Abstract
Th17 cells are principal mediators of many autoimmune conditions. Recently, memory Th17 cells have been revealed as crucial in mediating the chronicity of various refractory autoimmune disorders; however, the underlying mechanisms maintaining memory Th17 cells have remained elusive. Here, using a preclinical model of ocular autoimmune disease we show that both IL-7 and IL-15 are critical for maintaining pathogenic memory Th17 cells. Neutralization of these cytokines leads to substantial reduction of memory Th17 cells; both IL-7 and IL-15 provide survival signals via activating STAT5, and IL-15 provides additional proliferation signals via activating both STAT5 and Akt. Topical neutralization of ocular IL-7 or IL-15 effectively reduces memory Th17 cells at the inflammatory site and draining lymphoid tissues, while topical neutralization of IL-17 alone, the major pathogenic cytokine secreted by Th17 cells, does not diminish memory Th17 cells at the draining lymphoid tissues. Our results suggest that the effective removal of pathogenic memory Th17 cells via abolishing environmental IL-7 or IL-15 is likely to be a novel strategy in the treatment of autoimmune diseases.
Keywords: memory Th17, maintenance, IL-7, IL-15
1. INTRODUCTION
Antigen-experienced memory T cells survive the acute phase and can exert a more rapid and strong memory response to repeated exposures to the same antigen. Immunological memory is therefore a defining feature of adaptive immunity, and is additionally crucial for the development of vaccines. However, it can also be the underlying pathogenic process enabling chronic inflammation in autoimmunity. Thus, identifying critical factors that maintain pathogenic memory T cells may lead to the development of novel immunotherapies in autoimmunity.
T helper 17 (Th17) cells play a major immunopathogenic role in numerous autoimmune diseases, such as multiple sclerosis (MS) (1), rheumatoid arthritis (RA) (2), inflammatory bowel disease (3), uveitis/scleritis (4), dry eye disease (5), and their respective experimental models (2, 4, 6–11). A recent study has demonstrated the existence of human memory Th17 cells in graft-versus-host disease, ulcerative colitis, and cancer (12). However, little is known about the mechanisms involved in the maintenance of memory Th17 cells. Because of very low frequency, general “memory phenotype” (MP, CD44hiCD62L−) cells from unmanipulated subjects have been widely used to study memory CD4+ T cell generation and maintenance (13). To avoid their unknown history of activation, later studies used an adoptive transfer of T cells expressing a transgenic T cell receptor (TCR Tg) into lymphopenic hosts to generate antigen-specific memory CD4+ T cells (13, 14). However, neither MP nor TCR Tg memory CD4+ T cells represent natural memory T cells in human diseases. Here, we used a preclinical model of autoimmune ocular disease and generated disease-specific pathogenic memory Th17 cells within intact lymphoid compartments (11) to study the mechanisms of memory Th17 cell maintenance.
Recently, blockade of interleukin (IL)-7 receptor has been shown to improve EAE (15), CIA (16) and colitis (17), indicating an association between IL-7 signaling and autoimmunity. A proof-of-concept study has shown disease improvement in RA patients who were treated with anti-IL-15 Abs (18), suggesting a potential role of IL-15 signaling in Th17 response. IL-15 has been reported to promote (19) as well as inhibit (20) the differentiation of effector Th17 cells. But, the specific roles of IL-7 and IL-15 in maintaining pathogenic memory Th17 cells have not yet been defined. Here, we demonstrate for the first time that both IL-7 and IL-15 are non-redundant for maintaining functional memory Th17 cells via providing essential survival and proliferation signals. Furthermore, in vivo blockade of either IL-7 or IL-15 significantly abolishes the maintenance of memory Th17 cells and thus prevents chronic disease development. Our results suggest targeting IL-7 and IL-15 signaling as a novel therapeutic approach to treat memory Th17 cell-mediated inflammatory diseases.
2. MATERIALS AND METHODS
2.1 Animals
Female 6- to 8-week old C57BL/6 mice (Charles River Laboratories) were used for this study. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Preclinical model of autoimmune DED
Chronic dry eye disease (DED) developed in C57BL/6 mice to mirror the long-term fluctuating course of human DED as described previously (11) with some modifications. In brief, following the initial 14 days of environmental desiccating stress (relative humidity: < 20%, airflow: 15 L/min, temperature: 21 ~ 23°C) without administering scopolamine (a tear secretion inhibitor), mice were housed in the standard non-desiccated vivarium (relative humidity: 40 ~ 60%, no airflow, temperature: 21 ~ 23°C) for additional 14 days. Corneal epithelial disease was evaluated using fluorescein (Sigma-Aldrich) staining and scored using the National Eye Institute grading system (NEI, Bethesda, MD). Briefly, 1 μl of 2.5% fluorescein was applied into the lateral conjunctival sac of the mice and after 3 minutes corneas were examined with a slit lamp biomicroscope under cobalt blue light. Punctate staining was recorded in a masked fashion with the standard National Eye Institute grading system of 0 to 3 for each of the five areas of the cornea – central, superior, inferior, nasal and temporal. In the topical in vivo blocking experiments, DED mice were divided into 4 groups at day 14 and received the following Abs three times daily: 10μg of topical anti-IL-7 (AB-407, R&D Systems), anti-IL-15 (Clone # AIO.3, eBiosceience), anti-IL-17 (Clone # TC11-18H10.1, Biolegend), or control IgG (Abcam) for up to day 28.
2.3 Tissue and cell culture
Draining lymph nodes (DLN) and conjunctivae were collected from DED mice and cultured in RPMI (Invitrogen) supplemented with 10% FBS. Alternatively, single cell suspensions were prepared from DLN and CD4+ T cells were enriched using the negative isolation kit (Miltenyi Biotec). Subsequently, the CD44hiCD62L− subpopulations were further sorted using MoFlo FACS sorter (Dako Cytomation). The tissue explants or CD44hiCD62L−CD4+ cells were treated with anti-IL-7 (10 μg/ml, R&D Systems), anti-IL-15 (5 μg/ml, eBioscience), anti-IL-7 (10 μg/ml) + anti-IL-15 (5 μg/ml), anti-IL-7Rα (10 μg/ml, R&D Systems) + anti-IL-15Rα (10 μg/ml, R&D Systems) Abs, IL-7 (20 ng/ml, PeproTech), IL-15 (20 ng/ml, PeproTech), or IL-7 (20 ng/ml) + IL-15 (20 ng/ml) for 72 hours. Memory Th17 cells were then examined by flow cytometry.
2.4 Flow cytometry analysis
Conjunctivae tissues were first digested in RPMI (Invitrogen) with 2mg/ml DNase and 2mg/ml Collagenase (Roche) at 37°C. The following Abs were used for flow cytometry analysis: FITC-conjugated anti-CD4, PerCP-Cy5.5-conjugated anti-CD44, PE-conjugated anti- IL-7Rα, APC-conjugated anti- IL-15Rα, PE- conjugated anti-CD62L, FITC-conjugated anti-Ki-67, Brilliant Violet 421-conjugated anti-Annexin V (BioLegend), PE-Cy7-conjugated anti- IL-17, and PE-conjugated anti- IL-17 (eBioscience). For intracellular IL-17 staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin (Sigma-Aldrich) for 6 hours at 37°C and 5% CO2 in the presence of GolgiStop™ (4 μl per 6 mL cell culture, BD Biosciences) to inhibit cytokine secretion. Stained cell were examined with an LSR II flow cytometer (BD Biosciences), and the results were analyzed using FlowJo software (Tree Star).
2.5 Real-time PCR
Draining lymph nodes and conjunctivae were harvested from mice, frozen in TRIzol® Reagent (Invitrogen) and stored at −80°C until use. Total RNA was isolated with an RNeasy® Micro kit (Qiagen) according to the manufacturer's recommendations and reverse transcribed using a SuperScript™ III kit (Invitrogen). Real-time PCR was performed using TaqMan® Universal PCR Master Mix and predesigned primers for IL-7 (Mm01295803_m1), IL-15 (Mm00434210_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm99999915_g1) (Applied Biosystems) in a LightCycler® 480 II System (Roche Applied Science). The GAPDH gene was used as an endogenous control for each reaction. The results of quantitative PCR were analyzed by the comparative CT method in which the target change = 2−ΔΔCT. The results were normalized by the CT value of GAPDH, and the mean CT of relative mRNA level in the normal group was used as the calibrator.
2.6 ELISA
For protein extraction, draining lymph nodes and conjunctivae were harvested and stored in cold sterile PBS containing protease inhibitors (Sigma-Aldrich) at −80°C until used. The samples were homogenized on ice and centrifuged. The supernatant was assayed using commercial ELISA kits for levels of the total protein (Thermo Scientific), IL-7, and IL-15 (eBioscience).
2.7 Statistical analysis
An unpaired, two-tailed Student's t test was used, and differences were considered significant at p < 0.05.
3. RESULTS
3.1 Pathogenic memory Th17 cells express IL-7 and IL-15 receptors
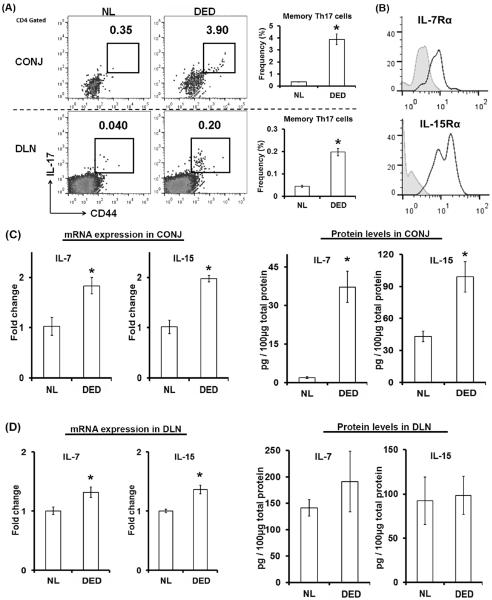
Using our well-established preclinical model of autoimmune dry eye disease (DED) in wild-type mice (11), we observed a prominent memory Th17 (CD44hiIL-17+CD4+) population at both the peripheral inflamed site (conjunctivae) and draining lymph nodes (Fig. 1A). Effector IFN-γ+IL-17− or IFN-γ+IL-17+ CD4+ T cells were not significantly increased in chronic DED (data not shown). Further analysis of these memory Th17 cells showed that they expressed the specific IL-7 receptor subunit α (IL-7Rα) and IL-15 receptor subunit α (IL-15Rα; Fig. 1B), suggesting a potential role of IL-7 and IL-15 in maintaining memory Th17 cells.
Figure 1. Memory Th17 cells express IL-7 and IL-15 receptors in an inflamed environment.
(A) Compared to the normal control (NL), there was a significant memory Th17 population (CD44hiIL-17+CD4+) at both the inflamed eye (conjunctivae, CONJ) and the draining lymph nodes (DLNs) in autoimmune dry eye disease (DED). Representative flow cytometry dot plots are shown on the left. Data presented on bar graphs are combined results from three independent experiments (n = 5 mice / experiment) and represent mean±SEM. (B) Representative histographs showing IL-7Rα and IL-15Rα expression (blue lines) on memory Th17 cells from DED mice. Gray areas represent isotype controls. IL-15Rα expression on memory Th17 is highly variable. (C) mRNA and protein expression of IL-7 in CONJ were quantified by real-time RT-PCR and ELISA, respectively, and mRNA data were normalized to normal mice (NL). (D) mRNA and protein expression of IL-15 in DLN were quantified by real-time RT-PCR and ELISA, respectively, and mRNA data were normalized to normal mice (NL). Data shown in (C) and (D) are combined from two independent experiments (n = 5 mice / experiment) and represent mean±SEM. *, p < 0.05.
We next examined IL-7 and IL-15 cytokine levels in conjunctivae and draining lymph nodes. Conjunctivae of DED mice exhibited a significant 2-fold up-regulation of both IL-7 and IL-15 mRNA (Fig. 1C). Protein levels of these two cytokines in DED conjunctivae were increased even more dramatically; especially the IL-7 level increased almost 20-fold compared to normal conjunctivae (Fig. 1C). In draining lymph nodes, we found a moderate up-regulation of both cytokines. However, the absolute levels of both cytokines were high in the lymph nodes of normal and DED mice (Fig. 1D), indicating that both IL-7 and IL-15 are constitutively expressed by lymphoid tissues, consistent with the concept that both cytokines are required for the maintenance of naïve T cells (21) in lymphoid tissues.
3.2 IL-7 and IL-15 are essential in maintaining memory Th17 cells
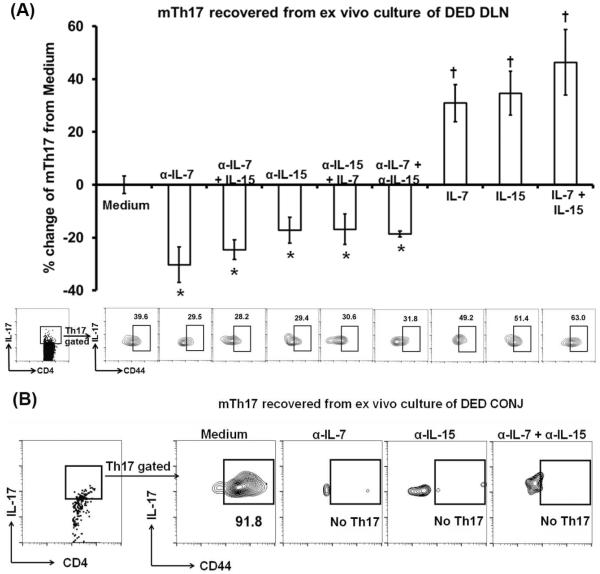
To determine whether IL-7 and/or IL-15 play a role in maintaining memory Th17 cells, we cultured draining lymph nodes from DED in the presence of anti-IL-7, anti-IL-15, or both Abs. After 72 hours we assessed the frequencies of memory Th17 cells by flow cytometry, and found that blocking IL-7, IL-15, or both leads to a 20~30% reduction of memory Th17 cells without significant differences among these three groups (Fig. 2A). When we added recombinant IL-7, IL-15, or both to our culture frequencies of memory Th17 cells significantly increased in all groups after the 72 hours (Fig. 2A).
Figure 2. Memory Th17 cells are maintained by both IL-7 and IL-15.
(A) Whole and intact DLNs were collected from autoimmune DED and cultured in complete RPMI-1640 with anti-IL-7, anti-IL-7 and exogenous IL-15, anti-IL-15, anti-IL-15 and exogenous IL-7, anti-IL-7 and anti-IL-15 Abs, exogenous IL-7, IL-15, or IL-7 and IL-15 for 72 hours. Thereafter, the DLNs were analyzed for memory Th17 cell (mTh17) frequencies by flow cytometry. Representative flow cytometry graphs are shown on the bottom. Data presented on the top bar graphs are normalized to medium only group and combined from two independent experiments (n = 4–5 wells in each group in each experiment) and represent mean±SEM. *, decrease with p < 0.05 as compared to medium control (with isotype IgG); †, increase with p < 0.05 as compared to medium control. No significant differences were observed between anti-IL-7 and anti-IL-7 + IL-15, between anti-IL-15 and anti-IL-15 + IL-7, or between IL-7, IL-15, and IL-7 + IL-15 groups. (B) CONJ were collected from DED mice and cultured in complete RPMI-1640 with anti-IL-7, anti-IL-15, or anti-IL-7 and anti-IL-15 Abs for 72 hours. Thereafter, CONJ were analyzed for memory Th17 cell (mTh17) frequencies by flow cytometry. Data are representative of 2 experiments (n = 5 pooled eyes in each group).
In addition, we cultured conjunctivae from DED mice in the presence of anti-IL-7, anti-IL-15, or both Abs for 72 hours, and found a dramatic reduction in memory Th17 cells (Fig. 2B).
3.3 Distinct roles of IL-7 and IL-15 in regulating proliferation and survival of memory Th17 cells
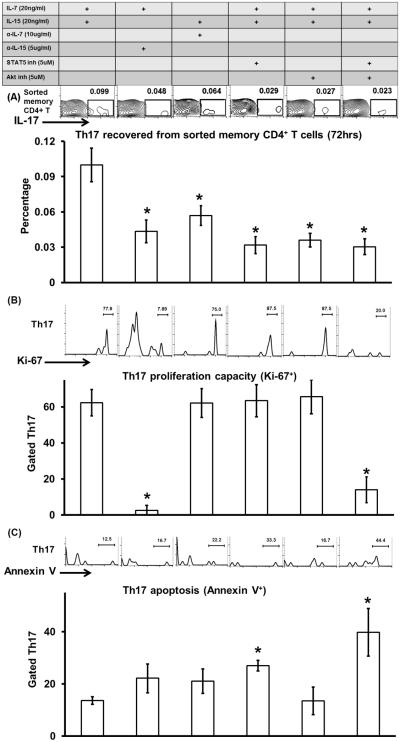
To determine how IL-7 and IL-15 maintain memory Th17 cells, we isolated memory CD4+ T cells (CD44hiCD62L−CD4+) from draining lymph nodes of DED mice and cultured them in the presence of different cytokines, antibodies, and signaling inhibitors. Here, we used sorted memory CD4+ T cells to exclude the effects of endogenous IL-7 and IL-15 and their potential indirect effects on T cells via dendritic cells or B cells. It has been reported that both Janus kinase 1/3–signal transducer and activator of transcription 5 (Jak1/3-STAT5) and phosphoinositide 3-kinase–Akt (PI3K-Akt) signaling pathways are involved in naïve and memory CD8+ T cell maintenance (21, 22–25). Thus, we cultured DED-pathogenic memory CD4+ T cells with: IL-7 and IL-15, IL-7 and anti-IL-15 Ab, IL-15 and anti-IL-7 Ab, IL-7, IL-15 and STAT5 inhibitor (pimozide) (26, 27), IL-7, IL-15 and Akt inhibitor (MK-2206) (27 – 29), IL-7, IL-15, STAT5 and Akt inhibitors. After 72 hours, we collected the cells and determined the frequencies of Th17 cells. In comparison with the IL-7 and IL-15-treated group, all groups showed significantly lower Th17 cells (Fig. 3A), demonstrating that both IL-7 and IL-15 are required for the maintenance of memory Th17 cells, and further indicating that IL-7 and IL-15 maintains memory Th17 cells via both the Jak1/3-STAT5 and PI3K-Akt signaling pathway.
Figure 3. Mechanisms by which IL-7 and IL-15 maintain memory Th17 cells.
Memory CD4+ T cells (CD44hiCD62L−CD4+) were isolated from the draining lymph nodes of DED mice and then cultured in the presence of different cytokines, Abs, and signaling inhibitors as listed in the table. After 72 hours, the cells were collected and analyzed by flow cytometry. Representative flow graphs (top) and bar graphs (bottom) show frequencies of memory Th17 cell (A), frequencies of proliferating Ki-67+ memory Th17 cell (B), and frequencies of Annexin V+ memory Th17 cell (C). Data represent mean±SEM (n = 4 wells in each group). *, p < 0.05 as compared to IL-7+IL-15 group.
We next delineated differential signals provided by IL-7 and IL-15 for the maintenance of memory Th17 cells. Th17 proliferation was assessed by staining Ki-67+ cells in cultures treated as above; IL-7 and IL-15-treated cultures (62.3±7.2%) as well as IL-15 and anti-IL-7 Ab-treated cultures (62.1±7.9%) showed significantly more proliferation than those treated with IL-7 and anti-IL-15 Ab (2.6±2.6%). Increased proliferation due to IL-15 was not blocked by either STAT5 or Akt inhibitor alone, but by their combined treatment (Fig. 3B).
In addition, Th17 survival was assessed by staining apoptotic Annexin V+ cells in cytokine-treated cultures. As compared to IL-7 and IL-15-treated cells the frequencies of Annexin V+ memory Th17 cells were increased when cells were cultured with either IL-7 or IL-15 alone (Fig. 3C), indicating that both IL-7 and IL-15 contribute to the survival of memory Th17 cells. Addition of STAT5 inhibitor, but not Akt inhibitor to the IL-7 and IL-15 culture significantly increased Annexin V+ Th17 cell frequencies. A combination of STAT5 and Akt inhibitors treatment showed a similar increase in Annexin V+ cell frequencies as STAT5 inhibitor alone (Fig. 3C). Thus, both IL-7 and IL-15 promoted the survival of memory Th17 cells via the STAT5 signaling pathway.
3.4 Topical blockade of IL-7 or IL-15 provides sustained amelioration of DED
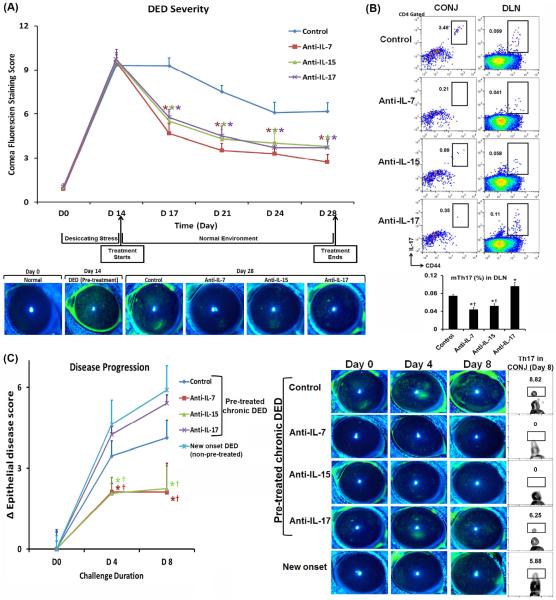
Finally, to test the functional effect of IL-7 and IL-15 in DED ocular surface autoimmunity, we examined the therapeutic effects of topical treatment with anti-IL-7 and anti-IL-15 Abs in DED mice. Because our ex vivo data indicated that both IL-7 and IL-15 are required for the maintenance of memory Th17 cells, we blocked either IL-7 or IL-15. We started treatment at the time of disease progressing into the chronic phase on day 14 (11) and continued the treatment until day 28. Control IgG was used as a negative control and anti-IL-17 treatment as positive control. Topical anti-IL-17 Ab has been shown effective in treating DED by our group (30). We found that both topical anti-IL-7 and anti-IL-15 monotherapies resulted in a significant reduction of disease severity assessed by the corneal epithelial disease score, and that their efficacies were comparable to topical anti-IL-17 treatment (Fig. 4A). Topical IL-7 or IL-15 blockade consistently decreased the principal DED mediator – memory Th17 cells in the draining lymph nodes and at the ocular surface (conjunctivae); in contrast, topical anti-IL-17 treatment showed an increase of memory Th17 cells compared to the control group on day 28 (Fig. 4B).
Figure 4. Sustained efficacy of topical anti-IL-7 or anti-IL-15 Ab treatment on ameliorating DED.
Autoimmune DED was induced on day 0 and treated with anti-IL-7, anti-IL-15, anti-IL-17 Ab, or control IgG from day 14 to 28. (A) Clinical disease severity was evaluated by corneal fluorescein staining with the representative images exhibited. The summary data shown represent mean±SEM from one representative experiment out of two performed (n = 10–14 eyes in each group). *, p < 0.05 as compared to IgG control. (B) Frequencies of memory Th17 cells (mTh17) at both CONJ and DLN were assessed by flow cytometry. *, p < 0.05 as compared to IgG control; †, p < 0.05 as compared to anti-IL-17 Ab. (C) At the end of the treatment (day 28), all groups were re-challenged with desiccating stress for 8 days. Naïve mice served as a primary challenge control. Ocular disease severity was evaluated by corneal fluorescein staining with the disease score changes calculated as Δ epithelial disease score = disease score after re-challenge − disease score before re-challenge (left) and the representative images shown (right). Summary data represent mean±SEM from 6–8 eyes. Conjunctivae tissues were analyzed for Th17 cell frequencies by flow cytometry after 8 days of re-challenge. *, p < 0.05 as compared to non-pre-treated, new onset DED group; †, p < 0.05 as compared to anti-IL-17 Ab pre-treatment.
To determine whether anti-IL7 or anti-IL-15 but not anti-IL-17 treatment has a long-term effect on diminishing DED pathogenesis, we re-challenged those treated DED mice by placing them back into the desiccating environment for 8 days without any further Ab treatment, to mimic the exacerbation observed in the human chronic disease. All groups received no treatment during re-challenge, and an additional group of naïve mice served as a primary challenge control. Both anti-IL-7 and anti-IL-15 Ab pre-treated groups showed significantly reduced disease progression and severity as compared to control IgG or anti-IL-17 Ab pre-treated groups (Fig. 4C, left). Although anti-IL17 Ab treatment diminished disease severity, this group exhibited rapid disease deterioration to an even higher level than seen in control IgG pre-treated mice once we stopped the Ab treatment and re-challenged these mice with desiccating stress. This effect was accompanied by re-infiltration of Th17 cells to the ocular surface, suggesting that blocking IL-17 does not eliminate memory Th17 cells. In contrast, no Th17 cell infiltration was observed in either anti-IL-7 or anti-IL-15 Ab pre-treated groups (Fig. 4C, right).
4. DISCUSSION
It is known that the immunological memory response is critical to chronical inflammation in autoimmune diseases (11, 12, 31, 32). Despite extensive investigations on various factors involved in the differentiation and expansion of effector Th17 cells, very little data is available on maintenance of memory Th17 cells (12). In this study, we demonstrate for the first time that two cytokines, IL-7 and IL-15, are required for the maintenance of immunopathogenic memory Th17 cells. They provide survival and proliferation signals via activating STAT5 and Akt pathways. Local neutralization of either of these two cytokines shows significant and long-lasting therapeutic effects.
To date, memory Th17 cells have been identified in the human (12, 33) and experimental autoimmune diseases (11, 32). However, the functionality of memory Th17 cells and their persistence requirements in pathophysiological conditions have been poorly investigated in either humans or animals. Animal studies have discovered memory Th17 cells as the principal mediators in sustaining chronic central nerve system inflammation (32) and ocular inflammation (11). Herein we explored the mechanisms by which memory Th17 cells are maintained in ocular surface autoimmune disease. Our results demonstrate that memory Th17 cells express receptors for IL-7 and IL-15. In contrast, effector CD4+ T cells do not express the IL-7 receptor (34). The expression of the IL-15 receptor on effector CD4+ T cells is unknown, although it is expressed by effector CD8+ T cells (35, 36). In addition, previous studies have shown that total memory CD4+ T cells express high levels of IL-7 receptor (37, 38) but low levels of IL-15 receptor (38). It has been suggested that IL-15 plays a less prominent role than IL-7 in the maintenance of memory CD4+ T cells (21, 38), and that IL-15 is only essential for the homeostasis of memory CD4+ T cells in the absence of IL-7 (39). However, our current findings show comparable expression levels for IL-7 and IL-15 receptors by memory Th17 cells, indicating that heterogeneous memory CD4+ T cell subsets may have distinct maintenance mechanisms. Additionally, our results documenting dramatically up-regulated IL-7 and IL-15 levels at the inflammatory site, and high levels in draining lymphoid tissues, further suggest that both cytokines are critical for long-term maintenance of pathogenic memory Th17 cells in autoimmunity. In fact, ex vivo treatment with either anti-IL-7 or anti-IL-15 Ab in lymph node cultures leads to a comparable significant reduction of memory Th17 cells, demonstrating that both cytokines are required for the maintenance of memory Th17 cells.
Studies on CD8+ memory T cells have demonstrated that both proliferation and survival mechanisms are involved in their maintenance. It has been reported that IL-15, but not IL-7, is essential to homeostatic proliferation of memory CD8+ T cells, while IL-7 plays a more prominent role in supporting memory CD8+ T cell survival (22, 36, 40). Large amounts of IL-7 alone can promote homeostatic proliferation of memory CD8+ T cells (38, 41). On the other hand, data derived from MP or TCR Tg memory CD4+ T cells are limited and controversial. Originally, neither IL-7 nor IL-15 was thought essential for proliferation or survival of memory CD4+ T cells (42, 43). Later, IL-7 was shown as an important survival factor for memory CD4+ T cells (37, 44). In a memory CD8+ T cell-dominant virus infection model, IL-7 was found to promote both survival and proliferation of antigen-specific memory CD4+ T cells, while IL-15 was found to play only accessory functions in the homeostasis of memory CD4+ T cells (using IL-15 deficient mice as a tool) (38), or it was required for memory CD4+ T cell proliferation when IL-7 was deficient (using TCR Tg cells as a tool) (39). In our autoimmune disease model, we use wild-type animals and found that both IL-7 and IL-15 are equally critical for maintaining pathogenic memory Th17 cells. Specifically, IL-7 mainly promotes memory Th17 cell survival via activating STAT5 signaling, and IL-15 provides signals for both cell survival (via STAT5 activation) and proliferation (via STAT5 and Akt activation).
Neutralization of different components of the Th17 pathway have been shown effective in a diverse group of immunoinflammatory diseases, such as animals with EAE (6, 45), CIA (2), and colitis (8). Clinical trials targeting IL-17 have also demonstrated promising efficacy in psoriasis, psoriatic arthritis, MS, Crohn's disease, RA, uveitis (non-infectious), and ankylosing spondylitis (45). Our current results suggest to target “memory” pathogenic Th17 cells as a novel strategy in autoimmunity. Although a recent study showed the requirement of IL-23 in the recall response of memory Th17 cells in EAE, it is unclear whether IL-23 is required for the maintenance of memory Th17 cells (32). In the present study, we not only show the comparable efficacy of topical neutralization of either IL-7 or IL-15 in ameliorating ocular inflammation as blockade of IL-17, but we also demonstrate a longer lasting effect of anti-IL-7 or anti-IL-15 treatment than anti-IL-17 treatment. Our result showing that topical neutralization of IL-17 significantly diminishes Th17 cells at the inflammatory site is consistent with findings from a clinical trial in patients with psoriasis (46), suggesting a positive self-feedback of IL-17 to Th17 cells. However, this feedback effect seems only limited to the local area because topical anti-IL-17 treatment at the inflammatory site cannot inhibit memory Th17 cells in draining lymphoid tissues, which significantly compromises its clinical efficacy, evidenced by faster and more severe disease exacerbation in the recall response. In contrast, topical anti-IL-7 or anti-IL-15 treatment can reduce memory Th17 cells in both inflammatory site and the draining lymphoid tissues, and thus provides a sustained inhibition of disease progression even after the treatment is stopped.
5. CONCLUSIONS
Our new compelling findings have established the critical roles of both IL-7 and IL-15 in the maintenance of memory Th17 cells, and the previously undescribed therapeutic advantages of targeting IL-7 and IL-15 further support a promising clinical translation in Th17 cell-mediated autoimmune and inflammatory disorders.
Highlights.
Pathogenic memory Th17 cells express both IL-7 and IL-15 receptors.
Neutralization of local IL-7 or IL-15 diminishes memory Th17 cells in autoimmunity.
IL-7 and IL-15 maintain pathogenic memory Th17 cells via STAT5 and Akt.
Targeting IL-7 and IL-15 may be useful for treating Th17 cell-mediated autoimmunity.
ACKNOWLEDGEMENTS
We thank Dr. Susanne Eiglmeier for critical reading and editing of this manuscript. This work was supported by National Institutes of Health (grant EY20889).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS YC, SKC, and RD designed research; YC and XT performed research; YC, SKC, and RD analyzed data; and YC, SKC, and RD wrote the paper.
REFERENCES
- 1.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 5.De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, 3rd, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human Th17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: what are they and what can they do? Semin Immunol. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walline CC, Kanakasabai S, Bright JJ. IL-7Rα confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 2011;12:1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartgring SA, Willis CR, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7-aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation. Arthritis Res Ther. 2012;14:R137. doi: 10.1186/ar3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki M, Yajima T, Tanabe M, Fukui K, Okada E, Okamoto R, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol. 2003;171:1556–1563. doi: 10.4049/jimmunol.171.3.1556. [DOI] [PubMed] [Google Scholar]
- 18.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- 19.Harris KM, Fasano A, Mann DL. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin Immunol. 2010;135:430–439. doi: 10.1016/j.clim.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandiyan P, Yang XP, Saravanamuthu SS, Zheng L, Ishihara S, O'Shea JJ, et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol. 2012;189:4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 23.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 24.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp RA, Pearson CF, Cornish GH, Seddon BP. Evidence of STAT5-dependent and -independent routes to CD8 memory formation and a preferential role for IL-7 over IL-15 in STAT5 activation. Immunol Cell Biol. 2010;88:213–219. doi: 10.1038/icb.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogami A, Oshikawa G, Okada K, Fukutake S, Umezawa Y, Nagao T, et al. FLT3-ITD confers resistance to the PI3K/Akt pathway inhibitors by protecting the mTOR/4EBP1/Mcl-1 pathway through STAT5 activation in acute myeloid leukemia. Oncotarget. 2015;6:9189–9205. doi: 10.18632/oncotarget.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 29.Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemoto Y, Kanai T, Kameyama K, Shinohara T, Sakamoto N, Totsuka T, et al. Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis. J Immunol. 2009;183:5059–5068. doi: 10.4049/jimmunol.0803684. [DOI] [PubMed] [Google Scholar]
- 32.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 2013;3:1378–1388. doi: 10.1016/j.celrep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 36.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 41.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 43.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 45.Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: Lessons from genetics and therapeutic interventions. Immunity. 2015;43:1040–1051. doi: 10.1016/j.immuni.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]