Abstract
Clinical development of anti‐angiogenic agents has been a major landmark in cancer therapy for several types of cancers. Signals mediated by both vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP)‐9 and 10 have been implicated in tumor angiogenesis. However, previous studies have shown that targeting the individual signals was not sufficiently effective in retarding tumor growth in certain preclinical and clinical conditions. In the present study, we developed a novel decoy chimeric receptor that traps both VEGF and BMP‐9/10. Single targeting of either VEGF or BMP‐9/10 signals significantly reduced the formation of tumor vessels in a mouse xenograft model of human pancreatic cancer; however, it did not show significant therapeutic effects on tumor growth. In contrast, dual targeting of the angiogenic signals resulted in more significant inhibition of tumor angiogenesis, leading to delay of tumor growth. Our findings suggest that simultaneous blockade of VEGF and BMP‐9/10 signals is a promising therapeutic strategy for the cancers that are resistant to anti‐VEGF and BMP‐9/10 therapies.
Keywords: ALK1, angiogenesis, BMP‐9/10, Fc‐chimera, vascular endothelial growth factor
Tumor vessels provide oxygen and nutrients to cancer cells, and become main routes for cancer cells to metastasize to distant organs.1 Therefore, it is crucial to develop effective strategies to target angiogenic signals in order to inhibit tumor growth and metastasis. During the formation of blood vessels, vascular endothelial growth factor (VEGF) plays central roles in promoting the proliferation and migration of blood vascular endothelial cells through the activation of VEGF receptor 2 (VEGFR2).2 Because of their critical roles in tumor angiogenesis, components of VEGF signaling axis have been attractive antitumor drug targets. Several strategies have been developed to target VEGF signals, including blocking antibodies against VEGF (bevacizumab), kinase inhibitors for VEGFR2, and decoy receptors. Decoy receptor for VEGF, called VEGF‐trap, is a chimeric protein consisting of the second Ig‐like domain of VEGFR1 (Fms‐related tyrosine kinase 1; FLT1), the third Ig‐like domain of VEGFR2, and the Fc portion of human IgG. VEGF‐trap sequestrates VEGF proteins in cancer microenvironment, leading to regression of tumor vessels.
Such inhibitors of VEGF signals have been clinically used for cancer therapies. Therapeutic effects of bevacizumab have been observed when bevacizumab was used in combination with other anticancer drugs.2 However, in some cases, it does not elongate overall survival despite combining with other chemotherapeutic regimens.3 Moreover, increasing lines of evidence have shown that anti‐angiogenic treatments result in the appearance of more aggressive and invasive tumors.4 Recent reports provide several mechanisms underlying the development of tumor resistance against anti‐angiogenic therapies. Growth of tumor vessels depends not only on VEGF but also on various types of other angiogenic factors. Therefore, inhibition of only VEGF‐related signals becomes compensated by other angiogenic factors, implying the importance of combinatorial treatments that target multiple angiogenic pathways. Koh and colleagues reported that dual targeting of VEGF and angiopoietins using double anti‐angiogenic protein consisting of extracellular domains of FLT1 and Tie2, a receptor for angiopoietins, inhibited tumor angiogenesis and metastasis more effectively than single targeting of VEGF or angiopoietins in a xenograft model of human ovarian carcinoma.5 However, identification of new targets and development of more effective methods to inhibit tumor angiogenesis is still highly required.
Multiple lines of evidence have suggested that members of the bone morphogenetic protein (BMP) family, particularly BMP‐9 and ‐10, play important roles in the development and maintenance of vascular systems.6 BMP‐9 and ‐10 transduce their signals through heteromeric complexes of serine/threonine kinase receptors known as type I and type II receptors. In contrast to other members of the BMP family, BMP‐9 and ‐10 preferentially bind to the type I receptor activin receptor‐like kinase 1 (ALK1). ALK1 is preferentially expressed in endothelial cells, and high level of expression has been also found at the site of angiogenesis during embryogenesis. Bone morphogenetic protein‐9 and ‐10 are produced in liver and heart, respectively, and are found in the peripheral blood circulatory system.7 It is of note that BMP‐9 expression is elevated during the progression of endocrine pancreatic tumor in the RIP1‐Tag2 transgenic mouse model.8 We have previously reported that BMP‐9 promotes multiple types of angiogenesis, including tumor angiogenesis, by stimulating the proliferation of endothelial cells.9 In accordance with our findings, pharmacological targeting of ALK1 signals using ALK1‐Fc chimeric protein, RAP‐041, impaired the growth, progression, and metastasis of tumors by inhibition of angiogenesis.8, 10 Targeting BMP‐9/10 signals is now widely recognized as a novel promising anti‐angiogenic therapy in cancer treatment.11, 12, 13 However, it remains unclear whether single targeting of BMP‐9/10/ALK1 signals is sufficient to treat various types of tumors.
In the present study, we examined the effects of targeting VEGF and BMP‐9/10 signals on tumor angiogenesis and growth using xenograft models of human pancreatic carcinomas. We showed that dual targeting of VEGF and BMP‐9/10 signals using Fc chimeric fusion protein was capable of inhibiting the growth of tumors resistant to single targeting of VEGF or BMP‐9/10. These findings suggest that combinatorial therapies are more effective in targeting tumor angiogenesis, and likely applied to the treatment of wide variety of cancers.
Materials and Methods
Cells, cell culture, and reagents
The BxPC3 human pancreatic adenocarcinoma cells were obtained from ATCC (Manassas, VA, USA), and were maintained in RPMI‐1640 supplemented with 10% FBS. Human umbilical vein endothelial cells were purchased from Lonza (Basel, Switzerland) and grown using the EGM‐2 BulletKit (Lonza).14 Both BMP‐9 and VEGF were purchased from R&D Systems (Minneapolis, MN, USA) and used as indicated in Figure 1.
Figure 1.
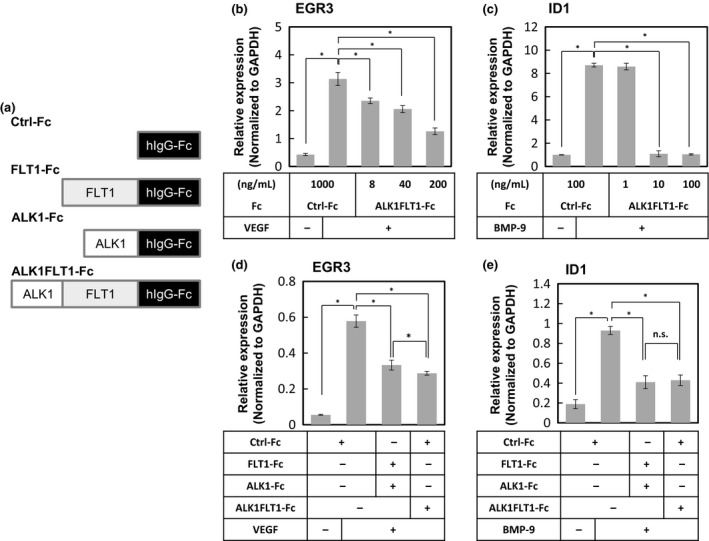
Effects of double decoy receptor ALK1FLT1‐Fc on vascular endothelial growth factor (VEGF) and bone morphogenetic protein‐9 (BMP‐9) signals in endothelial cells. (a) Schematic illustrations of constructed Fc chimeric proteins. (b–e) The conditioned media of HEK293T cells transiently transfected with expression vectors encoding control‐Fc (Ctrl‐Fc), Fms‐related tyrosine kinase 1 (FLT1)‐Fc, activin receptor‐like kinase 1 (ALK1)‐Fc, or ALK1FLT1‐Fc were collected, followed by determination of Fc protein concentrations by ELISA. HUVECs cultured with Fc fusion proteins were treated with BMP‐9 for 4 h or VEGF for 1 h, followed by quantitative RT‐PCR analyses for EGR3 (b, d) and ID1 (c, e), respectively. Error bars indicate SD. *P < 0.05. n.s., not significant.
Lentivirus production and infection
A lentiviral expression system was used to establish the BxPC3 cells expressing Ctrl‐Fc, FLT1‐Fc, ALK1‐Fc, and ALK1FLT1‐Fc chimeric receptors as previously described.9 Briefly, each cDNA was subcloned into the pENTR201 vector (Invitrogen, Carlsbad, CA, USA), and subsequently transferred into the pCSII‐EF‐RfA lentiviral expression vector (a gift from Dr. Hiroyuki Miyoshi, RIKEN). For lentiviral infection, 1 × 105 BxPC3 cells were infected with lentivirus vectors in suspension and plated in 6‐well culture plates.
Production of Fc chimeric protein
HEK293T cells were transfected using FuGENE HD transfection reagent (Promega, Madison, WI, USA) according to the manufacturer's instructions. Four hours after transfection with pCSII‐EF‐RfA encoding Fc chimeric proteins, conditioned medium was changed to serum‐free Opti‐MEM. Supernatant was collected 24 h after the medium change, and the concentrations of Fc chimeric proteins were determined by Human IgG ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX, USA).
Isolation of RNA and RT‐PCR analysis
Total RNAs were extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany). RNAs were reverse‐transcribed by oligo d(T) primer using PrimeScript II 1st strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). Quantitative RT‐PCR analysis was carried out using Power SYBR Green (Applied Biosystems, Foster City, CA, USA) and the ABI PRISM 7500 Fast Real‐Time PCR System (Applied Biosystems). All expression data were normalized to those for GAPDH. The primer sequences are shown in Table S1.
Tumor grafted BALB/c nude mice model
BALB/c female nude mice aged 5–6 weeks were obtained from Oriental Yeast (Tokyo, Japan). A total of 5 × 106 BxPC3 tumor cells in 0.2 mL Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and PBS (1:1) were s.c. inoculated into the left flank of each mouse. Tumor growth was assessed by caliper measurements and calculated from minor axis and major radius.
Immunohistochemistry of tumor section
Staining of tumor sections was done as previously described.9 Briefly, tumor samples excised from BALB/c nude mice were snap‐frozen in a dry‐ice acetone bath. Frozen samples were further sectioned at 10‐μm thickness in a cryostat and subsequently incubated with primary mAbs to platelet and endothelial cell adhesion molecule 1 (PECAM1) (Mec13.3) (BD Pharmingen, Franklin Lakes, NJ, USA), followed by incubation with secondary Alexa Fluor 488 goat anti‐rat IgG (H+L) antibody (Invitrogen). Specimens were then examined using an LSM 510 META confocal microscope (Carl Zeiss, Feldbach, Switzerland). All images were imported into Adobe Photoshop as JPEGs or TIFFs for figure assembly. Images were processed using ImageJ (NIH, https://imagej.nih.gov/ij/) to quantify PECAM1‐positive areas.
Statistical analysis
Values are presented as mean ± SD. Significant differences between means were determined using an unpaired Student's t‐test or one‐way anova followed by the Student–Newman–Keuls test or GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at *P < 0.05.
Results
Functional validation of a novel dual inhibitor of BMP‐9/10 and VEGF signals
In order to target two angiogenic signals mediated by VEGF and BMP‐9/10, we generated a double decoy receptor containing the Fc region of human IgG1 fused to the second immunoglobulin‐like (Ig2) domain of human FLT1, and an extracellular domain of human ALK1 (ALK1FLT1‐Fc) (Fig. 1a). We examined its ability to target VEGF and BMP‐9/10 signals simultaneously, using conditioned medium from the HEK293T cells transfected with expression vectors encoding control Fc proteins (Ctrl‐Fc) and the ALK1FLT1‐Fc fusion proteins. When HUVECs were treated with VEGF (Fig. 1b) and BMP‐9 (Fig. 1c), the expression of EGR3, one of the early growth response family of transcription factors known to be rapidly induced by VEGF,15 and that of ID1, a target gene of BMP family signals, were upregulated in the presence of Ctrl‐Fc. The ALK1FLT1‐Fc fusion protein decreased the expression of EGR3 (Fig. 1b) and ID1 (Fig. 1c) induced by VEGF and BMP‐9, respectively, in a dose‐dependent manner. Furthermore, in order to compare the inhibitory potential of dual ALK1FLT1‐Fc trap with those of single Fc traps, we prepared VEGF‐trap (FLT1‐Fc) and BMP‐9/10 trap (ALK1‐Fc) (Fig. 1a). We found that ALK1FLT1‐Fc is capable of targeting VEGF (Fig. 1d) and BMP‐9 (Fig. 1e) signals to the same extent as the combination of equivalent amounts of FLT1‐Fc and ALK1‐Fc. These results suggest that ALK1FLT1‐Fc functions as a potent dual inhibitor of VEGF and BMP‐9/10.
Tumor angiogenesis significantly reduced by ALK1FLT1‐Fc in a mouse xenograft model of human pancreatic cancer
We next characterized the anti‐angiogenic potential of ALK1FLT1‐Fc using a tumor xenograft model. In order to examine their potentials, we used BxPC3 human pancreatic tumor cells. As these cells contain a homozygous deletion of the SMAD4 gene,16 Fc chimeric proteins secreted from BxPC3 cells cannot modulate the Smad pathways by themselves. BxPC3 human pancreatic carcinoma cells were transduced with lentiviruses expressing Ctrl‐Fc, FLT1‐Fc, ALK1‐Fc, and ALK1FLT1‐Fc, and transplanted to immunodeficient mice, followed by evaluation of blood vessel formation. As shown in Figure 2, BxPC3 FLT1‐Fc and ALK1‐Fc tumors showed significant and similar levels of decrease in PECAM1‐positive areas as compared to that of BxPC3 Ctrl‐Fc. Introduction of ALK1FLT1‐Fc resulted in the most significant decrease of tumor angiogenesis among all of the Fc‐traps analyzed (Fig. 2), suggesting that dual targeting of VEGF and BMP‐9/10 in the cancer microenvironment inhibits tumor angiogenesis more effectively than single targeting.
Figure 2.
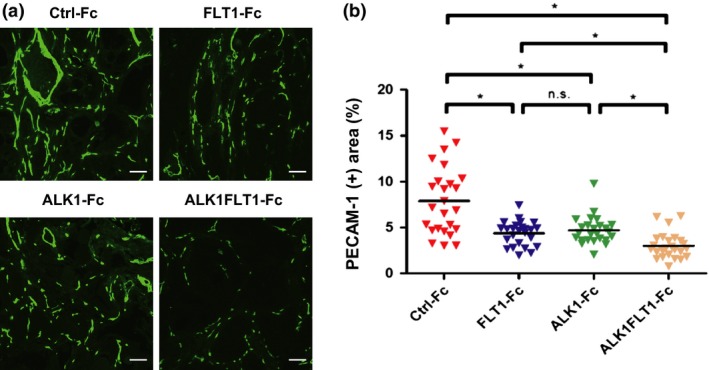
Effects of various Fc chimeric proteins on tumor angiogenesis in a mouse xenograft model of human pancreatic cancer. BxPC3 cells expressing control (Ctrl‐Fc), Fms‐related tyrosine kinase 1 (FLT1)‐Fc, activin receptor‐like kinase 1 (ALK1)‐Fc or ALK1FLT1‐Fc were inoculated s.c. in BALB/c nude mice. After 7 weeks of BxPC3 cell inoculations, tumors were excised and examined for vascular density. (a) Immunostaining for platelet and endothelial cell adhesion molecule 1 (PECAM1; green) of sections obtained from each type of BxPC3 tumor. Scale bar = 100 μm (n = 4 for each group). (b) Graphic representation of PECAM1‐positive area (%). Each value represents the positive area in each observed field. *P < 0.05. n.s., not significant.
Dual inhibition of VEGF and BMP‐9/10 signals inhibits growth of human pancreatic tumor xenografts
We also studied the effect of ALK1FLT1‐Fc on tumor growth in a human pancreatic tumor xenograft model. Growth of tumors derived from BxPC3 cells expressing only FLT1‐Fc or ALK1‐Fc did not differ from that of Ctrl‐Fc (Fig. 3). However, expression of the dual ALK1FLT1‐Fc trap significantly retarded the growth of tumor xenografts, likely due to effective inhibition of tumor angiogenesis by dual targeting of VEGF and BMP‐9/10, as shown in Figure 2.
Figure 3.
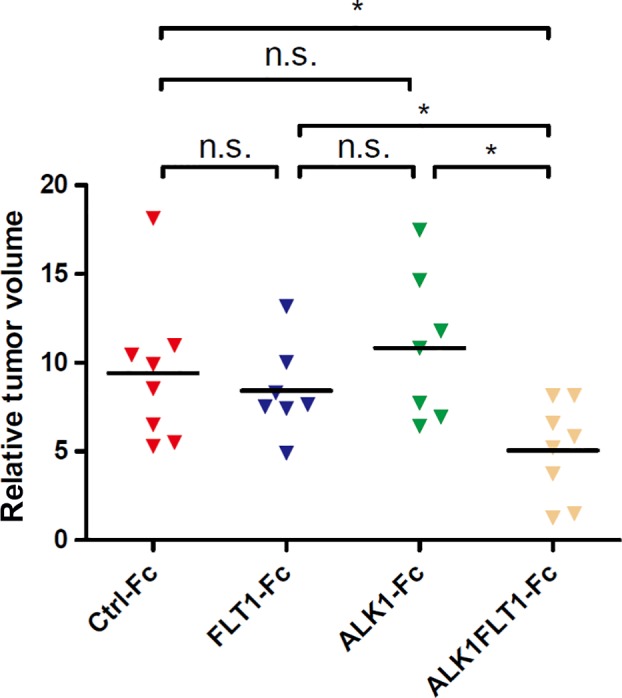
Effects of various Fc chimeric proteins on the growth of BxPC3 human pancreatic cancer xenografts. BxPC3 cells expressing control (Ctrl)‐Fc, activin receptor‐like kinase 1 (ALK1)‐Fc, Fms‐related tyrosine kinase 1 (FLT1)‐Fc, or ALK1FLT1‐Fc were inoculated s.c. in BALB/c nude mice. Tumor growth was assessed on day 49 after transplantation by caliper measurements and calculated from minor axis and major radius. *P < 0.05. n.s., not significant.
Discussion
Both VEGF and BMP‐9/10 signals have been implicated in tumor angiogenesis, and have been targeted to successfully retard the growth of multiple types of cancers.2, 8, 10 However, in the present study, single targeting of either ALK1‐Fc or FLT1‐Fc did not impair tumor growth in a BxPC3 xenograft model (Fig. 3) regardless of their ability to inhibit tumor angiogenesis (Fig. 2). Previous reports have also shown that targeting of VEGF signals only did not show clear antitumor effects in multiple human cancer xenograft models.2 Several lines of evidence show that multiple angiogenic factors compensate the angiogenic activities of VEGF after anti‐VEGF therapies. In order to target multiple angiogenic signals simultaneously, multikinase inhibitors such as sunitinib (an inhibitor of VEGFR and platelet‐derived growth factor receptors) have been developed and already launched for cancer therapies.
Dalantercept (ACE‐041), a human counterpart of RAP‐041 (ALK1‐Fc decoy receptor), has been preclinically and clinically evaluated as a novel anti‐angiogenic agent in various types of cancers.17 However, phase II evaluation of dalantercept for the monotherapy treatment of advanced or recurrent endometrial cancer has shown its insufficient activity to warrant further investigation.18 Combination therapies appear to be more promising. Indeed, an ongoing phase II study in renal carcinoma based on treatment with dalantercept in combination with axitinib resulted in significant reduction of tumor vessel formation19, which would support our concept of involvement of BMP‐9/10 and VEGF signals.
Recently, the treatment cost of biotechnology‐based drugs has become a serious problem; the combination of them is not acceptable for most cancer patients without financial support. Therefore, dual targeting drugs, such as VEGF and angiopoietin‐2 bispecific antibody20 and ALK1FLT1‐Fc in the present study, are expected to be novel therapies from the aspect of efficacy as well as cost. Further preclinical and clinical investigations are warranted to explore application of ALK1FLT1‐Fc in the treatment of various types of cancers.
Disclosure Statement
Y.A. is an employee of Nippon Kayaku, Co., Ltd. The other authors have no conflict of interest.
Supporting information
Table S1. List of PCR primers used in this study.
Acknowledgments
We thank Mr. Yasuyuki Morishita for technical assistance, Dr. Hiroyuki Miyoshi for the lentiviral vectors, Dr. Katarzyna Anna Inoue for manuscript preparation, and the members of Department of Molecular Pathology of the University of Tokyo, Laboratory of Oncology of Tokyo University of Pharmacy and Life Sciences, and Department of Biochemistry of Tokyo Medical and Dental University for critical discussion. This research was supported by Grants‐in‐Aid for Scientific Research on Innovative Areas, Integrative Research on Cancer Microenvironment Network (22112002) (to K.M.) and Cellular, and Molecular Basis for Neuro‐vascular Wiring (23122504) (to T.W.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Japan Science Technology Agency, Precursory Research for Embryonic Science and Technology (to T.W.), Japan Society for the Promotion of Science Core‐to‐Core Program “Cooperative International Framework in TGF‐β Family Signaling” (to K.M.), and the Yasuda Medical Foundation (to K.M.).
Cancer Sci 108 (2017) 151–155
Funding Information
Ministry of Education, Culture, Sports, Science, and Technology of Japan (22112002, 23122504); Japan Science Technology Agency; Japan Society for the Promotion of Science; Yasuda Medical Foundation
Contributor Information
Kohei Miyazono, Email: miyazono@m.u-tokyo.ac.jp.
Tetsuro Watabe, Email: t-watabe@umin.ac.jp.
References
- 1. Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 2010; 10: 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shibuya M, Claesson‐Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 2006; 312: 549–60. [DOI] [PubMed] [Google Scholar]
- 3. Miller K, Wang M, Gralow J et al Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357: 2666–76. [DOI] [PubMed] [Google Scholar]
- 4. Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koh YJ, Kim HZ, Hwang SI et al Double antiangiogenic protein, DAAP, targeting VEGF‐A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell 2010; 18: 171–84. [DOI] [PubMed] [Google Scholar]
- 6. Ye L, Jiang WG. Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett 2016; 380: 586–97. [DOI] [PubMed] [Google Scholar]
- 7. David L, Mallet C, Mazerbourg S et al Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor‐like kinase 1 (ALK1) in endothelial cells. Blood 2007; 109: 1953–61. [DOI] [PubMed] [Google Scholar]
- 8. Cunha SI, Pardali E, Thorikay M et al Genetic and pharmacological targeting of activin receptor‐like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 2010; 207: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki Y, Ohga N, Morishita Y et al BMP‐9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci 2010; 123: 1684–92. [DOI] [PubMed] [Google Scholar]
- 10. Cunha SI, Bocci M, Lövrot J et al Endothelial ALK1 is a therapeutic target to block metastatic dissemination of breast cancer. Cancer Res 2015; 75: 2445–56. [DOI] [PubMed] [Google Scholar]
- 11. Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 2011; 117: 6999–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bendell JC, Gordon MS, Hurwitz HI et al Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor‐like kinase‐1 ligand trap, in patients with advanced cancer. Clin Cancer Res 2014; 20: 480–9. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt RS, Atkins MB. Molecular pathways: can activin‐like kinase pathway inhibition enhance the limited efficacy of VEGF inhibitors? Clin Cancer Res 2014; 20: 2838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshimatsu Y, Lee YG, Akatsu Y et al Bone morphogenetic protein‐9 inhibits lymphatic vessel formation via activin receptor‐like kinase 1 during development and cancer progression. Proc Natl Acad Sci USA 2013; 110: 18940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suehiro J, Hamakubo T, Kodama T et al Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response‐3. Blood 2010; 115: 2520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleeff J, Wildi S, Friess H et al Ligand induced upregulation of the type II transforming growth factor (TGF‐β) receptor enhances TGF‐β responsiveness in COLO‐357 cells. Pancreas 1999; 18: 364–70. [DOI] [PubMed] [Google Scholar]
- 17. Gupta S, Gill D, Pal SK et al Activin receptor inhibitors–dalantercept. Curr Oncol Rep 2015; 17: 14. [DOI] [PubMed] [Google Scholar]
- 18. Makker V, Filiaci VL, Chen LM et al Phase II evaluation of dalantercept, a soluble recombinant activin receptor‐like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study 0229N. Gynecol Oncol 2015; 138: 24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henner Voss M, Plimack ER, Rini BI et al DART Study: A phase II randomized trial of dalantercept plus axitinib versus placebo plus axitinib in advanced clear cell renal cell carcinoma (RCC): Results from Part 1. J Clin Oncol 33, 2015; suppl 7; abstr 407 http://meetinglibrary.asco.org/content/141662-159 [Google Scholar]
- 20. Kontermann RE. Dual targeting strategies with bispecific antibodies. mAbs 2012; 4: 182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of PCR primers used in this study.


