Abstract
Alcohol consumption is an established risk factor, and also a potential prognostic factor, for squamous cell carcinoma of the head and neck (HNSCC). However, little is known about whether the prognostic impact of alcohol consumption differs by treatment method. We evaluated the association between alcohol drinking and survival by treatment method to the primary site in 427 patients with HNSCC treated between 2005 and 2013 at Aichi Cancer Center Central Hospital (Nagoya, Japan). The impact of alcohol on prognosis was measured by multivariable Cox regression analysis adjusted for established prognostic factors. Among all HNSCC patients, the overall survival rate was significantly poorer with increased levels of alcohol consumption in multivariable analysis (trend P = 0.038). Stratification by treatment method and primary site revealed that the impact of drinking was heterogeneous. Among laryngopharyngeal cancer (laryngeal, oropharyngeal, and hypopharyngeal cancer) patients receiving radiotherapy (n = 141), a significant dose–response relationship was observed (trend P = 0.034). In contrast, among laryngopharyngeal cancer patients treated with surgery (n = 80), no obvious impact of alcohol was observed. This heterogeneity in the impact of alcohol between surgery and radiotherapy was significant (for interaction, P = 0.048). Furthermore, among patients with oral cavity cancer treated by surgery, a significant impact of drinking on survival was seen with tongue cancer, but not with non‐tongue oral cancer. We observed a significant inverse association between alcohol drinking and prognosis among HNSCC patients, and its impact was heterogeneous by treatment method and primary site.
Keywords: Adverse effect, alcohol, head and neck cancer, prognostic factor, treatment
Squamous cell carcinoma of the head and neck (HNSCC), which includes cancers of the oral cavity, oropharynx, hypopharynx, and larynx, is the sixth most common malignancy worldwide.1 In 2014, 9600 people died of head and neck cancer in Japan, accounting for 6.7% of all cancer deaths.2 Like smoking,3 alcohol drinking is an established risk factor for head and neck cancer.4, 5
A number of reports have indicated that alcohol consumption is also a prognostic factor for head and neck cancer.6, 7, 8, 9, 10 In their meta‐analysis of the association between alcohol consumption and survival in Asia and North America,7 Li et al. reported that heavy drinkers have a significantly worse prognosis than non‐drinkers. Leoncini et al. reported the potential influence of alcohol consumption on survival by each primary site of HNSCC, with hazard ratios (HRs) of 1.60 for the oral cavity, 1.60 for the oropharynx, and 4.19 for the hypopharynx.6 We have also reported a similarly poorer prognosis with alcohol drinking among Japanese patients.10
However, these studies did not report whether the impact of alcohol consumption on prognosis varied by treatment method. Definitive treatment for head and neck cancer is either surgery or radiotherapy/chemoradiotherapy (RT/CRT), depending on the primary site,11 and treatment and site are important prognostic factors. Lifestyle factors such as smoking or drinking also influence the therapeutic effect of RT/CRT in cancer of the aerodigestive tract.12, 13, 14 Accordingly, we considered it important to evaluate the heterogeneity of the influence of alcohol consumption on prognosis in combination with treatment method to the primary site. To our knowledge, however, no such study has yet been published.
Using data from the Hospital–based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) in Japan, we evaluated the association between alcohol consumption and HNSCC prognosis by treatment method. All treatments were in accordance with the National Comprehensive Cancer Network (NCCN) clinical practice guideline.
Materials and Methods
Study group
Patients were selected from the database of the HERPACC, which has been described in detail elsewhere.15, 16 Version 3 of HERPACC enrolled first‐visit outpatients at Aichi Cancer Center Hospital (ACCH; Nagoya, Japan) from December 2005 to March 2013, and had a participation rate of 66%. Participants completed the self‐administered HERPACC questionnaire on lifestyle factors, which included items on demographic characteristics, family and individual medical history, height, weight, amount of daily exercise, smoking and drinking habits, vitamin use, and consumption of selected foods and beverages before any diagnostic procedure were carried out. Trained interviewers checked the responses. All participants gave written informed consent to participate. This study was approved by the Institutional Ethics Committee of ACCH.
For the present study, we selected subject patients from among HERPACC version 3 participants using the same criteria as our previous study among HERPRACC version 2 participants,10 as follows: (i) primary and locoregional head and neck cancer in the oral cavity, oropharynx, hypopharynx, or larynx; (ii) histological diagnosis of squamous cell carcinoma; (iii) no previous definitive therapy of the primary site; and (iv) performance status (PS) of 0–2 according to the Eastern Cooperative Oncology Group criteria. Patients with metastatic head and neck cancer were excluded from the analysis, and cases of nasopharyngeal, salivary gland, nasal, and paranasal cancer were also excluded due to their distinct etiology. Finally, 427 cases satisfied the eligibility criteria for analysis and 230 cases were excluded.
Alcohol consumption and other exposure data
Alcohol consumption and other lifestyle factors were evaluated using the self‐administered HERPACC questionnaire. All patients were asked about their alcohol consumption, drinking behavior (frequency per week or per month), and intake of various common beverages (Japanese sake, shochu, beer, wine, and whiskey) before the development of their current symptoms. Alcohol consumption was converted to ethanol per day and used to divide subjects into the four categories of non‐, light (<23 g ethanol/day), moderate (23–46 g ethanol/day), and heavy drinker (>46 g ethanol/day).
Subjects were categorized by smoking status into the three groups of never, former, and current smokers. Former smokers were defined as those who quit smoking at least 1 year before the survey. Cumulative smoking was evaluated as pack‐years (PY), the product of the number of packs of cigarettes smoked per day and the number of years of smoking. In this study, subjects were divided into the four categories of never, PY < 20, PY < 40, and PY ≥ 40.
Therapy
Study patients were generally treated according to the NCCN guideline. Specifically, patients with oral cavity cancer (tongue, and others) at The International Union Against Cancer (UICC) stage T 1–2 and N0 were treated by surgery or RT, while those at other stages were treated by surgery only. In exceptional cases, patients who requested RT received this treatment, subject to physician assessment. Patients with laryngopharyngeal cancer (oropharyngeal, hypopharyngeal, and laryngeal cancer) at any stage were treated by either surgery or RT with or without concomitant chemotherapy. When the surgical margin was positive or extracapsular spread of lymph node metastasis was identified in pathologic tissue, postoperative RT was carried out.
After definitive RT/platinum‐based CRT (66–70 Gy), therapeutic effect was evaluated by computed tomography and endoscopy and residual disease was treated by salvage surgery. If platinum‐based induction chemotherapy (IC) was undertaken before definitive therapy, the therapeutic effect was evaluated after IC according to Response Evaluation Criteria in Solid Tumors.17 Patients with complete response or partial response were treated by RT, and those with stable disease or progressive disease were treated by surgery.
Lesions were considered to be a second primary cancer (SPC) if they were distinct, solid cancers that were histologically proven to be inconsistent with recurrent or metastatic disease. An SPC was classified as synchronous SPC if identified within 6 months of primary HNSCC diagnosis, and metachronous SPC if beyond this 6‐month period. In our evaluation, we limited the classification of SPCs as alcohol related‐cancers in accordance with the International Agency for Research on Cancer, namely to oral cavity, pharyngeal, laryngeal, esophagus, liver, and colorectal cancers.18
Statistical analysis
The primary endpoint of this study was overall survival (OS; interval between the date of first therapy and death from any case, or date of last follow‐up) and the secondary endpoints were disease‐specific survival (DSS; interval between the date of first therapy and death from head and neck cancer, or date of last follow‐up), disease‐free survival (DFS; interval between the date of first therapy and the date of locoregional or metastatic relapse, death from any cause, or date of last follow‐up), and incidence of metachronous SPC (interval between the date of first therapy and date of onset of metachronous SPC, or date of last follow‐up).
Vital status, disease progression, treatment method, and SPC were confirmed by checking medical records. In the case of loss of follow‐up, vital status was confirmed by census registration undertaken annually.
Point estimates of OS, DSS, DFS, and incidence of metachronous SPC were estimated by the Kaplan–Meier method.19 To assess the association between alcohol consumption and prognosis, we estimated multivariable HRs and 95% confidence intervals (CIs) using Cox proportional hazard models. Alcohol consumption was a major exposure of interest. Confounders considered in the multivariate analysis were age, gender, Eastern Corporative Oncology Group PS, smoking status, Union for International Cancer Control stage, definitive therapy and IC, energy, and synchronous SPC. Onset of metachronous SPC was considered in multivariate analysis as a time‐varying covariate.
We first analyzed the association between alcohol consumption and the prognosis of all patients with HNSCC. We then classified patients by primary site and definitive therapy according to the NCCN guideline, and analyzed each treatment group. Additionally, we examined the interaction between alcohol consumption and treatment method for laryngopharyngeal cancer using a multivariate Cox regression model.
Distribution of patient characteristics was assessed by the χ2‐test or Fisher's exact test, as appropriate. All analyses were carried out using Stata SE version 13 (StataCorp, College Station, TX, USA). P‐values <0.05 were considered statistically significant.
Results
Patient characteristics and overall clinical outcomes
Demographic characteristics and selected lifestyle habits of patients are shown in Table 1. Among 427 subjects, 206 patients (48%) had oral cavity cancer, of whom 145 patients (36%) had cancer of the tongue.
Table 1.
Characteristics of 427 patients with head and neck squamous cell carcinoma and levels of alcohol consumption
| n = 427 (%) | Drinking categories† | P‐value‡ | ||||
|---|---|---|---|---|---|---|
| Non‐drinker | Light | Moderate | Heavy | |||
| n = 113 (%) | n = 93 (%) | n = 74 (%) | n = 147 (%) | |||
| Age, years | ||||||
| <60 | 195 (45.7) | 53 (47.0) | 48 (51.6) | 29 (39.2) | 65 (44.2) | 0.420 |
| ≥60 | 232 (54.3) | 60 (53.0) | 45 (48.4) | 45 (60.8) | 82 (55.8) | |
| Sex | ||||||
| Male | 334 (78.1) | 54 (47.8) | 72 (77.4) | 67 (90.5) | 141 (95.9) | <0.001 |
| Female | 93 (21.9) | 59 (52.2) | 21 (22.6) | 7 (9.5) | 6 (4.1) | |
| Performance status | ||||||
| 0 | 305 (71.3) | 78 (68.7) | 69 (74.2) | 50 (67.6) | 108 (73.5) | 0.419 |
| 1 | 109 (25.6) | 30 (27.0) | 21 (22.6) | 20 (27.0) | 38 (25.9) | |
| 2 | 13 (3.0) | 5 (4.3) | 3 (3.2) | 4 (5.4) | 1 (0.7) | |
| Primary site | ||||||
| Oral cavity | 206 (48.3) | 72 (62.6) | 51 (54.8) | 31 (41.9) | 53 (36.1) | <0.001 |
| Tongue | 145 (34.0) | 45 (40.0) | 42 (45.2) | 20 (27.0) | 38 (25.9) | |
| Non‐tongue§ | 61 (14.2) | 26 (22.6) | 9 (9.7) | 11 (14.9) | 15 (10.2) | |
| Laryngopharynx | 221 (51.5) | 42 (45.2) | 42 (45.2) | 42 (45.2) | 94 (63.9) | |
| Oropharynx | 73 (17.0) | 14 (12.2) | 18 (19.4) | 16 (21.6) | 25 (17.0) | |
| Hypopharynx | 96 (22.4) | 10 (8.7) | 12 (12.9) | 18 (24.3) | 56 (38.1) | |
| Larynx | 52 (12.4) | 19 (16.5) | 12 (12.9) | 9 (12.2) | 13 (8.8) | |
| UICC stage | ||||||
| 1 | 77 (18.2) | 24 (21.7) | 18 (19.4) | 14 (18.9) | 21 (14.3) | 0.208 |
| 2 | 94 (21.9) | 27 (23.5) | 28 (30.1) | 12 (16.2) | 27 (18.4) | |
| 3 | 60 (14.0) | 18 (15.7) | 11 (11.8) | 9 (12.2) | 22 (15.0) | |
| 4 | 196 (45.9) | 44 (39.1) | 36 (38.7) | 39 (52.7) | 77 (52.4) | |
| T stage | ||||||
| 1 | 98 (22.8) | 27 (23.9) | 26 (28.0) | 19 (25.7) | 26 (17.7) | 0.001 |
| 2 | 177 (41.3) | 51 (45.1) | 48 (51.6) | 28 (37.8) | 50 (34.0) | |
| 3 | 67 (15.6) | 11 (9.7) | 7 (7.5) | 11 (14.9) | 38 (25.9) | |
| 4 | 84 (19.6) | 23 (20.4) | 12 (12.9) | 16 (21.6) | 33 (22.4) | |
| N stage | ||||||
| 0 | 225 (52.4) | 71 (62.8) | 52 (55.9) | 31 (41.9) | 71 (48.3) | 0.145 |
| 1 | 51 (11.9) | 11 (9.7) | 13 (14.0) | 10 (13.5) | 17 (11.6) | |
| 2 | 140 (32.6) | 30 (26.5) | 24 (25.8) | 31 (41.9) | 55 (37.4) | |
| 3 | 11 (2.6) | 1 (0.9) | 4 (4.3) | 2 (2.7) | 4 (2.7) | |
| Smoking status | ||||||
| Never | 109 (25.4) | 63 (54.8) | 30 (32.3) | 8 (10.8) | 8 (5.4) | <0.001 |
| Former | 146 (34.3) | 26 (23.5) | 36 (38.7) | 31 (41.9) | 53 (36.1) | |
| Current | 172 (40.1) | 24 (20.9) | 27 (29.0) | 35 (47.3) | 86 (58.5) | |
| Smoking category | ||||||
| Non–low (0 ≤ and ≤5 PY) | 119 (27.7) | 65 (56.5) | 35 (37.6) | 11 (14.9) | 8 (5.4) | <0.001 |
| Light (5 ≤ and <20 PY) | 60 (14.0) | 13 (11.3) | 19 (20.4) | 10 (13.5) | 18 (12.2) | |
| Moderate (20 ≤ and <40 PY) | 95 (22.1) | 21 (18.3) | 10 (10.8) | 28 (37.8) | 36 (24.5) | |
| Heavy (≥40 PY) | 140 (32.6) | 14 (12.2) | 27 (29.0) | 21 (28.4) | 78 (53.1) | |
| Unknown | 15 (3.5) | 2 (1.7) | 2 (2.2) | 4 (5.4) | 7 (4.8) | |
| Smoking status | ||||||
| Never | 109 (25.4) | 63 (54.8) | 30 (32.3) | 8 (10.8) | 8 (5.4) | <0.001 |
| Former | 146 (34.3) | 26 (23.5) | 36 (38.7) | 31 (41.9) | 53 (36.1) | |
| Current | 172 (40.1) | 24 (20.9) | 27 (29.0) | 35 (47.3) | 86 (58.5) | |
| Therapy | ||||||
| Induction chemotherapy | ||||||
| Yes | 144 (33.6) | 22 (19.1) | 28 (30.1) | 28 (37.8) | 66 (44.9) | <0.001 |
| No | 285 (66.4) | 93 (80.9) | 65 (69.9) | 46 (62.2) | 81 (55.1) | |
| Definitive therapy | ||||||
| Op | 269 (62.9) | 82 (71.3) | 62 (66.7) | 45 (60.8) | 81 (55.1) | 0.041 |
| RT | 159 (37.1) | 33 (28.7) | 31 (33.3) | 29 (39.2) | 66 (44.9) | |
| Second primary cancer¶ | ||||||
| Synchronous cancer | 40 (0.5) | 5 (0.9) | 3 (1.1) | 6 (0.0) | 26 (0.0) | <0.001 |
†Light, <23 g ethanol/day; moderate, 23–46 g ethanol/day; heavy, >46 g ethanol/day. ‡χ2‐test. §Gingiva, oral floor, and buccal mucosa. ¶Alcohol‐related cancer (head and neck, esophagus, liver, and colon). Op, surgery; PY, pack‐years; RT, radiotherapy; UICC, The International Union Against Cancer.
The prevalence of the factors of male, hypopharyngeal cancer, synchronous and metachronous SPC, and current or heavy smoker increased with increasing levels of drinking. In contrast, no other significant difference was seen among drinking categories.
With regard to treatment, 270 patients (62%) underwent surgery and 62 (14%) received postoperative RT. One hundred and fifty nine patients (38%) underwent RT, of whom 99 (23%) received chemoradiotherapy. After RT, 36 patients (8%) had residual disease and underwent salvage surgery. One hundred and forty four patients (33%) underwent induction chemotherapy, of whom 75 (17%) were responders (complete response or partial response) and received RT, while 69 (16%) were non‐responders (stable disease or progressive disease) and received surgery.
Metachronous SPC occurred in 21 patients (7%), 10 of whom had head and neck cancer, 7 had esophageal cancer, and 4 had colorectal cancer.
Median follow‐up time was 46 months (range, 0–198 months). Five‐year OS, DSS, and DFS of all patients was 0.68 (95% CI, 0.62–0.73), 0.72 (95% CI, 0.67–0.77), and 0.49 (95% CI, 0.43–0.54), respectively.
Impact of alcohol consumption on prognosis
As shown in Figure 1(A), there was a significant difference in OS according to drinking level (log–rank, P = 0.006). Five‐year OS rates of non‐, light, moderate, and heavy drinkers were 0.78 (95% CI, 0.68–0.86), 0.73 (0.60–0.82), 0.64 (0.51–0.76), and 0.58 (0.49.1–0.67), respectively (Fig. 1A).
Figure 1.
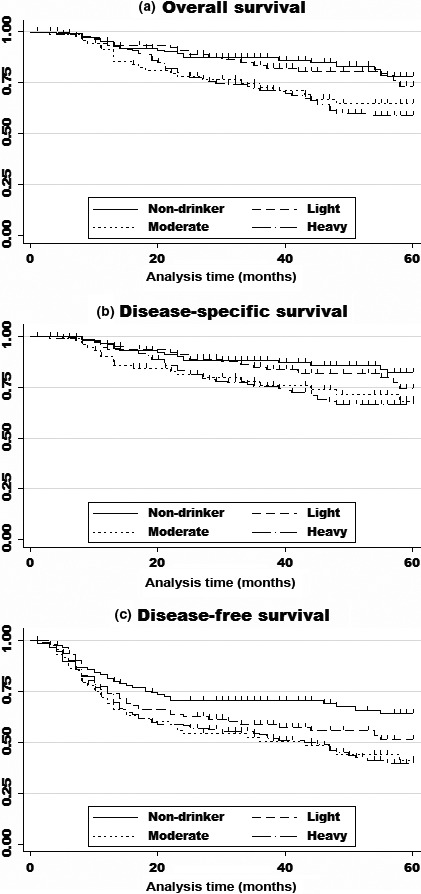
Kaplan–Meier survival curves of overall survival (A), disease‐specific survival (B), and disease‐free survival (C) for all 429 patients with head and neck squamous cell carcinoma. (A) Kaplan–Meier survival curve of overall survival. Heavy (>46 g ethanol/day; n = 147) and moderate (23–46 g ethanol/day; n = 89) alcohol consumption were significantly associated with poorer survival compared with non‐drinking (hazard ratio [HR], 1.92; 95% confidence interval [CI], 1.02–3.91, P = 0.044; and HR, 2.00; 95% CI, 1.02–3.59, P = 0.042, respectively). (B) Kaplan–Meier survival curve of disease‐specific survival. Heavy and moderate alcohol consumption were significantly associated with poorer survival than non‐drinking (HR, 2.12; 95% CI, 1.02–4.40, P = 0.043; and HR, 1.96; 95% CI, 0.99–3.86, P = 0.053, respectively). (C) Kaplan–Meier survival curve of disease‐free survival. Heavy and moderate alcohol consumption were significantly associated with poorer survival than non‐drinking (HR, 1.87; 95% CI, 1.16–2.91, P = 0.010; and HR, 1.87; 95% CI, 1.14–3.08, P = 0.014, respectively).
Table 2 (upper section) shows the results of univariable and multivariable analyses of alcohol consumption and OS. Univariate analysis showed a significantly poorer OS for moderate and heavy drinkers. Even after adjustment for covariates, moderate and heavy drinkers had significantly poorer survival than non‐drinkers, with HRs of 2.02 (1.02–3.91) for moderate and 1.91 (1.02–3.59) for heavy drinkers. A dose–response relationship between alcohol consumption and OS was observed with statistical significance (trend P = 0.038). As shown in Figure 1(B,C) and Table 2 (middle section), a similar tendency was observed with DSS and DFS.
Table 2.
Impact of alcohol consumption on overall, disease‐specific, and disease‐free survival, and incidence of metachronous second primary cancer (SPC) by multivariable proportional hazard models
| n (%) | 5‐year values (95% CI) | Crude | Adjusted† | |||
|---|---|---|---|---|---|---|
| HR (95% CI)† | P‐value | HR (95% CI)† | P‐value | |||
| Overall survival | ||||||
| Non‐drinker | 113 (26) | 0.78 (0.68–0.86) | 1.00 (Reference) | 1.00 (Reference) | ||
| Light | 93 (21) | 0.73 (0.60–0.82) | 1.26 (0.68–2.30) | 0.454 | 1.34 (0.71–2.60) | 0.367 |
| Moderate | 74 (17) | 0.64 (0.51–0.76) | 2.05 (1.14–3.69) | 0.016 | 2.00 (1.02–3.91) | 0.044 |
| Heavy | 147 (34) | 0.59 (0.49–0.67) | 2.17 (1.31–3.61) | 0.003 | 1.91 (1.02–3.59) | 0.042 |
| Trend P = 0.001 | Trend P = 0.038 | |||||
| Disease‐specific survival | ||||||
| Non‐drinker | 113 (26) | 0.82 (0.72–0.89) | 1.00 (Reference) | 1.00 (Reference) | ||
| Light | 93 (21) | 0.74 (0.61–0.84) | 1.39 (0.73–2.63) | 0.306 | 1.55 (0.78–3.05) | 0.210 |
| Moderate | 74 (17) | 0.68 (0.54–0.79) | 1.99 (1.05–3.78) | 0.033 | 2.12 (1.02–4.40) | 0.043 |
| Heavy | 147 (34) | 0.66 (0.57–0.74) | 2.04 (1.18–3.55) | 0.011 | 1.96 (0.99–3.86) | 0.053 |
| Trend P = 0.006 | Trend P = 0.066 | |||||
| Disease‐free survival | ||||||
| Non‐drinker | 113 (26) | 0.64 (0.53–0.73) | 1.00 (Reference) | 1.00 (Reference) | ||
| Light | 93 (21) | 0.51 (0.39–0.62) | 1.55 (1.01–2.39) | 0.046 | 1.65 (1.04–2.60) | 0.033 |
| Moderate | 74 (17) | 0.41 (0.27–0.53) | 1.94 (1.22–3.03) | 0.003 | 1.87 (1.13–3.08) | 0.014 |
| Heavy | 147 (34) | 0.40 (0.31–0.49) | 1.91 (1.30–2.80) | 0.001 | 1.84 (1.16–2.91) | 0.010 |
| Trend P = 0.001 | Trend P = 0.020 | |||||
| Incidence of metachronous SPC | ||||||
| Non‐drinker | 113 (26) | 0.03 (0.01–0.09) | 1.00 (Reference) | 1.00 (Reference) | ||
| Light | 93 (21) | 0.04 (0.01–0.15) | 1.10 (0.22–5.53) | 0.887 | 1.94 (0.33–11.96) | 0.471 |
| Moderate | 74 (17) | 0.03 (0.01–0.22) | 1.70 (0.34–8.44) | 0.515 | 1.75 (0.22–13.54) | 0.594 |
| Heavy | 147 (34) | 0.07 (0.03–0.15) | 2.98 (0.82–10.70) | 0.095 | 2.54 (0.42–15.30) | 0.309 |
| Trend P = 0.049 | Trend P = 0.331 | |||||
Alcohol consumption graded as: light, <23 g ethanol/day; moderate, 23–46 g ethanol/day; and heavy, >46 g ethanol/day. †Adjusted by sex, age, Union for International Cancer Control performance status, stage, primary site, smoker, energy, synchoronous and metachronous, SPC, definitive therapy, and induction chemotherapy. CI, confidence interval; HR, hazard ratio.
Regarding the incidence of metachronous SPC, a suggestive association with alcohol consumption was observed in crude analysis (Fig. S1, Table 2, lower section), but this was not statistically significant on multivariable analysis (HR, 2.54; 95% CI, 0.42–15.30 for heavy drinkers).
Stratified analysis by potential confounders
To evaluate the consistency of the association with alcohol consumption, we evaluated Cox proportional hazards models for OS stratified by potential confounders (Fig. S2). Compared with non‐drinkers, heavy and moderate drinkers had poor survival in all subgroups, except in those aged <60 years. Survival was suggested to be poorer in the non‐smoker than in the smoker group (P for interaction = 0.103; Table S1).
Distribution of primary site and definitive therapy
Table 3 shows the distribution of primary site and definitive therapy according to alcohol consumption. Among patients with oral cavity cancer, 189 patients received surgery and the remaining 17 received RT. Among those with laryngopharyngeal cancer (oropharyngeal, hypopharyngeal, and laryngeal cancer), 80 patients were treated by surgery and 142 by RT.
Table 3.
Distribution of primary site, definitive therapy, and alcohol consumption in 427 patients with head and neck squamous cell carcinoma
| Primary site | Definitive therapy | n = 427 (%) | Drinking categories | P‐value† | |||
|---|---|---|---|---|---|---|---|
| Non‐drinker | Light | Moderate | Heavy | ||||
| n = 115 (%) | n = 93 (%) | n = 74 (%) | n = 147 (%) | ||||
| Oral cavity | Op | 189 (44.3) | 67 (59.1) | 48 (51.6) | 28 (37.8) | 46 (31.3) | 0.420 |
| RT | 17 (4.0) | 4 (3.5) | 3 (3.2) | 3 (4.1) | 7 (4.8) | ||
| Tongue | Op | 132 (31.0) | 41 (36.5) | 39 (41.9) | 18 (24.3) | 34 (23.1) | 0.958 |
| RT | 13 (3.0) | 4 (3.5) | 3 (3.2) | 2 (2.7) | 4 (2.7) | ||
| Non‐tongue‡ | Op | 57 (13.3) | 26 (22.6) | 9 (9.7) | 10 (13.5) | 12 (8.2) | 0.072 |
| RT | 4 (0.9) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 3 (2.0) | ||
| Laryngopharynx (oropharynx, hypopharynx, larynx) | Op | 80 (18.6) | 14 (12.2) | 14 (15.1) | 17 (23.0) | 35 (23.8) | 0.908 |
| RT | 141 (33.1) | 28 (25.2) | 28 (30.1) | 26 (305.4) | 59 (40.1) | ||
| Oropharynx | Op | 29 (6.8) | 3 (2.6) | 7 (7.5) | 9 (12.2) | 10 (6.8) | 0.285 |
| RT | 44 (10.3) | 11 (9.6) | 11 (11.8) | 7 (9.5) | 15 (10.2) | ||
| Hypopharynx | Op | 40 (9.3) | 6 (5.2) | 5 (5.4) | 6 (8.1) | 23 (15.6) | 0.592 |
| RT | 56 (13.1) | 4 (3.5) | 7 (7.5) | 12 (16.2) | 33 (22.4) | ||
| Larynx | Op | 11 (2.6) | 5 (4.3) | 2 (2.2) | 2 (2.7) | 2 (1.4) | 0.829 |
| RT | 42 (9.8) | 13 (12.2) | 10 (10.8) | 7 (9.5) | 11 (7.5) | ||
Alcohol consumption graded as: light, <23 g ethanol/day; moderate, 23–46 g ethanol/day; and heavy, >46 g ethanol/day. †χ2‐test. ‡Oral cancer and not tougue consist of gingiva, oral floor, and buccal mucosa. Op, surgery; RT, radiotherapy.
As the NCCN guideline recommends RT as an optional treatment for primary oral cancer and only 17 of our patients were under this category, we decided that further evaluation of the interaction of therapy and alcohol consumption on prognosis should focus on patients with oral cavity cancer treated by surgery and laryngopharyngeal cancer treated by either surgery or RT. We further divided patients with oral cavity cancer by subsite (tongue and non‐tongue oral cancer, consisting of the buccal mucosa, floor of mouth, and gingiva).
Impact of alcohol consumption on prognosis by primary site and definitive therapy
Oral cavity
In all patients with oral cavity cancer who received surgery (n = 189), light, moderate, and heavy drinkers had poorer survival than non‐drinkers, although the trend was not significant. Among these patients, this trend was significant in moderate drinkers after adjustment for covariates (HR, 3.61; 95% CI, 1.27–10.22; Table 4, upper left). Furthermore, when we stratified these patients by subsite (tongue and non‐tongue), those with tongue cancer (n = 132) showed increased HRs for OS compared to non‐drinkers, with HRs of 3.20 (95% CI, 0.96–10.6) for light, 11.70 (95% CI, 2.99–45.74) for moderate, and 3.07 (95% CI, 0.75–12.15) for heavy drinkers (Fig. S3). In patients with non‐tongue oral cancer (n = 57), in contrast, the contribution of alcohol consumption was not clear. These results in OS were also consistently observed for DSS and DFS.
Table 4.
Impact of alcohol consumption on survival in 429 patients with head and neck squamous cell carcinoma according to treatment method to primary site
| n (%) | Overall survival | Disease‐specific survival | Disease‐free survival | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5‐year values (95% CI) | HR (95% CI)† | P‐value | 5‐year values (95% CI) | HR (95%CI)† | P‐value | 5‐year values (95% CI) | HR (95% CI)† | P‐value | ||
| Oral cavity (Op) | ||||||||||
| Non‐drinker | 68 (35) | 0.79 (0.65–0.87) | 1.00 (Reference) | 0.79 (0.65–0.88) | 1.00 (Reference) | 0.67 (0.54–0.78) | 1.00 (Reference) | |||
| Light | 48 (25) | 0.72 (0.54–0.85) | 1.92 (0.77–4.76) | 0.157 | 0.73 (0.54–0.85) | 1.89 (0.76–4.68) | 0.171 | 0.61 (0.43–0.74) | 1.99 (1.02–3.90) | 0.043 |
| Moderate | 28 (14) | 0.57 (0.32–0.76) | 3.61 (1.27–10.22) | 0.016 | 0.48 (0.24–0.69) | 4.15 (1.55–11.17) | 0.005 | 0.33 (0.14–0.54) | 3.17 (1.45–6.94) | 0.004 |
| Heavy | 46 (24) | 0.68 (0.49–0.81) | 2.02 (0.72–5.70) | 0.184 | 0.76 (0.58–0.87) | 1.36 (0.47–3.96) | 0.572 | 0.53 (0.36–0.68) | 1.94 (0.90–4.18) | 0.090 |
| Trend P = 0.173 | Trend P = 0.429 | Trend P = 0.077 | ||||||||
| Tongue (Op) | ||||||||||
| Non‐drinker | 42 (31) | 0.83 (0.62–0.93) | 1.00 (Reference) | 0.83 (0.63–0.92) | 1.00 (Reference) | 0.68 (0.50–0.81) | 1.00 (Reference) | |||
| Light | 39 (29) | 0.66 (0.43–0.81) | 3.20 (0.96–10.60) | 0.057 | 0.66 (0.43–0.81) | 3.19 (0.93–10.92) | 0.064 | 0.54 (0.35–0.70) | 2.19 (0.99–4.88) | 0.054 |
| Moderate | 18 (13) | 0.43 (0.13–0.70) | 11.70 (2.99–45.74) | <0.001 | 0.40 (0.13–0.67) | 11.63 (3.04–44.53) | <0.001 | 0.15 (0.01–0.43) | 6.36 (2.43–16.65) | <0.001 |
| Heavy | 34 (25) | 0.67 (0.44–0.82) | 3.07 (0.76–12.42) | 0.117 | 0.75 (0.54–0.88) | 2.04 (0.48–8.58) | 0.332 | 0.51 (0.31–0.68) | 2.44 (0.93–6.40) | 0.071 |
| Trend P = 0.126 | Trend P = 0.345 | Trend P = 0.055 | ||||||||
| Non‐tongue‡ (Op) | ||||||||||
| Non‐drinker | 26 (42) | 0.72 (0.50–0.85) | 1.00 (Reference) | 0.72 (0.49–0.85) | 1.00 (Reference) | 0.65 (0.43–0.80) | 1.00 (Reference) | |||
| Light | 9 (14) | 1.00 | 1.00 | 0.88 (0.39–0.98) | 0.21 (0.03–1.88) | 0.166 | ||||
| Moderate | 10 (17) | 0.74 (0.29–0.93) | 0.09 (0.08–1.07) | 0.057 | 0.59 (0.19–0.85) | 0.26 (0.39–2.17) | 0.213 | 0.61 (0.20–0.86) | 0.56 (0.94–3.31) | 0.522 |
| Heavy | 12 (21) | 0.71 (0.34–0.90) | 0.31 (0.05–1.94) | 0.209 | 0.77 (0.35–0.94) | 0.21 (0.03–1.51) | 0.123 | 0.60 (0.22–0.84) | 0.58 (0.13–2.54) | 0.467 |
| Trend P = 0.322 | Trend P = 0.222 | Trend P = 0.468 | ||||||||
| Laryngopharyngeal cancer (oropharyngeal, hypopharyngeal, laryngeal) | ||||||||||
| Non‐drinker | 43 (19) | 0.82 (0.60–0.93) | 1.00 (Reference) | 0.95 (0.81–0.98) | 1.00 (Reference) | 0.63 (0.42–0.78) | 1.00 (Reference) | |||
| Light | 43 (18) | 0.71 (0.49–0.85) | 1.43 (0.49–4.19) | 0.513 | 0.73 (0.51–0.86) | 2.67 (0.67–10.64) | 0.164 | 0.39 (0.22–0.55) | 2.27 (1.08–4.79) | 0.030 |
| Moderate | 43 (19) | 0.69 (0.51–0.81) | 1.89 (0.66–5.40) | 0.233 | 0.80 (062–0.90) | 2.38 (0.57–9.86) | 0.232 | 0.43 (0.25–0.59) | 2.05 (0.96–4.35) | 0.062 |
| Heavy | 94 (42) | 0.54 (0.42–0.65) | 2.35 (0.88–6.25) | 0.086 | 0.62 (0.50–0.72) | 4.09 (1.11–15.08) | 0.034 | 0.33 (0.23–0.44) | 2.35 (1.17–4.69) | 0.016 |
| Trend P = 0.054 | Trend P = 0.025 | Trend P = 0.047 | ||||||||
| Op | ||||||||||
| Non‐drinker | 14 (17) | 0.84 (0.50–0.96) | 1.00 (Reference) | 0.84 (0.49–0.96) | 1.00 (Reference) | 0.31 (0.05–0.62) | 1.00 (Reference) | |||
| Light | 14 (17) | 0.36 (0.07–0.68) | 1.35 (0.23–8.13) | 0.737 | 0.41 (0.07–0.73) | 1.05 (0.15–7.32) | 0.958 | 0.21 (0.04–0.46) | 1.67 (0.54–5.20) | 0.377 |
| Moderate | 17 (21) | 0.88 (0.59–0.97) | 0.60 (0.08–4.33) | 0.615 | 0.93 (0.63–0.99) | 0.50 (0.06–4.31) | 0.531 | 0.44 (0.17–0.69) | 0.67 (0.20–2.20) | 0.507 |
| Heavy | 35 (43) | 0.48 (0.25–0.68) | 1.45 (0.28–7.43) | 0.653 | 0.62 (0.39–0.79) | 1.46 (0.28–7.61) | 0.648 | 0.36 (0.16–0.56) | 0.98 (0.34–2.82) | 0.967 |
| Trend P = 0.592 | Trend P = 0.511 | Trend P = 0.666 | ||||||||
| RT | ||||||||||
| Non‐drinker | 29 (20) | 0.81 (0.50–0.94) | 1.00 (Reference) | 1.00 | 0.78 (0.52–0.91) | 1.00 (Reference) | ||||
| Light | 28 (19) | 0.89 (0.69–0.96) | 0.86 (0.16–4.69) | 0.862 | 0.88 (0.69–0.96) | Cannot be estimated§ | 0.46 (0.23–0.66) | 3.02 (0.94–10.01) | 0.067 | |
| Moderate | 26 (18) | 0.59 (0.36–0.76) | 2.94 (0.69–12.51) | 0.145 | 0.74 (0.50–0.87) | 0.42 (0.21–0.61) | 4.37 (1.37–13.93) | 0.013 | ||
| Heavy | 59 (41) | 0.56 (0.41–0.68) | 3.11 (0.76–12.69) | 0.113 | 0.62 (0.48–0.74) | 0.31 (0.19–0.44) | 5.03 (1.64–15.40) | 0.005 | ||
| Trend P = 0.034 | Trend P = 0.009 | Trend P = 0.004 | ||||||||
Alcohol consumption graded as: light, <23 g ethanol/day; moderate, 23–46 g ethanol/day; and heavy, >46 g ethanol/day. †Adjusted by sex, age, Union for International Cancer Control performance status, stage, primary site, smoker, energy, synchoronous and metachronous, second primary cancer, definitive therapy, and induction chemotherapy. ‡Oral cancer and non‐tongue consist of gingiva, oral floor, and buccal mucosa. §Non‐drinker was no event and hazard ratio (HR) cannot be estimated. CI, confidence interval; Op, surgery; RT, radiotherapy.
Laryngopharyngeal
Among all subjects with laryngopharyngeal cancer, the survival rate decreased as drinking levels increased with marginal statistical significance (trend P = 0.054; Table 4, lower left).
On stratification by treatment method, a significant dose–response relationship was observed among patients receiving RT (n = 143; trend P = 0.034), with moderate and heavy alcohol consumption associated with poorer survival (HR, 2.94 and 95% CI, 0.69–12.51 for moderate drinkers; HR, 3.11 and 95% CI, 0.76–12.69 for heavy drinkers; Fig. S4A). In contrast, among patients treated by surgery (n = 80), no association between alcohol consumption and OS was observed (Fig. S3B).
To clearly assess the interaction between treatment method and alcohol consumption on prognosis, we undertook an analysis with dichotomization by non–light versus moderate–heavy alcohol consumption. Among patients receiving RT, survival of moderate–heavy drinkers was significantly poorer than that of non–light drinkers (5‐year OS, 0.56 and 0.85, respectively; HR relative to non–light drinkers, 3.32 [95% CI, 1.22–8.98]) (Table 5, upper). In contrast, among patients receiving surgery, there was no obvious difference in survival between non–light and moderate–heavy drinkers (5‐year OS, 0.60 and 0.62, respectively; HR relative to non–light drinkers, 0.94 [95% CI, 0.34–2.63]) (Fig. 2). A significant interaction between alcohol consumption and therapy on prognosis were observed (P for interaction = 0.048 for OS [Table 5, upper]). A similar tendency was observed with DSS and DFS.
Table 5.
Interaction between alcohol consumption and treatment method of laryngopharyngeal cancer
| n (%) | 5‐year values (95% CI) | HR (95% CI)† | P‐value | P for interaction | |
|---|---|---|---|---|---|
| Overall survival | |||||
| Op‡ | |||||
| Non–light | 28 (35) | 0.60 (0.34–0.79) | 1.00 (Reference) | 0.048 | |
| Moderate–heavy | 52 (65) | 0.62 (0.44–0.76) | 0.94 (0.34–2.64) | 0.875 | |
| RT‡ | |||||
| Non–light | 56 (40) | 0.85 (0.69–0.93) | 1.00 (Reference) | ||
| Moderate–heavy | 85 (60) | 0.56 (0.44–0.66) | 3.32 (1.22–8.98) | 0.018 | |
| Disease‐specific survival | |||||
| Op‡ | |||||
| Non–light | 28 (35) | 0.63 (0.36–0.82) | 1.00 (Reference) | 0.036 | |
| Moderate–heavy | 52 (65) | 0.78 (0.55–0.84) | 1.08 (0.35–3.33) | 0.890 | |
| RT‡ | |||||
| Non–light | 56 (40) | 0.94 (0.083–0.98) | 1.00 (Reference) | ||
| Moderate–heavy | 85 (60) | 0.65 (0.53–0.75) | 4.56 (1.27–16.44) | 0.020 | |
| Disease‐free survival | |||||
| Op‡ | |||||
| Non–light | 28 (35) | 0.27 (0.10–0.48) | 1.00 (Reference) | 0.022 | |
| Moderate–heavy | 52 (65) | 0.39 (0.23–0.55) | 0.64 (0.30–1.35) | 0.241 | |
| RT‡ | |||||
| Non–light | 56 (40) | 0.79 (0.54–0.91) | 1.00 (Reference) | ||
| Moderate–heavy | 85 (60) | 0.31 (0.19–0.43) | 2.25 (1.21–4.16) | 0.010 | |
Alcohol consumption classified as: non–light, non‐drinker and <23 g ethanol/day; and moderate–heavy, ≥23 g ethanol/day. †Adjusted by sex, age, Union for International Cancer Control performance status, stage, primary site, smoker, energy, second primary cancer, and induction chemotherapy. ‡Laryngopharyngeal cancer (oropharyngeal, hypopharyngeal, and laryngeal). CI, confidence interval; HR, hazard ratio; Op, surgery; RT, radiotherapy.
Figure 2.
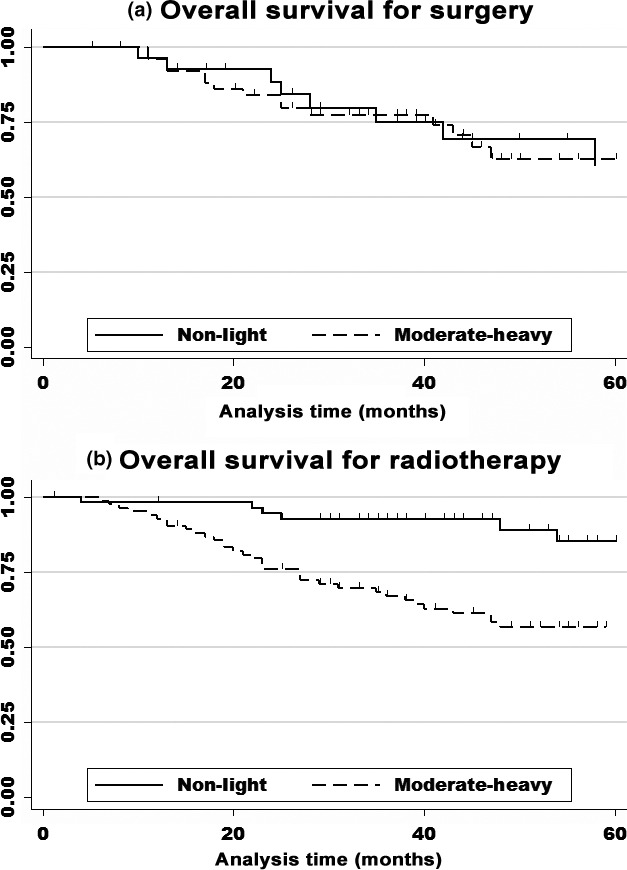
Kaplan–Meier survival curves for patients with laryngopharyngeal cancer by treatment method. (A) Surgery: no obvious difference was observed between non–light drinkers (0 and <23 g ethanol/day; n = 28) and moderate–heavy drinkers (23–46 and >46 g ethanol/day; n = 52) (5‐year overall survival: 0.60 and 0.62, respectively; hazard ratio relative to non–light drinkers, 0.94 [95% confidence interval, 0.34–2.63], P = 0.875). (B) Radiotherapy: a significant association was observed between non–light drinkers (n = 56) and moderate–heavy drinkers (n = 85) (5‐year overall survival, 0.56 and 0.85, respectively; hazard ratio relative to non–light drinkers, 3.26 [95% confidence interval, 1.19–8.98], P = 0.018).
Discussion
In this study, we found significantly poor survival, in terms of OS, DSS, and DFS, among HNSCC patients with higher alcohol consumption after adjustment for clinical covariates. This association was consistently observed in strata of age, sex, smoking status, and stage. More importantly, the association between alcohol consumption and prognosis differed by definitive treatment in each primary site. Among patients with laryngopharyngeal cancer, alcohol consumption had a more significant impact on prognosis in patients treated by RT than surgery; among patients with oral cavity cancer treated by surgery, a standard therapy for oral cavity cancer, alcohol consumption had a more significant impact in patients with tongue cancer than non‐tongue cancer. To our knowledge, this is the first study to indicate a heterogeneous impact of alcohol consumption on the prognosis of HNSCC by treatment method.
Several reports have noted an association between alcohol consumption and survival of HNSCC. In their meta‐analysis, Li et al. reported that heavy drinking was a poor prognostic factor, with an HR of 4.19 (95% CI, 2.32–7.55) compared to non‐drinkers with HNSCC, whereas moderate drinkers had an HR of 1.51 (95% CI, 0.77–2.96).7 Another study reported an association between alcohol drinking and prognosis in patients with head and neck cancer who received RT:14 Fortin et al. evaluated the prognostic value of smoking and drinking in 1871 patients with head and neck cancer who received RT, and reported that active drinkers had a poorer prognosis compared to non‐drinkers on multivariate analysis (HR, 1.3; 95% CI, 1.13–1.54).14 In our previous study, a positive dose relationship was evident in the prognosis of Aldehyde dehydrogenase‐2 (ALDH2) Glu/Glu patients with HNSCC.10 These findings consistently show an association between poor prognosis and heavy drinking. Our present results are consistent with these earlier findings.
The background mechanisms behind the association behind alcohol drinking and survival of all HNSCC patients remain unclear, however, several mechanisms appear plausible for alcohol drinking and prognosis. First, several molecular effects of ethanol are proposed, including enhanced cell proliferation and altered expression of cytokeratin suggesting inhibition of squamous cell differentiation,20, 21 interference with DNA repair machinery and DNA synthesis,12, 22, 23 and impaired antioxidant defense and enhanced production of reactive oxygen species.24 A second possible explanation is that alcohol consumption impairs patients’ nutrient status and immune system, leading to the inability to destroy cancer cells.25 Among head and neck cancer, it has been reported that alcohol has significant immunomodulatory effects, by impairing cellular immunity,26 particularly antigen‐specific immune responses.27
As previous studies did not evaluate potential heterogeneity in the association between alcohol consumption and prognosis by treatment method, we evaluated this association by treatment method as indicated in the NCCN guideline. We found that alcohol consumption had a considerable impact on prognosis in subjects with laryngopharyngeal cancer who received RT, but no impact among patients who were treated with surgery. Although the cause of this heterogeneous association is unclear, several possibilities can be considered. First, the prevalence of human papilloma virus (HPV)‐related oropharyngeal cancer among laryngopharyngeal cancer patients treated by RT might be high. Clinically, it is known that HPV‐related oropharyngeal cancers are sensitive to RT,28, 29, 30 and prevalence is high among non‐smokers and non‐drinkers.31, 32 In our study, although we did not evaluate HPV status, it is likely that non‐drinkers receiving RT accounted for a substantial portion of HPV‐related oropharyngeal cancer cases. An association between alcohol and HPV status on survival has been reported.33, 34 Broglie et al. evaluated patients with oropharyngeal cancer who were treated with RT and found that p16, a potential surrogate marker of HPV infection, and lifestyle factors (smoking and drinking) were associated with survival.34 After multivariate analysis, including p16 status, alcohol dinking was a poor prognostic factor, as was N classification.34 These results are congruent with our study. Further studies to examine the association between HPV infection and alcohol on prognosis are warranted.
A second possibility is genetic alteration induced by ethanol and an acetaldehyde‐introduced heterogeneous impact of alcohol drinking between radiation therapy and surgery. A previous study of 136 patients with head and neck cancer reported that acetaldehyde induced upregulation of miRNA30 and miRNA934, which are related with proliferation, cisplatin sensitivity, and tumor suppressor genes.35 The association of cisplatin sensitivity with radiation sensitivity among head and neck cancer patients is well known.36, 37 Taken together, these reports lead us to hypothesize that these genomic alterations induced by heavier acetaldehyde exposure might lead to radiation resistance.
We also found that the impact of alcohol drinking between tongue and non‐tongue oral cavity cancer patients treated by surgery was heterogeneous. Patients with tongue cancer showed a significant association between alcohol consumption and prognosis, whereas those with non‐tongue cancer showed no clear association. The reason for this difference of impact by subsite is unclear. To our knowledge, this is the first study to report this heterogeneity, and further investigations to replicate and explain it are warranted.
Several methodological strengths of our study should be mentioned. First, potential confounding by age, sex, PS, smoking, energy, and SPC were adjusted in the analysis. In particular, SPC was a major alcohol‐related comorbidity,38 so we treated the onset of synchronous SPC as a usual covariate and the onset of metachronous SPC as a time‐varying covariate in multivariable analysis. Second, to exclude the effect of other alcohol‐related comorbidities such as coronary disease, cerebral vessel disease, and pulmonary disease, we also evaluated DSS and DFS, and found a similar tendency. Third, alcohol consumption, the exposure of interest, was measured before treatment, ensuring the chronological relationship between exposure and outcome. Moreover, clinicians associated with the study cases were blinded to exposure status, limiting the possibility of information bias among researchers.
Two potential limitations of this study also warrant mention. First, as previously mentioned, we did not evaluate HPV status, which is potentially related to alcohol consumption in oropharyngeal cancer. Second, our information about drinking was limited to pretreatment behavior and we did not evaluate behavioral change after diagnosis. Evidence is limited about the impact of drinking after diagnosis on prognosis. However, most of the previous studies reported that patients did not change their drinking behavior even after diagnosis of HNSCC.39, 40 For example, López‐Pelayo et al. reported that more than 80% of drinkers continued drinking even after HNSCC was diagnosed.40 In other reports, older patients and those with a longer and heavier drinking habit prior to diagnosis were more likely to continue drinking after diagnosis.39, 41, 42 Therefore, it may be likely that a substantial number of heavy drinkers before diagnosis continued drinking after diagnosis in our study. There have been several reports on the prognostic impact of continued drinking. Continued drinking after diagnosis of HNSCC increases treatment complications, the likelihood of recurrent cancer,43, 44 second primary cancer,45 and the risk of mortality with relative risk 2.7 compared to non‐drinkers.46 By all means, worse prognosis observed in our study might be explained by the potential number of heavy drinkers who continued drinking after diagnosis. Moreover, if the negative association between drinking behavior after diagnosis and prognosis is true and HNSCC patients have the potential to quit drinking after diagnosis, one may say that our evaluation by using drinking behavior before diagnosis might give bias to a null association. Prospective evaluations of alcohol drinking after diagnosis on prognosis are needed.
In conclusion, we found that alcohol consumption was strongly associated with prognosis of HNSCC overall, and that its impact was heterogeneous by treatment method and primary site. Further study to validate these results and clarify the mechanisms behind these observed heterogeneous impacts of alcohol on prognosis by treatment method and primary site is warranted. In particular, we emphasize the importance of evaluation of HPV status in this context to depict the differential sensitivity to RT by alcohol consumption.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Kaplan–Meier survival curve of onset of metachronous cancer in patients with head and neck squamous cell carcinoma.
Fig. S2. Subgroup analysis of impact of drinking categories on overall survival in patients with head and neck squamous cell carcinoma.
Fig. S3. Impact of drinking categories on survival according to treatment method at the primary site in patients with head and neck squamous cell carcinoma.
Fig. S4. Kaplan–Meier survival curves of overall survival (A), disease‐specific survival (B), and disease‐free survival (C) for laryngopharyngeal cancer patients.
Table S1. Interaction between alcohol consumption and potential confounders on overall survival of patients with head and neck squamous cell carcinoma
Acknowledgments
We thank the many doctors, nurses, and technical and administration staff of Aichi Cancer Center Hospital for their daily administration of the HERPACC study. This study was supported by: Grants‐in‐Aid for Scientific Research on Priority Areas and on Innovative Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan; a Grant‐in‐Aid for Scientific Research (grant no. 26253041) from the Ministry of Education, Culture, Sports, Science and Technology; and a Grant‐in‐Aid for the Third Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan. The grantors were not involved in the study design, subject enrollment, study analysis or interpretation, or submission of the manuscript for this study.
Cancer Sci 108 (2017) 91–100
Funding Information
Ministry of Education, Science, Sports, Culture and Technology of Japan; Ministry of Health, Labor and Welfare of Japan.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Registry and Statistics Cancer Information Service, National Cancer Center, Japan.
- 3. Wyss A, Hashibe M, Chuang SC, et al Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol 2013; 178: 679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oze I, Matsuo K, Hosono S, et al Comparison between self‐reported facial flushing after alcohol consumption and ALDH2 Glu504Lys polymorphism for risk of upper aerodigestive tract cancer in a Japanese population. Cancer Sci 2010; 101: 1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen N, Anderson LM, Beland FA, et al Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 2010; 96: 1–1379. [PMC free article] [PubMed] [Google Scholar]
- 6. Leoncini E, Vukovic V, Cadoni G, et al Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol 2015; 39: 367–74. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Mao Y, Zhang Y, et al Alcohol drinking and upper aerodigestive tract cancer mortality: a systematic review and meta‐analysis. Oral Oncol 2014; 50: 269–75. [DOI] [PubMed] [Google Scholar]
- 8. Inoue M, Nagata C, Tsuji I, et al Impact of alcohol intake on total mortality and mortality from major causes in Japan: a pooled analysis of six large‐scale cohort studies. J Epidemiol Community Health 2012; 66: 448–56. [DOI] [PubMed] [Google Scholar]
- 9. Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all‐cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 2010; 21: 2295–302. [DOI] [PubMed] [Google Scholar]
- 10. Kawakita D, Oze I, Hosono S, et al Prognostic value of drinking status and aldehyde dehydrogenase 2 polymorphism in patients with head and neck squamous cell carcinoma. J Epidemiol 2016; 26: 292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancers Cancer v1. 2015. [Accessed 1 January 2016.] Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf).
- 12. Shitara K, Matsuo K, Hatooka S, et al Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci 2010; 101: 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura K, Kodaira T, Tomita N, et al Clinical results of definitive intensity‐modulated radiation therapy for oropharyngeal cancer: retrospective analysis of treatment efficacy and safety. Jpn J Clin Oncol 2015; 46(1): 78–85. [DOI] [PubMed] [Google Scholar]
- 14. Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2009; 74: 1062–9. [DOI] [PubMed] [Google Scholar]
- 15. Tajima K, Hirose K, Inoue M, Takezaki T, Hamajima N, Kuroishi T. A model of practical cancer prevention for out‐patients visiting a hospital: the hospital‐based epidemiologic research program at Aichi cancer center (HERPACC). Asian Pac J Cancer Prev 2000; 1: 35–47. [PubMed] [Google Scholar]
- 16. Hamajima N, Matsuo K, Saito T, et al Gene‐environment Interactions and Polymorphism Studies of Cancer Risk in the Hospital‐based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC‐II). Asian Pac J Cancer Prev 2001; 2: 99–107. [PubMed] [Google Scholar]
- 17. Therasse P, Arbuck SG, Eisenhauer EA, et al New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 18. Cancer IAfRo, Cancer IAfRo . Alcoholic Beverage Consumption and Ethyl Carbamate (Urethane). IARC Monograph 96 on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer (IARC), 2010. [Google Scholar]
- 19. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Association 1958; 53: 457–81. [Google Scholar]
- 20. Kornfehl J, Temmel A, Formanek M, Knerer B. Effects of ethanol treatment of proliferation and differentiation in a head and neck squamous cell carcinoma cell line. Alcohol Clin Exp Res 1999; 23: 1102–7. [PubMed] [Google Scholar]
- 21. Liu Y, Chen H, Sun Z, Chen X. Molecular mechanisms of ethanol‐associated oro‐esophageal squamous cell carcinoma. Cancer Lett 2015; 361: 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol 2005; 35: 195–203. [DOI] [PubMed] [Google Scholar]
- 23. Nozoe T, Korenaga D, Kabashima A, Sugimachi K. Smoking‐related increase of O 6‐methylguanine‐DNA methyltransferase expression in squamous cell carcinoma of the esophagus. Cancer Lett 2002; 184: 49–55. [DOI] [PubMed] [Google Scholar]
- 24. Seitz HK, Stickel F. Molecular mechanisms of alcohol‐mediated carcinogenesis. Nat Rev Cancer 2007; 7: 599–612. [DOI] [PubMed] [Google Scholar]
- 25. Meadows GG, Zhang H. Effects of alcohol on tumor growth, metastasis, immune response, and host survival. Alcohol Res Curr Rev 2015; 37: 311–22. [PMC free article] [PubMed] [Google Scholar]
- 26. Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol 1999; 34: 830–41. [DOI] [PubMed] [Google Scholar]
- 27. Wustrow TP. Antigen‐specific plaques formation of cultured mononuclear cells in head and neck cancer. Acta Otolaryngol 1991; 111: 420–7. [DOI] [PubMed] [Google Scholar]
- 28. Lassen P, Eriksen JG, Hamilton‐Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV‐associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1992–8. [DOI] [PubMed] [Google Scholar]
- 29. Licitra L, Perrone F, Bossi P, et al High‐risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 2006; 24: 5630–6. [DOI] [PubMed] [Google Scholar]
- 30. Petrelli F, Sarti E, Barni S. Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: an updated systematic review and meta‐analysis of 30 trials. Head Neck 2014; 36: 750–9. [DOI] [PubMed] [Google Scholar]
- 31. Andrews E, Seaman WT, Webster‐Cyriaque J. Oropharyngeal carcinoma in non‐smokers and non‐drinkers: a role for HPV. Oral Oncol 2009; 45: 486–91. [DOI] [PubMed] [Google Scholar]
- 32. D'Souza G, Kreimer AR, Viscidi R, et al Case‐control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944–56. [DOI] [PubMed] [Google Scholar]
- 33. Saito Y, Yoshida M, Ushiku T, et al Prognostic value of p16 expression and alcohol consumption in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer 2013; 119: 2005–11. [DOI] [PubMed] [Google Scholar]
- 34. Broglie MA, Soltermann A, Rohrbach D, et al Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity‐modulated chemoradiation. Head Neck 2013; 35: 1698–706. [DOI] [PubMed] [Google Scholar]
- 35. Saad MA, Kuo SZ, Rahimy E, et al Alcohol‐dysregulated miR‐30a and miR‐934 in head and neck squamous cell carcinoma. Mol Cancer 2015; 14: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urba SG, Moon J, Giri PG, et al Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: a Southwest Oncology Group Trial. J Clin Oncol 2005; 23: 88–95. [DOI] [PubMed] [Google Scholar]
- 37. Urba S, Wolf G, Eisbruch A, et al Single‐cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol 2006; 24: 593–8. [DOI] [PubMed] [Google Scholar]
- 38. Chung CS, Liao LJ, Lo WC, et al Risk factors for second primary neoplasia of esophagus in newly diagnosed head and neck cancer patients: a case‐control study. BMC Gastroenterol 2013; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller PM, Day TA, Ravenel MC. Clinical implications of continued alcohol consumption after diagnosis of upper aerodigestive tract cancer. Alcohol Alcohol 2006; 41: 140–2. [DOI] [PubMed] [Google Scholar]
- 40. Lopez‐Pelayo H, Miquel L, Altamirano J, Blanch JL, Gual A, Lligona A. Alcohol consumption in upper aerodigestive tract cancer: Role of head and neck surgeons’ recommendations. Alcohol 2016; 51: 51–6. [DOI] [PubMed] [Google Scholar]
- 41. Gritz ER, Carmack CL, de Moor C, et al First year after head and neck cancer: quality of life. J Clin Oncol 1999; 17: 352. [DOI] [PubMed] [Google Scholar]
- 42. Allison PJ. Factors associated with smoking and alcohol consumption following treatment for head and neck cancer. Oral Oncol 2001; 37: 513–20. [DOI] [PubMed] [Google Scholar]
- 43. Falk RT, Pickle LW, Brown LM, Mason TJ, Buffler PA, Fraumeni JF Jr. Effect of smoking and alcohol consumption on laryngeal cancer risk in coastal Texas. Cancer Res 1989; 49: 4024–9. [PubMed] [Google Scholar]
- 44. Deleyiannis FW‐B, Thomas DB, Vaughan TL, Davis S. Alcoholism: independent predictor of survival in patients with head and neck cancer. J Natl Cancer Inst 1996; 88: 542–9. [DOI] [PubMed] [Google Scholar]
- 45. Do K‐A, Johnson MM, Doherty DA, et al Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control 2003; 14: 131–8. [DOI] [PubMed] [Google Scholar]
- 46. Mayne ST, Cartmel B, Kirsh V, Goodwin WJ Jr. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev 2009; 18: 3368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier survival curve of onset of metachronous cancer in patients with head and neck squamous cell carcinoma.
Fig. S2. Subgroup analysis of impact of drinking categories on overall survival in patients with head and neck squamous cell carcinoma.
Fig. S3. Impact of drinking categories on survival according to treatment method at the primary site in patients with head and neck squamous cell carcinoma.
Fig. S4. Kaplan–Meier survival curves of overall survival (A), disease‐specific survival (B), and disease‐free survival (C) for laryngopharyngeal cancer patients.
Table S1. Interaction between alcohol consumption and potential confounders on overall survival of patients with head and neck squamous cell carcinoma


