was not referenced due to limited space. Authors thank the Reviewers for their helpful insights. ABL is a Fellow and JM, CAB, MSL and OC are Researchers from CONICET. Work in our labs was funded by grants from Agencia Nacional de Promoción Científica y Tecnológica, Argentina and National Institute of Health, USA (RO1AI104531 to OC). The funders had no role in studies design, data collection and analysis, decision to publish or preparation of the manuscript.
GLOSSARY
- αGal glycotope
O-linked oligosaccharide bearing the structure Galα1-3Galβ1-4GlcNAcα. This glycotope is highly antigenic and the main target of the humoral response in chronic Chagasic patients
- Contractile Vacuole Complex, CVC
organelle connected to the flagellar pocket of trypanosomatids that is involved in regulating parasite osmolarity and that contribute to the traffic of GPI-anchored proteins between the Golgi complex and the plasma membrane
- Glycoinositolphospholipids, GIPLs
a group of glycolipids of unknown function and structurally similar to free GPI-anchors
- Glycosylphosphatidylinositol, GPI
glycolipid composed of phosphatidylinositol, carbohydrates and ethanolamine phosphate that is attached to the C-terminus of a protein during post-translational modification in the endoplasmic reticulum
- Gp85s
very large group of surface glycoproteins of ~85 kDa involved in parasite-host interaction, and bearing some structural similarity to TS, though not displaying trans-sialylation capacity neither SAPA
- Insect-dwelling stages
include the replicative epimastigote and the infective metacyclic trypomastigote forms, found in the midgut and hindgut, respectively, of the triatomine vector
- Lipid rafts
a membrane organizing principle based on differential lipidic composition that allows the compartmentalization of proteins, receptors, etc
- Mucin-associated surface proteins, MASPs
glycoproteins of chimeric structure displayed on the surface of T. cruzi
- Mucins
proteins that bear a dense array of oligosaccharides O-linked to Ser and/or Thr residues, and hence well-suited for protection and lubrication of epithelia or, when tethered to the plasma membrane as in T. cruzi, for mediating cell-to-cell interactions.
- Membrane vesicles, MVs
vesicles that are shed by the parasite. They contain associated proteins
- Sialic acids, SA
group of negatively charged, 9-carbon monosaccharides, being N-acetylneuraminic acid the most commonly found
- Shed acute phase antigen, SAPA
the C-terminus of TS that is composed by a variable number of a 12-mer repetitive motif
- tGPI-mucins
a group of biochemically defined and poorly characterized 60–200 kDa heavily glycosylated proteins expressed by the trypomastigote, whose peptide scaffold is provided by TcMUC-encoded polypeptides
- Tol-T
surface T. cruzi antigens restricted to the flagellum and bearing structural similarity to bacterial TolA
- Trypomastigote
extracellular, non-replicative and infective form of T. cruzi, (see Figure 2).
- trans-Sialidase, TS
an enzyme that transfer α2-3 linked SA residues among terminal β-galactose units located in oligosaccharides
- Trypomastigote small surface antigen, TSSA
mucin-like protein belonging to the TcMUC group of genes, which presents polymorphisms among T. cruzi strains
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg S, Avila AR. Aspects of Trypanosoma cruzi stage differentiation. Adv Parasitol. 2011;75:285–305. doi: 10.1016/B978-0-12-385863-4.00013-7. [DOI] [PubMed] [Google Scholar]
- 2.Brunoro GV, et al. Reevaluating the Trypanosoma cruzi proteomic map: The shotgun description of bloodstream trypomastigotes. J Proteomics. 2015;115:58–65. doi: 10.1016/j.jprot.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Noireau F, et al. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano PS, et al. Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay. IUBMB Life. 2012;64:387–396. doi: 10.1002/iub.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 6.Canepa GE, et al. Structural features affecting trafficking, processing, and secretion of Trypanosoma cruzi mucins. J Biol Chem. 2012;287:26365–26376. doi: 10.1074/jbc.M112.354696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman JS, Bangs JD. Form and function in the trypanosomal secretory pathway. Curr Opin Microbiol. 2012;15:463–468. doi: 10.1016/j.mib.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engstler M, et al. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Previato JO, et al. Glycoinositolphospholipid from Trypanosoma cruzi: structure, biosynthesis and immunobiology. Adv Parasitol. 2004;56:1–41. doi: 10.1016/s0065-308x(03)56001-8. [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Chioccola VL, et al. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 11.Buscaglia CA, et al. Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat Rev Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- 12.Campetella O, et al. A superfamily of Trypanosoma cruzi surface antigens. Parasitol Today. 1992;8:378–381. doi: 10.1016/0169-4758(92)90175-2. [DOI] [PubMed] [Google Scholar]
- 13.dos Santos SL, et al. The MASP family of Trypanosoma cruzi: changes in gene expression and antigenic profile during the acute phase of experimental infection. PLoS Negl Trop Dis. 2012;6:e1779. doi: 10.1371/journal.pntd.0001779. doi:1710.1371/journal.pntd.0001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattos EC, et al. The Gp85 surface glycoproteins from Trypanosoma cruzi. Subcell Biochem. 2014;74:151–180. doi: 10.1007/978-94-007-7305-9_7. [DOI] [PubMed] [Google Scholar]
- 15.Canepa GE, et al. Involvement of TSSA (trypomastigote small surface antigen) in Trypanosoma cruzi invasion of mammalian cells. Biochem J. 2012;444:211–218. doi: 10.1042/BJ20120074. [DOI] [PubMed] [Google Scholar]
- 16.Quanquin NM, et al. Immunization of mice with a TolA-like surface protein of Trypanosoma cruzi generates CD4(+) T-cell-dependent parasiticidal activity. Infect Immun. 1999;67:4603–4612. doi: 10.1128/iai.67.9.4603-4612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pablos LM, Osuna A. Multigene families in Trypanosoma cruzi and their role in infectivity. Infect Immun. 2012;80:2258–2264. doi: 10.1128/IAI.06225-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caradonna KL, Burleigh BA. Mechanisms of host cell invasion by Trypanosoma cruzi. Adv Parasitol. 2011;76:33–61. doi: 10.1016/B978-0-12-385895-5.00002-5. [DOI] [PubMed] [Google Scholar]
- 19.Previato JO, et al. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985;16:85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- 20.de Lederkremer RM, Agusti R. Glycobiology of Trypanosoma cruzi. Adv Carbohydr Chem Biochem. 2009;62:311–366. doi: 10.1016/S0065-2318(09)00007-9. [DOI] [PubMed] [Google Scholar]
- 21.Schenkman S, et al. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson S, et al. Role of sialic acid in the resistance of Trypanosoma cruzi trypomastigotes to complement. J Immunol. 1994;153:3141–3147. [PubMed] [Google Scholar]
- 23.Schenkman S, et al. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991;65:1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- 24.Schenkman S, et al. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;59:293–303. doi: 10.1016/0166-6851(93)90227-o. [DOI] [PubMed] [Google Scholar]
- 25.Campo V, et al. Differential accumulation of mutations localized in particular domains of the mucin genes expressed in the vertebrate host stage of Trypanosoma cruzi. Mol Biochem Parasitol. 2004;133:81–91. doi: 10.1016/j.molbiopara.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Di Noia JM, et al. AU-rich elements in the 3′-untranslated region of a new mucin-type gene family of Trypanosoma cruzi confers mRNA instability and modulates translation efficiency. J Biol Chem. 2000;275:10218–10227. doi: 10.1074/jbc.275.14.10218. [DOI] [PubMed] [Google Scholar]
- 27.Buscaglia CA, et al. The surface coat of the mammal-dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. J Biol Chem. 2004;279:15860–15869. doi: 10.1074/jbc.M314051200. [DOI] [PubMed] [Google Scholar]
- 28.Nakayasu ES, et al. GPIomics: global analysis of glycosylphosphatidylinositol-anchored molecules of Trypanosoma cruzi. Mol Syst Biol. 2009;5:261. doi: 10.1038/msb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban I, et al. Molecular diversity of the Trypanosoma cruzi TcSMUG family of mucin genes and proteins. Biochem J. 2011;438:303–313. doi: 10.1042/BJ20110683. [DOI] [PubMed] [Google Scholar]
- 30.Acosta-Serrano A, et al. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol Biochem Parasitol. 2001;114:143–150. doi: 10.1016/s0166-6851(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 31.Almeida IC, et al. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas’ disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J. 1994;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souto-Padrón T, et al. Distribution of alpha-galactosyl-containing epitopes on Trypanosoma cruzi trypomastigote and amastigote forms from infected Vero cells detected by Chagasic antibodies. J Eukaryot Microbiol. 1994;41:47–54. doi: 10.1111/j.1550-7408.1994.tb05933.x. [DOI] [PubMed] [Google Scholar]
- 33.Schocker NS, et al. Synthesis of Galalpha(1,3)Galbeta(1,4)GlcNAcalpha-, Galbeta(1,4)GlcNAcalpha- and GlcNAc-containing neoglycoproteins and their immunological evaluation in the context of Chagas disease. Glycobiology. 2016;26:39–50. doi: 10.1093/glycob/cwv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lantos AB, et al. Sialic acid glycobiology unveils Trypanosoma cruzi trypomastigote membrane physiology. PLoS Pathog. 2016;12:e1005559. doi: 10.1371/journal.ppat.1005559. doi:1005510.1001371/journal.ppat.1005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Parajo MF, et al. Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci. 2014;127:4995–5005. doi: 10.1242/jcs.146340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 37.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 38.Levental KR, Levental I. Giant plasma membrane vesicles: models for understanding membrane organization. Curr Top Membr. 2015;75:25–57. doi: 10.1016/bs.ctm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnat M, et al. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spira F, et al. Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat Cell Biol. 2012;14:640–648. doi: 10.1038/ncb2487. [DOI] [PubMed] [Google Scholar]
- 43.Goldston AM, et al. Sink or swim: lipid rafts in parasite pathogenesis. Trends Parasitol. 2012;28:417–426. doi: 10.1016/j.pt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souto-Padrón T, de Souza W. Freeze-fracture localization of filipin-cholesterol complexes in the plasma membrane of Trypanosoma cruzi. J Parasitol. 1983;69:129–137. [PubMed] [Google Scholar]
- 45.de Martins VP, et al. Developmental expression of a Trypanosoma cruzi phosphoinositide-specific phospholipase C in amastigotes and stimulation of host phosphoinositide hydrolysis. Infect Immun. 2010;78:4206–4212. doi: 10.1128/IAI.00473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maric D, et al. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J Biol Chem. 2011;286:33109–33117. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niyogi S, et al. Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi. PLoS Pathog. 2014;10:e1004224. doi: 10.1371/journal.ppat.1004224. doi:1004210.1001371/journal.ppat.1004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyler KM, et al. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serricchio M, et al. Flagellar membranes are rich in raft-forming phospholipids. Biol Open. 2015;4:1143–1153. doi: 10.1242/bio.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–2065. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper RC, et al. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J Cell Biol. 1995;128:499–508. doi: 10.1083/jcb.128.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasser MI, Landfear SM. Sequences required for the flagellar targeting of an integral membrane protein. Mol Biochem Parasitol. 2004;135:89–100. doi: 10.1016/j.molbiopara.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Tran KD, et al. Both sequence and context are important for flagellar targeting of a glucose transporter. J Cell Sci. 2012;125:3293–3298. doi: 10.1242/jcs.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stepanek L, Pigino G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science. 2016;352:721–724. doi: 10.1126/science.aaf4594. [DOI] [PubMed] [Google Scholar]
- 55.Hanrahan O, et al. The glycosylphosphatidylinositol-PLC in Trypanosoma brucei forms a linear array on the exterior of the flagellar membrane before and after activation. PLoS Pathog. 2009;5:e1000468. doi: 10.1371/journal.ppat.1000468. doi:1000410.1001371/journal.ppat.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie K, et al. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques. Mol Membr Biol. 2003;20:13–18. doi: 10.1080/0968768021000055698. [DOI] [PubMed] [Google Scholar]
- 57.Fujita M, Kinoshita T. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012;1821:1050–1058. doi: 10.1016/j.bbalip.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Labriola C, et al. Trypanosoma cruzi calreticulin is a lectin that binds monoglucosylated oligosaccharides but not protein moieties of glycoproteins. Mol Biol Cell. 1999;10:1381–1394. doi: 10.1091/mbc.10.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves MJ, Colli W. Role of the gp85/trans-sialidase superfamily of glycoproteins in the interaction of Trypanosoma cruzi with host structures. Subcell Biochem. 2008;47:58–69. doi: 10.1007/978-0-387-78267-6_4. [DOI] [PubMed] [Google Scholar]
- 60.Alves MJ, et al. Comprehensive glycoprofiling of the epimastigote and trypomastigote stages of Trypanosoma cruzi. J Proteomics. 2016 doi: 10.1016/j.jprot.2016.05.034. pii: S1874–3919(16)30238-X. [DOI] [PubMed] [Google Scholar]
- 61.Bayer-Santos E, et al. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res. 2013;12:883–897. doi: 10.1021/pr300947g. [DOI] [PubMed] [Google Scholar]
- 62.Silverman JM, et al. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008;9:R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goncalves MF, et al. Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol. 1991;72:43–53. doi: 10.1016/0014-4894(91)90119-h. [DOI] [PubMed] [Google Scholar]
- 64.Barros HC, et al. Release of membrane-bound trails by Trypanosoma cruzi amastigotes onto modified surfaces and mammalian cells. J Eukaryot Microbiol. 1996;43:275–285. doi: 10.1111/j.1550-7408.1996.tb03990.x. [DOI] [PubMed] [Google Scholar]
- 65.Frevert U, et al. Stage-specific expression and intracellular shedding of the cell surface trans-sialidase of Trypanosoma cruzi. Infect Immun. 1992;60:2349–2360. doi: 10.1128/iai.60.6.2349-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freitas LM, et al. Genomic analyses, gene expression and antigenic profile of the trans-sialidase superfamily of Trypanosoma cruzi reveal an undetected level of complexity. PLoS One. 2011;6:e25914. doi: 10.1371/journal.pone.0025914. doi:25910.21371/journal.pone.0025914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cremona ML, et al. A single tyrosine differentiates active and inactive Trypanosoma cruzi trans-sialidases. Gene. 1995;160:123–128. doi: 10.1016/0378-1119(95)00175-6. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez P, et al. Improving protein pharmacokinetics by genetic fusion to simple amino acid sequences. J Biol Chem. 2004;279:3375–3381. doi: 10.1074/jbc.M311356200. [DOI] [PubMed] [Google Scholar]
- 69.Leguizamón MS, et al. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muiá RP, et al. Identification of glycoproteins targeted by Trypanosoma cruzi trans-sialidase, a virulence factor that disturbs lymphocyte glycosylation. Glycobiology. 2010;20:833–842. doi: 10.1093/glycob/cwq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tribulatti MV, et al. The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas’ disease by reducing the platelet sialic acid contents. Infect Immun. 2005;73:201–207. doi: 10.1128/IAI.73.1.201-207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leguizamón MS, et al. trans-Sialidase from Trypanosoma cruzi induces apoptosis in cells from the immune system in vivo. J Infect Dis. 1999;180:1398–1402. doi: 10.1086/315001. [DOI] [PubMed] [Google Scholar]
- 73.Mucci J, et al. Thymocyte depletion in Trypanosoma cruzi infection is mediated by trans-sialidase-induced apoptosis on nurse cells complex. Proc Natl Acad Sci U S A. 2002;99:3896–3901. doi: 10.1073/pnas.052496399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mucci J, et al. A sexual dimorphism in intrathymic sialylation survey is revealed by the trans-sialidase from Trypanosoma cruzi. J Immunol. 2005;174:4545–4550. doi: 10.4049/jimmunol.174.8.4545. [DOI] [PubMed] [Google Scholar]
- 75.Risso MG, et al. Immune system pathogenesis is prevented by the neutralization of the systemic trans-sialidase from Trypanosoma cruzi during severe infections. Parasitology. 2007;134:503–510. doi: 10.1017/S0031182006001752. [DOI] [PubMed] [Google Scholar]
- 76.Chuenkova MV, Pereiraperrin M. Neurodegeneration and neuroregeneration in Chagas disease. Adv Parasitol. 2011;76:195–233. doi: 10.1016/B978-0-12-385895-5.00009-8. [DOI] [PubMed] [Google Scholar]
- 77.Risso MG, et al. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J Infect Dis. 2004;189:2250–2259. doi: 10.1086/420831. [DOI] [PubMed] [Google Scholar]
- 78.Buschiazzo A, et al. Trypanosoma cruzi trans-sialidase in complex with a neutralizing antibody: structure/function studies towards the rational design of inhibitors. PLoS Pathog. 2012;8:e1002474. doi: 10.1371/journal.ppat.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitcovsky TA, et al. A functional network of intramolecular cross-reacting epitopes delays the elicitation of neutralizing antibodies to Trypanosoma cruzi trans-sialidase. J Infect Dis. 2002;186:397–404. doi: 10.1086/341463. [DOI] [PubMed] [Google Scholar]
- 80.Burgos JM, et al. Differential distribution of genes encoding the virulence factor trans-sialidase along Trypanosoma cruzi Discrete typing units. PLoS One. 2013;8:e58967. doi: 10.1371/journal.pone.0058967. doi:58910.51371/journal.pone.0058967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruiz Diaz P, et al. Trypanosoma cruzi trans-sialidase prevents elicitation of Th1 cell response via interleukin 10 and downregulates Th1 effector cells. Infect Immun. 2015;83:2099–2108. doi: 10.1128/IAI.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todeschini AR, et al. Costimulation of host T lymphocytes by a trypanosomal trans-sialidase: involvement of CD43 signaling. J Immunol. 2002;168:5192–5198. doi: 10.4049/jimmunol.168.10.5192. [DOI] [PubMed] [Google Scholar]
- 83.Gao W, Pereira MA. Trypanosoma cruzi trans-sialidase potentiates T cell activation through antigen-presenting cells: role of IL-6 and Bruton’s tyrosine kinase. Eur J Immunol. 2001;31:1503–1512. doi: 10.1002/1521-4141(200105)31:5<1503::AID-IMMU1503>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 84.Freire-de-Lima L, et al. Trypanosoma cruzi subverts host cell sialylation and may compromise antigen-specific CD8+ T cell responses. J Biol Chem. 2010;285:13388–13396. doi: 10.1074/jbc.M109.096305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bermejo DA, et al. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORgammat and Ahr that leads to IL-17 production by activated B cells. Nat Immunol. 2013;14:514–522. doi: 10.1038/ni.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zomer A, van Rheenen J. Implications of extracellular vesicle transfer on cellular heterogeneity in cancer: what are the potential clinical ramifications? Cancer Res. 2016;76:2071–2075. doi: 10.1158/0008-5472.CAN-15-2804. [DOI] [PubMed] [Google Scholar]
- 88.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 89.Regev-Rudzki N, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 90.Atayde VD, et al. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 2015;13:957–967. doi: 10.1016/j.celrep.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szempruch AJ, et al. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell. 2016;164:246–257. doi: 10.1016/j.cell.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Twu O, et al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 2013;9:e1003482. doi: 10.1371/journal.ppat.1003482. doi:1003410.1001371/journal.ppat.1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Silva MR, et al. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. Biomed Res Int. 2014;2014:305239. doi: 10.1155/2014/305239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandez-Calero T, et al. Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Mol Biochem Parasitol. 2015;199:19–28. doi: 10.1016/j.molbiopara.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Trocoli Torrecilhas AC, et al. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009;11:29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 96.Nogueira PM, et al. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J Extracell Vesicles. 2015;4:28734. doi: 10.3402/jev.v4.28734. doi:28710.23402/jev.v28734.28734. [DOI] [PMC free article] [PubMed] [Google Scholar]