Abstract
Wnt5b is a member of the same family of proteins as Wnt5a, the overexpression of which is associated with cancer aggressiveness. Wnt5b is also suggested to be involved in cancer progression, however, details remain unclarified. We analyzed the biochemical properties of purified Wnt5b and the mode of secretion of Wnt5b by cancer cells. Wnt5b was glycosylated at three asparagine residues and lipidated at one serine residue, and these post‐translational modifications of Wnt5b were essential for secretion. Purified Wnt5b showed Dvl2 phosphorylation and Rac activation abilities to a similar extent as Wnt5a. In cultured‐cell conditioned medium, Wnt5b was detected in supernatant or precipitation fractions that were separated by centrifugation at 100 000 g. In PANC‐1 pancreatic cancer cells, 55% of secreted endogenous Wnt5b was associated with exosomes. Exosomes from wild‐type PANC‐1 cells, but not those from Wnt5b‐knockout PANC‐1 cells, activated Wnt5b signaling in CHO cells and stimulated migration and proliferation of A549 lung adenocarcinoma cells, suggesting that endogenous, Wnt5b‐associated exosomes are active. The exosomes were taken up by CHO cells and immunoelectron microscopy revealed that Wnt5b is indeed associated with exosomes. In Caco‐2 colon cancer cells, most Wnt5b was recovered in precipitation fractions when Wnt5b was ectopically expressed (Caco‐2/Wnt5b cells). Knockdown of TSG101, an exosome marker, decreased the secretion of Wnt5b‐associated exosomes from Caco‐2/Wnt5b cells and inhibited Wnt5b‐dependent cell proliferation. Exosomes secreted from Caco‐2/Wnt5b cells stimulated migration and proliferation of A549 cells. These results suggest that Wnt5b‐associated exosomes promote cancer cell migration and proliferation in a paracrine manner.
Keywords: CRISPR, exosome, TSG101, Wnt signaling, Wnt5b
Wnts are secretory proteins that are conserved evolutionally and regulate at least two intracellular signaling pathways: β‐catenin‐dependent and –independent.1, 2 Among 19 human or mouse Wnt family members, Wnt1 and Wnt3a activate the β‐catenin‐dependent pathway, whereas Wnt5a and Wnt11 activate the β‐catenin‐independent pathway.3, 4 Wnt5a is one of the most extensively studied Wnts and has been shown to be essential for embryonic development. Wnt5a is also involved in several post‐natal diseases, including cancer and inflammatory diseases.4, 5, 6 Wnt5b is a Wnt5a family member (80% identity) in mammals;7 one Wnt5 also exists in Drosophila and zebrafish (pipetail).8, 9 Wnt5b is essential for convergent extension in zebrafish,9 whereas in the mouse, Wnt5a, but not Wnt5b, is essential for development.10, 11
Evidence has accumulated that Wnt5b expression is involved in mouse chondrocyte maturation12, 13 and is associated with human diabetic mellitus and adipogenesis.14, 15 Similar to Wnt5a,16, 17, 18, 19, 20 it is likely that Wnt5b is involved in cancer progression. Periostin, interferon‐induced transmembrane protein 1, and Wnt5b were reported to be highly expressed in head and neck squamous cell carcinomas; their expression promoted the invasive ability of head and neck squamous cell carcinomas cells by inducing MMP‐10.21, 22 Wnt5b was also highly expressed in breast cancer with high invasive activity, and Wnt5b expression in basal‐like cancers was associated with brain metastasis.23 In breast cancers and mammary stem cells, secreted MMP‐3 interacted with and inactivated Wnt5b, thereby enhancing the β‐catenin‐dependent pathway.24 Lung cancer and pancreatic cancer cells produced Wnt5b, which promoted invasion activity, when they were treated with transforming growth factor‐β (TGF‐β).25 However, the biochemical properties and cancer‐associated activities of Wnt5b have not been well characterized compared with Wnt5a because Wnt5b has not yet been purified.
Wnt proteins are post‐translationally glycan‐ and lipid‐modified secretory proteins that are ready to self‐aggregate and bind heparin sulfate proteoglycans.26, 27, 28 Therefore, Wnts are not easily diffusible in the extracellular milieu. Several possible mechanisms have been proposed to explain the long‐distance movements of Wnts. Lipoprotein particles are large, globular complexes composed of a central core of hydrophobic lipids that are associated with apoproteins and surrounded by a monolayer of membrane phospholipids. In Drosophila, the Wingless (Wg) protein derived from wing discs was found to be copurified with lipoprotein particles.29 Larvae with reduced lipoprotein particles showed a narrow expression of Distalless, a target gene of Wnt signaling, in wing porch. In mammals, Wnt3a was released from mouse fibroblasts by the presence of lipoproteins in the culture medium, specifically through high‐density lipoprotein particles.30 A Wnt3a mutant that lacked palmitate was not associated with lipoproteins. The lipid modification of Wnt3a could be important for targeting Wnt3a to the phospholipids of lipoproteins. These results suggest that lipoprotein particles act as machinery for the efficient intercellular movement and stabilization of Wnt3a/Wg. In addition, it has been reported that some proteins, including swim and afamin, bound to and solubilized Wg and Wnts.31, 32
It is also possible that Wnt is secreted and spread by being attached to membranous vesicles.33 A substantial fraction of Wnts were found on extracellular vesicles known as exosomes.34, 35 As intraluminal vesicles that originate in large multivesicular bodies (MVBs), exosomes are released in the extracellular milieu following fusion of MVBs with cell surface membranes.36, 37 In larval neuromuscular junctions, Wg was released with Evi/Wntless‐containing extracellular vesicles and traveled from one cell to another at the synaptic cleft.38 Subsequent electron microscopy and proteomics studies revealed that these vesicles are exosomes.39 In addition, Wnt3a and Wg are observed on exosomes released from mammalian L and HEK293 and Drosophila S2 cells.40, 41 Fibroblasts secreted exosomes, which were internalized by breast cancer cells (BCCs) and associated with Wnt11. Exosome‐associated Wnt11, in turn, promoted BCC protrusion activity and motility to drive invasive behavior.42 Thus, exosome‐associated Wnt ligands could be involved in intercellular communication. However, whether Wnt5b is secreted with exosomes is unclear; if so, whether Wnt5b‐associated exosomes show some activity remains to be clarified.
Here, we characterized purified Wnt5b and found that Wnt5b is secreted with exosomes in a cell context. We also showed that Wnt5b‐associated exosomes promote cancer cell migration and proliferation in a paracrine manner.
Materials and Methods
Purification of Wnt5b
Wnt5b was purified to near homogeneity from Wnt5b conditioned medium (CM) through three successive column chromatography, including Blue Sepharose HP (GE Healthcare Bio‐Sciences, Buckinghamshire, UK), HiLoad Superdex 200 (GE Healthcare Bio‐Sciences) and HiTrap Heparin (GE Healthcare Bio‐Sciences) columns. Details of the purification of Wnt5b are described in Data S1.
Isolation of exosome fraction
Conditioned media from cultured cells were subjected to sequential centrifugation steps of 2000 and 10 000 g. The obtained supernatant was further centrifuged at 100 000 g in a SW55Ti or SW32Ti swinging bucket rotor (Beckman Coulter, Brea, CA, USA) for 3 h as described.43 Proteins of the supernatant were precipitated with Blue Sepharose to detect Wnts (indicated as “Sup” in figures). The precipitates were suspended in 1/1000 of their original volume in PBS (indicated as “P100” in figures) and considered exosomes.
For sucrose density gradient ultracentrifugation of exosome fractions, P100 was loaded on top of a discontinuous sucrose gradient (0.25–2.5 M) and centrifuged at 100 000 g in a SW55Ti swinging bucket rotor for 3 h.44 Eleven fractions of 1 mL were collected and protein contents were enriched by centrifugation at 100 000 g.
Semiquantitative RT‐PCR
Semiquantitative RT‐PCR was carried out as described previously.45 Forward and reverse primers are listed in Table S1.
Knockdown by siRNA
CHO and Caco‐2 cells were transfected with a mixture of siRNAs (20 nM each) against genes of interest using RNAiMAX (Invitrogen, Grand Island, NY, USA) and the cells were used for experiments 48 h post‐transfection. Target sequences are listed in Table S2.
Supplementary materials and methods are described in Data S1.
Results
Purification and characterization of Wnt5b
To characterize Wnt5b biochemically, we first purified Wnt5b from CM of L cells that stably expressed Wnt5b (L/Wnt5b cells) by the use of three successive conventional column chromatographies (Table 1, Fig. 1a). Using peptides based on different amino acid sequences between Wnt5a and Wnt5b as antigens, anti‐Wnt5 antibodies that specifically recognized Wnt5a and Wnt5b were generated (Fig. 1b). Purified Wnt5b induced Dvl2 phosphorylation in NIH3T3 cells (Fig. S1a) and activated Rac in HeLaS3 cells to a similar extent as purified Wnt5a (Fig. S1b).
Table 1.
Purification of Wnt5b
| Volume, mL | Protein concentration, mg/mL | Total protein, mg | Wnt5b concentration, μg/mL | Wnt5b, μg | |
|---|---|---|---|---|---|
| Wnt5b CM | 20 000 (in total) | 4.67 | 93 400 | 0.0288 | 576.0 |
| Blue Sepharose | 625 (in total) | 23.70† | 14.800 | 0.3620 | 226.5 |
| Gel filtration | 80 (in total) | 32.30† | 2.650 | 2.2100 | 176.8 |
| Heparin Sepharose | 3.0 | 52.00† | 0.156 | 50.0000 | 150.0 |
Concentration of Wnt5b protein in the conditioned medium was determined by comparing its signal intensity on a Wnt5b immunoblot to that of serial dilutions of a known amount of purified Wnt5b protein. †Concentration in μg/mL.
Figure 1.
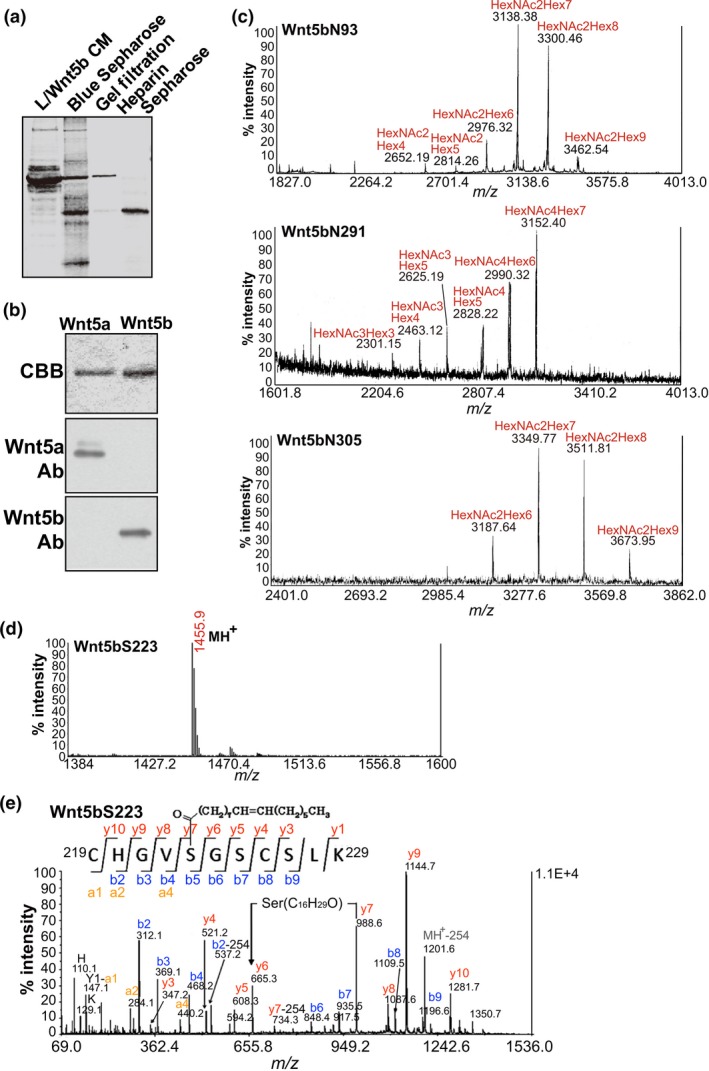
Determination of the glycan profiles of Wnt5b. (a) Coomassie Brilliant Blue (CBB) staining of an SDS polyacrylamide gel containing fractions from all chromatographies in the purification process. CM, conditioned medium. (b) Purified Wnt5a and Wnt5b proteins were stained with CBB and probed with anti‐Wnt5a and anti‐Wnt5b antibodies. (c) Wnt5b glycopeptides obtained from nano‐flow liquid chromatography were separately analyzed by MALDI mass spectrometry (MS). (d) MALDI mass spectrum of a peptide containing Ser223 of Wnt5b. (e) MALDI‐MS/MS spectrum from the ion at m/z 1455.9 in (d). The sequences from the N‐ and C‐termini and the position of the modified residue were read out based on y’’l and b m ions.
Wnt5b has four possible N‐glycosylation sites, namely Asn‐X‐Ser/Thr, including N93, N99, N291, and N305. Wnt5b peptides, which were digested with trypsin or Glu‐C, were subjected to nano‐flow liquid chromatography (LC) followed by MALDI mass spectrometry (MS)/MS and nano‐flow LC/electrospray ionization MS (LC/ESI‐MS). Approximately 70% of the entire Wnt5b amino acid sequence was covered (Table 2). Among the four possible glycosylation sites, N93, N291, and N305 were glycosylated with high‐mannose‐, hybrid‐, and high‐mannose‐type oligosaccharides, respectively (Fig. 1c). The MALDI‐MS/MS analysis of the peptide containing N93 and N99 observed at m/z 3300.46 revealed that N99 is not glycosylated (Fig. S1c). Further analyses of digested peptides using nano‐flow reversed‐phase LC and MALDI‐MS/MS confirmed that glycans were attached to N93, N291, and N305 of Wnt5b (Table 3).
Table 2.
Summary of observed masses of digested peptides of human Wnt5b, fractionated by nano‐flow reversed‐phase liquid chromatography, and their corresponding theoretical masses analyzed by MALDI mass spectrometry and electrospray ionization mass spectrometry
| Observed molecular mass | Theoretical molecular mass | Sequence | Modification | Position |
|---|---|---|---|---|
| 2152.15 | 2152.05 | KLC†QLYQEHMAYIGEGAK | 60–77 | |
| 1416.77 | 1416.69 | TGIKECQHQFR | 78–88 | |
| 2652.19 | 2652.06 | WNCSTADNASVFGR | HexNAc2Hex4 | 92–105 |
| 2814.26 | 2814.12 | HexNAc2Hex5 | ||
| 2976.32 | 2976.17 | HexNAc2Hex6 | ||
| 3138.38 | 3138.22 | HexNAc2Hex7 | ||
| 3300.46 | 3300.28 | HexNAc2Hex8 | ||
| 3462.54 | 3462.33 | HexNAc2Hex9 | ||
| 789.46 | 789.42 | VMQIGSR | 106–112 | |
| 1900.06 | 1899.98 | ETAFTHAVSAAGVVNAISR | 113–131 | |
| 1282.59 | 1282.53 | EGELSTCGCSR | 135–145 | |
| 2441.18 | 2441.09 | DLPRDWLWGGCGDNVEYGYR | 151–170 | |
| 735.39 | 735.36 | EFVDARER | 174–181 | |
| 1513.86 | 1513.78 | VLMNLQNNEAGRR | 195–207 | |
| 635.41 | 635.38 | RAVYK | 207–211 | |
| 807.40 | 807.36 | MADVACK | 212–218 | |
| 1454.90 | 1454.76 | CHGVSGSCSLK | C16:1‐fatty acid | 219–229 |
| 1464.83 | 1464.76 | TCWLQLAEFRK | 230–240 | |
| 1140.58 | 1140.52 | EKYDSAAAMR | 247–256 | |
| 1042.64 | 1042.59 | GRLELVNSR | 261–269 | |
| 2525.29 | 2525.18 | FTQPTPEDLVYVDPSPDYCLR | 270–290 | |
| 2301.15 | 2300.96 | NESTGSLGTQGR | HexNAc3Hex3 | 291–302 |
| 2463.12 | 2463.01 | HexNAc3Hex4 | ||
| 2625.19 | 2625.07 | HexNAc3Hex5 | ||
| 2828.22 | 2828.14 | HexNAc4Hex5 | ||
| 2990.32 | 2990.20 | HexNAc4Hex6 | ||
| 3152.40 | 3152.25 | HexNAc4Hex7 | ||
| 3187.64 | 3187.34 | STGSLGTQGRLCNKTSE‡ | HexNAc2Hex6 | 293–309 |
| 3349.77 | 3349.40 | HexNAc2Hex7 | ||
| 3511.81 | 3511.45 | HexNAc2Hex8 | ||
| 3673.95 | 3673.50 | HexNAc2Hex9 | ||
| 1803.75 | 1803.67 | TSEGMDGCELMCCGR | 307–321 | |
| 755.40 | 755.36 | GYNQFK | 322–327 | |
| 1238.61 | 1238.55 | FHWCCFVR | 338–345 | |
| 1583.85 | 1583.77 | KCTEIVDQYICK | 348–359 |
†Cys (C) is an S‐(propionamide) cysteine. ‡Peptide was obtained from the Glu‐C digest of Wnt5b.
Table 3.
Summary of observed and theoretical masses of glycopeptides of human Wnt5b and the relative abundance of each glycoform observed in MALDI mass spectrometry
| Observed molecular mass | Theoretical molecular mass | Sequence | Modification | Position | Relative abundance (%) |
|---|---|---|---|---|---|
| 2652.19 | 2652.06 | WNC†STADNASVFGR | HexNAc2Hex4 | N93 | 4.3 |
| 2814.26 | 2814.12 | HexNAc2Hex5 | 2.6 | ||
| 2976.32 | 2976.17 | HexNAc2Hex6 | 12.0 | ||
| 3138.38 | 3138.22 | HexNAc2Hex7 | 41.2 | ||
| 3300.46 | 3300.28 | HexNAc2Hex8 | 35.4 | ||
| 3462.54 | 3462.33 | HexNAc2Hex9 | 4.4 | ||
| 2301.15 | 2300.96 | NESTGSLGTQGR | HexNAc3Hex3 | N291 | 5.3 |
| 2463.12 | 2463.01 | HexNAc3Hex4 | 9.9 | ||
| 2625.19 | 2625.07 | HexNAc3Hex5 | 10.6 | ||
| 2828.22 | 2828.14 | HexNAc4Hex5 | 7.3 | ||
| 2990.32 | 2990.20 | HexNAc4Hex6 | 23.9 | ||
| 3152.40 | 3152.25 | HexNAc4Hex7 | 43.0 | ||
| 3187.64 | 3187.34 | STGSLGTQGRLCNKTSE‡ | HexNAc2Hex6 | N305 | 15.1 |
| 3349.77 | 3349.40 | HexNAc2Hex7 | 37.4 | ||
| 3511.81 | 3511.45 | HexNAc2Hex8 | 37.4 | ||
| 3673.95 | 3673.50 | HexNAc2Hex9 | 10.2 |
†Cys (C) is an S‐(propionamide) cysteine. ‡Peptide was obtained from the Glu‐C digest of Wnt5b.
A peptide containing S223, observed at m/z 1455.9 (Fig. 1d), was identified as a peptide with a monounsaturated fatty acid (C16:1, palmitoleic acid) (Fig. 1e), indicating that Wnt5b was modified with palmitoleic acid as well as Wnt3a, Wnt5a, and Wnt11.46, 47
Wnt secretion modes vary among cell lines
Wnts are secreted into the extracellular space through secretory vesicles or extracellular microvesicles.33 In cultured cells, Wnts were reported to be secreted with extracellular microvesicles, such as exosomes, which were recovered in the 100 000 g centrifugation precipitation fractions from CM. These fractions contained clathrin and tumor susceptibility gene 101 (TSG101), which play roles in the trafficking of exosomes.48, 49 Wnts released by the fusion of secretory vesicles with cell surface membranes were detected in supernatant fractions. In L cells stably expressing Wnts, most Wnt5b, Wnt5a, and Wnt3a proteins were recovered in supernatant fractions; Wnts were not detected in precipitation fractions, or only a small fraction was detected (Fig. 2a). In MDCK cells expressing Wnts, almost all Wnt1, Wnt3a, Wnt5a, and Wnt11 proteins were recovered in supernatant fractions (Fig. S2a). Some cancer cell lines, including HeLaS3 cervical cancer, A549 lung adenocarcinoma, and KKLS gastric cancer cells, expressed Wnt5a endogenously,16, 20, 50 and most Wnt5a secreted in CM was present in supernatant fractions (Fig. 2b). Therefore, in these cells, Wnt1, Wnt3a, Wnt5a, and Wnt11 might not be associated with extracellular microvesicles prepared by 100 000 g centrifugation, but it is possible that there are microvesicles associated with other Wnts in supernatant fractions when exosomes are prepared by other methods.51
Figure 2.
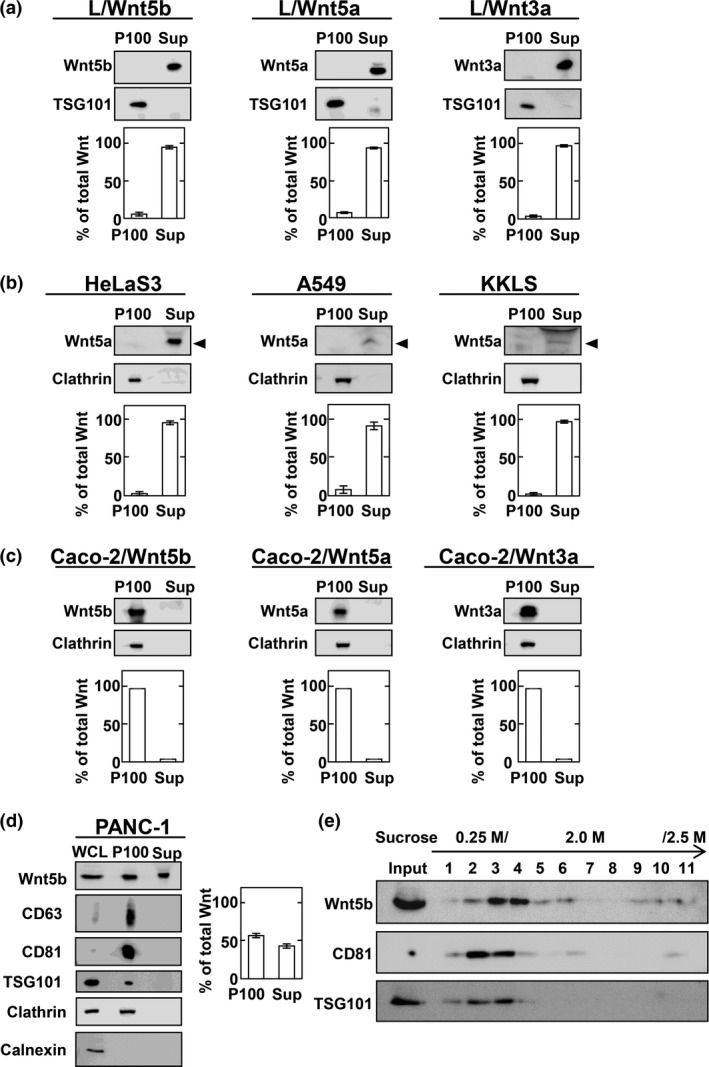
Wnt secretion modes vary among cell lines. (a) Conditioned medium (CM) of L cells stably expressing Wnts was separated into 100 000 g precipitates (P100) and supernatants (Sup). Each fraction was probed with anti‐Wnt5a/b or anti‐Wnt3a antibodies. Wnt signals were quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA) and results expressed as the ratio of Wnts in P100 and Sup fractions. The sum of Wnts secreted from cultured cells into P100 and Sup fractions was set to 100%. Results are shown as means ± SD of three independent experiments. (b) P100 and Sup prepared from HeLaS3, A549, and KKLS cells were probed with anti‐Wnt5a/b antibody. Arrowheads indicate the position of Wnt5a. (c) P100 and Sup from Caco‐2 cells transiently expressing indicated Wnts were probed with anti‐Wnt5a/b or anti‐Wnt3a antibodies. (d) P100 and Sup prepared from PANC‐1 cells were probed with the indicated antibodies. (e) P100 prepared from PANC‐1 cells was subjected to discontinuous sucrose gradient analysis, and samples of fractions were probed with the indicated antibodies.
Almost all Wnts were recovered in precipitation fractions when Wnt5b, Wnt5a, and Wnt3a were transiently expressed in Caco‐2 colon cancer cells (Fig. 2c). However, Wnt5b NQ (N93Q, N99Q, N291Q, and N305Q) mutant that lacks glycosylation and Wnt5b S223A mutant that lacks lipidation were hardly recovered in precipitation and supernatant fractions of CM (Fig. S2b,c), suggesting that glycosylation and lipidation are essential for Wnt5b secretion and its association with exosomes.
To analyze endogenous Wnt5b on exosomes, the expression levels of Wnt5b mRNA were quantified in various cancer cell lines. Among various types of cancer cells, Wnt5b mRNA was highly expressed endogenously in PANC‐1 cells (Fig. S2d). Wnt5b mRNA expression was the highest in PANC‐1 cells compared with other Wnt mRNA (Fig. S2d). Approximately 55% of endogenous Wnt5b released from PANC‐1 cells was recovered in precipitation fractions, which also contained CD63, CD81, and TSG101 that are known to be present on exosomes (Fig. 2d).48, 49, 52, 53 Therefore, whether secreted Wnts are recovered in supernatant or precipitation fractions depends on cell types. Wnt5b recovered in precipitation fractions from CM of PANC‐1 cells was subjected to discontinuous sucrose density gradient analysis, which revealed that CD81 and TSG101 are partially overlapped with Wnt5b (Fig. 2e). The reason why Wn5b and exosome markers, such as CD81 and TSG101, were not co‐sedimented, was unclear, but it might be due to Wnt5b not only being associated with exosomes containing CD81 and TSG101, but also other exosomes.
Development of a reporter assay system to measure Wnt5a and Wnt5b signaling
As Wnt5b‐associated exosomes prepared from 50 mL CM did not show Dvl2 phosphorylation or Rac activation activities (data not shown), we developed a highly sensitive reporter system to measure Wnt5a and Wnt5b signaling using CHO cells. As shown in a previous report,54 the N‐terminal region of Ror2 was fused with the transmembrane and intracellular region of low‐density lipoprotein receptor‐related protein 6 (LRP6) to thereby construct a Ror2‐LRP6 chimera (Fig. 3a). The chimera construct was stably expressed in CHO cells that had been already transfected with a TOP‐flash gene, which is a luciferase reporter plasmid that contains wild‐type T‐cell factor binding regions (CHO/Ror2‐LRP6‐TOP cells; Fig. 3a).
Figure 3.
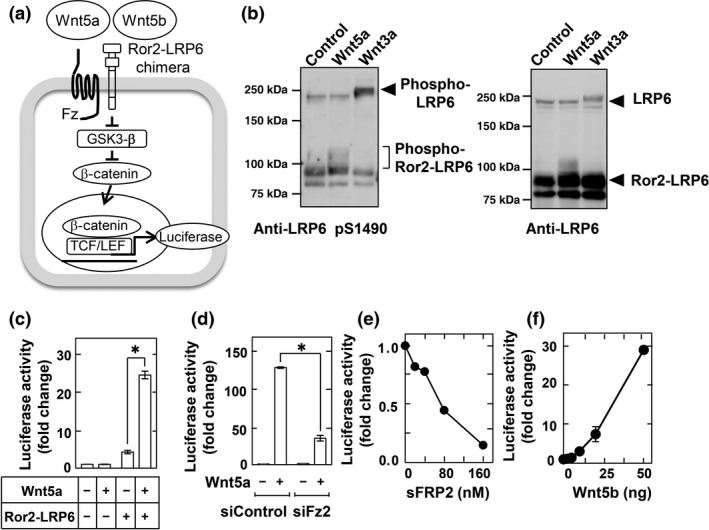
Development of reporter assay to measure Wnt5a and Wnt5b signaling. (a) Schema of the reporter system used to measure Wnt5a/b signaling using CHO cells stably expressing a Ror2– low‐density lipoprotein receptor‐related protein 6 (LRP6) chimera and TOP‐flash (CHO/Ror2‐LRP6‐TOP cells). GSK3‐β, glycogen synthase kinase 3β; TCF/LEF, T‐cell factor/lymphoid enhancer factor. (b) CHO/Ror2‐LRP6‐TOP cells were stimulated with 1 mL control, Wnt3a, or Wnt5a conditioned medium (CM) for 3 h, and cell lysates were probed with anti‐phospho‐LRP6 and anti‐LRP6 antibodies. (c) CHO/Ror2‐LRP6‐TOP cells and parental CHO cells stably expressing only TOP‐flash were stimulated with control or Wnt5a CM for 8 h. A luciferase assay was carried out and the activity was expressed as a fold increase compared with samples without Wnt5a and Ror2‐LRP6. Results are shown as means ± SD of three independent experiments. *P < 0.05. (d) CHO/Ror2‐LRP6‐TOP cells, which had been transfected with control or Frizzled‐2 (Fz2) siRNAs, were stimulated with control or Wnt5a CM for 8 h. (e) CHO/Ror2‐LRP6‐TOP cells were stimulated with Wnt5a CM for 8 h in the presence of the indicated concentrations of secreted Frizzled‐related protein‐2 sFRP2. (f) CHO/Ror2‐LRP6‐TOP cells were stimulated with the indicated concentrations of purified Wnt5b for 8 h.
Using CHO/Ror2‐LRP6‐TOP cells, Wnt5a activity was measured as control. Wnt5a CM induced the phosphorylation of the Ror2‐LRP6 chimera under the same conditions used in the induction of the phosphorylation of endogenous LRP6 by Wnt3a CM (Fig. 3b). Although Wnt5a CM did not increase luciferase activity without Ror2‐LRP6 chimera expression, chimera expression strongly promoted a Wnt5a‐dependent increase in luciferase activity (Fig. 3c). Knockdown of endogenous Frizzled 2 (Fz2) or the addition of secreted Frizzled‐related protein‐2, which inhibits the binding of Wnts and Fzs,6, 55 reduced the Wnt5a‐dependent luciferase activity (Fig. 3d,e). Therefore, in this reporter system, Wnt5a activates transcription factor T‐cell factor activity through the Ror2‐LRP6 chimera and endogenous Fz2. Similar to Wnt5a, purified Wnt5b increased luciferase activity in a dose‐dependent manner (Fig. 3f). Such a reporter system is therefore useful for quantitatively measuring Wnt5a or Wnt5b activity.
Wnt5b‐associated exosomes from PANC‐1 cells are active
To examine endogenous Wnt5b activity in PANC‐1 cells, the WNT5B gene was successfully knocked out by a CRISPR/Cas9 system (Fig. 4a); Wnt5b protein expression was completely lost (Fig. 4b). WNT5B knockout (KO) PANC‐1 cells showed reduced migration and invasion activity (Fig. 4c). Transforming growth factor‐β increased the expression of Snail and vimentin mRNA, and decreased E‐cadherin mRNA expression in PANC‐1 cells (Fig. 4d). In WNT5B KO PANC‐1 cells, TGF‐β‐dependent increases in Snail and vimentin mRNAs and the decrease in E‐cadherin mRNA were attenuated (Fig. 4d), suggesting the involvement of Wnt5b signaling in epithelial–mesenchymal transition induced by TGF‐β. However, WNT5B KO did not affect PANC‐1 cellular proliferation (Fig. 4e). These results are consistent with previous observations using Wnt5b siRNA.25
Figure 4.
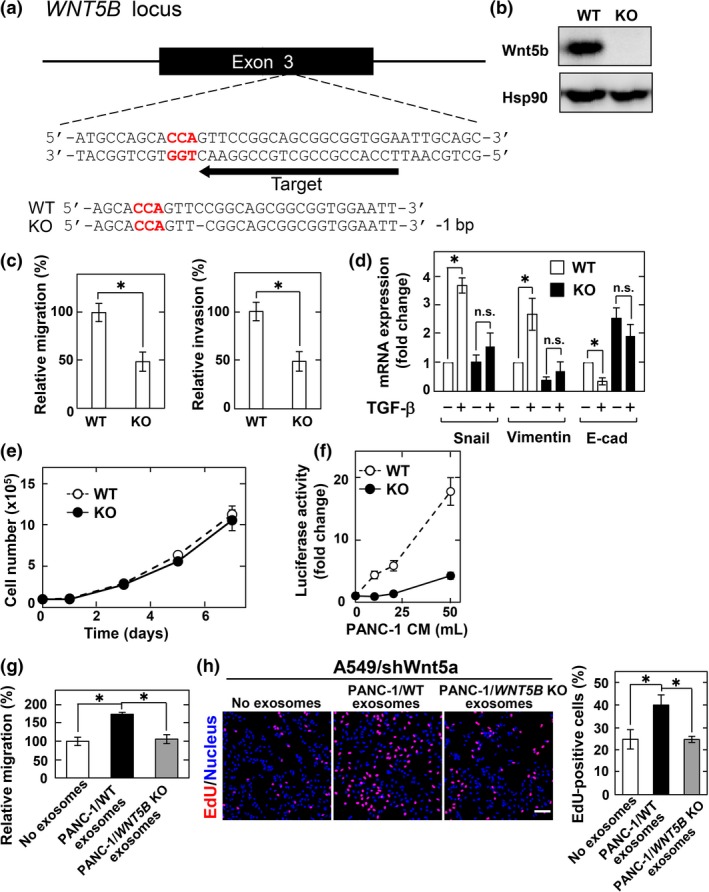
Wnt5b‐associated exosomes from PANC‐1 cells are active. (a) Schematic drawing of the targeting site of single guide RNA (sgRNA) at exon 3 of human WNT5B gene, and the nucleotide sequences of both alleles from wild‐type (WT) and WNT5B knockout (KO) PANC‐1 cells are shown. A dash represents a single base deletion. (b) Lysates from WT and WNT5B KO cells were probed with anti‐Wnt5b antibody. Hsp90, heat shock protein 90. (c) WT and WNT5B KO PANC‐1 cells were subjected to migration and invasion assays and relative migration and invasion activities of WNT5B KO PANC‐1 cells were expressed as percentages of those of wild‐type PANC‐1 cells. Results are shown as means ± SD of three independent experiments. *P < 0.05. (d) WT and WNT5B KO PANC‐1 cells were stimulated with 5 ng/mL transforming growth factor‐β (TGF‐β) for 48 h and mRNA levels of Snail, vimentin, and E‐cadherin (E‐cad) were measured by quantitative real‐time PCR. The results were expressed as fold‐changes compared with WT cells without TGF‐β stimulation. n.s., not significant. (e) WT and WNT5B KO PANC‐1 cells were subjected to cell proliferation assay. (f) CHO/Ror2‐LRP6‐TOP cells were treated with the P100 fraction recovered from the indicated volumes of PANC‐1 conditioned medium (CM) for 8 h. (g) A549/shWnt5a cells were subjected to migration assay in the presence of the exosomes isolated from CM of WT and WNT5B KO PANC‐1 cells and relative migration activity of A549/shWnt5a cells were expressed as percentages of those of A549/shWnt5a cells in the absence of the exosomes. (h) A549/shWnt5a cells were treated for 48 h with the exosomes isolated from CM of WT and WNT5B KO PANC‐1 cells, and then the cells were incubated with 5‐ethynyl‐2′‐deoxyuridine (EdU) for 1 h before fixation and stained with DRAQ5. EdU‐positive cells are expressed as percentages of positively stained cells compared with total DRAQ5 stained cells per field (n = 200–300). Scale bar = 100 μm.
Using CHO/Ror2‐LRP6‐TOP cells, Wnt5b‐associated exosome activity was measured. Precipitation fractions from CM of PANC‐1 cells increased luciferase activity in a dose‐dependent manner, but those from WNT5B KO PANC‐1 cells decreased luciferase activity dramatically (Fig. 4f). To clarify the biological significance of paracrine mechanisms of Wnt5b‐associated exosomes, we used A549 cells as target cells, because A549 cells were reported to migrate and proliferate in response to Wnt5a.20 Wnt5a was knocked down to reduce endogenous Wnt5a effects in A549 cells (A549/shWnt5a cells). Exosomes prepared from wild‐type PANC‐1 cells, but not those from Wnt5b KO PANC‐1 cells, stimulated cell migration of, and increased 5‐ethynyl‐2′‐deoxyuridine (EdU) incorporation into A549/shWnt5a cells (Fig. 4g,h). Therefore, Wnt5b secreted with exosomes is active.
To confirm that Wnt5b‐associated exosomes are taken up by cells, exosomes prepared from PANC‐1 cells were labelled with PKH26. Exosome uptake in CHO cells was observed in a time‐dependent manner at 37°C, but not at 4°C (Fig. 5a,b). Internalization of labeled exosomes was also detected as merged images of vertical sections by confocal stacks (Fig. 5c). To observe that Wnt5b is indeed associated with exosomes, we generated a plasmid vector expressing HA‐tagged Wnt5b (HA‐Wnt5b), by introducing HA tag sequence at position Arg36, located in a weakly conserved surface region opposite the Frizzled receptor binding site.56 HA‐Wnt5b was active comparable to non‐tagged Wnt5b in CHO/Ror2‐LRP6‐TOP cells (Fig. 5d). HA‐Wnt5b was recovered in precipitation fractions from CM of PANC‐1 cells expressing HA‐Wnt5b (Fig. 5e). Immunoelectron microscopy revealed that HA‐Wnt5b is present on exosomes isolated from PANC‐1 cells expressing HA‐Wnt5b (Fig. 5f).
Figure 5.
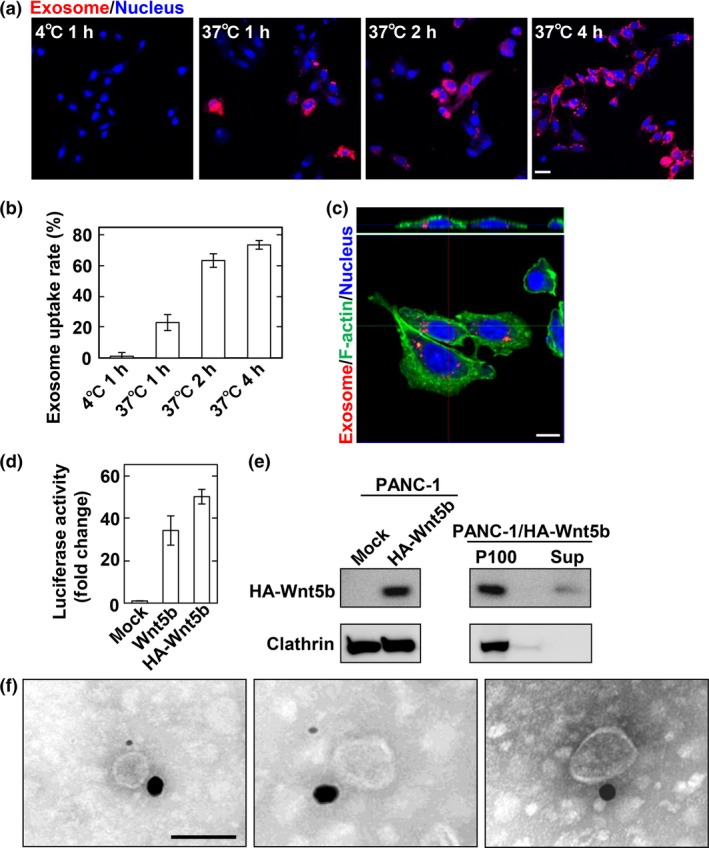
Wnt5b‐associated exosomes are taken up by CHO cells. (a, b) CHO cells were incubated with PKH26‐labeled PANC‐1 exosomes for the indicated times at 4°C or 37°C. Representative images (a) and quantification of exosome uptake (b) are shown. Scale bar = 20 μm. (c) CHO cells incubated with PKH26‐labeled PANC‐1 exosomes were stained with phalloidin (F‐actin, green) and DRAQ5 DNA Dye (nucleus, blue). Upper panel, vertical section of the CHO cells (0.3‐μm steps); lower panel, side views of the CHO cells are merges of 35 vertical sections of confocal stacks. Scale bar = 10 μm. (d) CHO/Ror2‐LRP6‐TOP cells were transfected with Wnt5b or HA‐Wnt5b for 48 h. A luciferase assay was carried out and the activity was expressed as a fold increase compared with mock‐transfected cells. (e) Left, lysates from mock and HA‐Wnt5b transfected PANC‐1 cells were probed with anti‐HA antibody. Right, The 100 000 g precipitates (P100) and supernatants (Sup) from PANC‐1 cells transiently expressing HA‐Wnt5b were probed with anti‐HA antibody. (f) Exosomes isolated from PANC‐1 cells transiently expressing HA‐Wnt5b were immunolabeled using anti‐HA and anti‐TSG101 antibodies followed by gold‐conjugated secondary antibodies and the samples were observed by electron microscopy. HA‐Wnt5b, 25‐nm gold particle; TSG101, 10‐nm gold particle. Scale bar = 100 nm.
Wnt5b‐associated exosomes from Caco‐2 cells are active
Caco‐2 cells expressed Wnt5a mRNA more highly than Wnt5b mRNA (see Fig. S2d). To reduce endogenous Wnt5a effects, Wnt5a was knocked down in Caco‐2 cells stably expressing GFP (Caco‐2/GFP cells) or Wnt5b (Caco‐2/Wnt5b cells) (Fig. S3a,b). Knockdown of Wnt5a did not affect cell migration and expression of Wnt5b did not affect it either, regardless of Wnt5a expression (Fig. S3c). Therefore, Wnt5b could not be involved in Caco‐2 cell motility. However, Wnt5b expression promoted cell proliferation compared with GFP expression, and knockdown of Wnt5b inhibited Wnt5b‐dependent cellular proliferation (Figs 6a,S4a).
Figure 6.
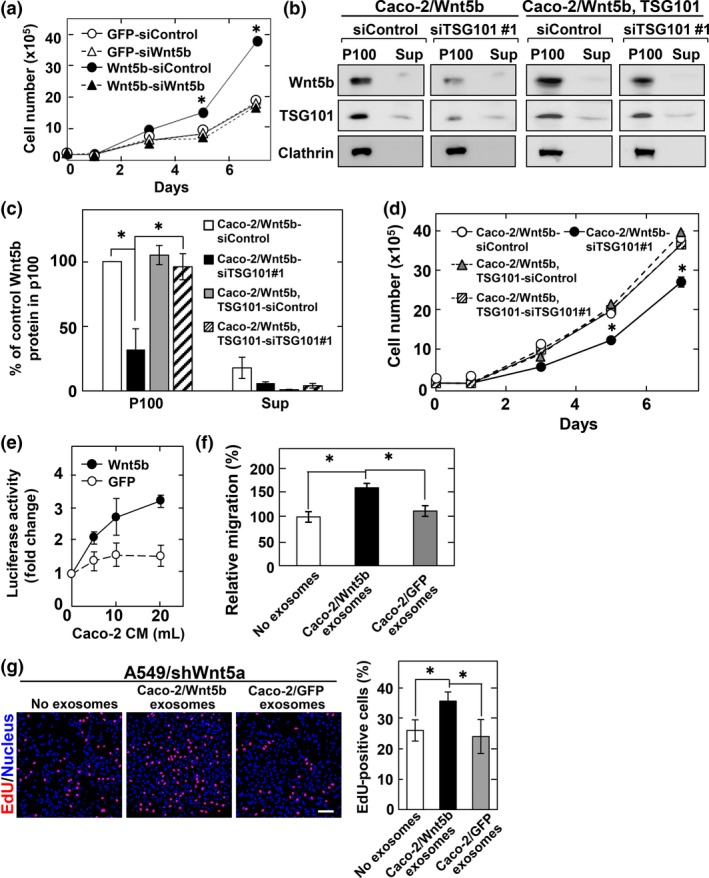
Wnt5b‐assocated exosomes from Caco‐2 cells are active. (a) Caco‐2 cells stably expressing GFP or Wnt5b were transfected with the indicated siRNAs and subjected to cell proliferation assay. Results are shown as mean ± SD of three independent experiments. *P < 0.05, between GFP‐siControl and Wnt5b‐siControl. (b, c) The 100 000 g precipitates (P100) and supernatants (Sup) prepared from Caco‐2 cells stably expressing Wnt5b (Caco‐2/Wnt5b cells) or Wnt5b and TSG101 (TSG101 rescue construct) (Caco‐2/Wnt5b, TSG101 cells), which were transfected with the indicated siRNAs, were probed with anti‐Wnt5b and anti‐clathrin antibodies. The Wnt5b signal secreted from control siRNA‐transfected cells into the P100 fraction was set to 100%. Representative images (b) and quantification of Wnt5b protein (c) are shown. (d) Caco‐2/Wnt5b or Caco‐2/Wnt5b, TSG101 cells transfected with the indicated siRNAs were subjected to cell proliferation assay. *P < 0.05. (e) CHO/Ror2‐LRP6‐TOP cells were treated for 8 h with the P100 fraction prepared from the indicated volumes of conditioned medium (CM) of Caco‐2/Wnt5b or Caco‐2/GFP cells. (f) A549/shWnt5a cells were subjected to migration assay in the presence of the exosomes isolated from CM of Caco‐2/Wnt5b or Caco‐2/GFP cells and relative migration activity of A549/shWnt5a was expressed as percentages of those of A549/shWnt5a cells in the absence of the exosomes. (g) A549/shWnt5a cells were treated for 48 h with the exosomes isolated from CM of Caco‐2/Wnt5b or Caco‐2/GFP cells, and then the cells were incubated with 5‐ethynyl‐2′‐deoxyuridine (EdU) for 1 h before fixation and stained with DRAQ5. EdU‐positive cells are expressed as percentages of positively stained cells compared with total DRAQ5 stained cells per field (n = 200–300). Scale bar = 100 μm.
TSG101 was knocked down by two siRNAs (#1 and #2). Knockdown of TGS101 by siRNA#1 suppressed Wnt5b‐associated exosomes (Figs 6b,c,S4b) and inhibited Wnt5b‐dependent, but not Wnt5b‐independent, cell proliferation (Figs 6d,S4c). The inhibitory phenotypes of TSG101 siRNA#1 were rescued by exogenous mouse TSG101 expression (Figs 6b–d,S4d), excluding siRNA off‐target effects. Similar phenotypes were observed with knockdown of TGS101 by siRNA#2 (Fig. S4b,c,e–g). Taken together with the observations that most secreted Wnt5b in CM was recovered in precipitation fractions (see Fig. 2c), Wnt5b‐associated exosomes might have activities on cancer cell proliferation.
To address this possibility, precipitation fractions from CM of Caco‐2/Wnt5b cells were prepared. The fractions increased luciferase activity in a dose‐dependent manner, but those from Caco‐2/GFP cells only slightly increased luciferase activity (Fig. 6e). Furthermore, exosomes prepared from Caco‐2/Wnt5b cells, but not those from Caco‐2/GFP cells, stimulated cell migration and increased EdU incorporation into A549/shWnt5a cells (Fig. 6f,g). Taken together, Wnt5b secretory modes depend on cell types, with active Wnt5b‐associated exosomes.
Discussion
In this study, we purified Wnt5b for the first time and revealed its biochemical properties and activities. Purified Wnt5b induced Dvl2 phosphorylation, Rac activation, and luciferase activity at comparable levels to purified Wnt5a. At least in vitro, little difference exists between the biochemical activities of purified Wnt5a and Wnt5b; the large difference noted in KO mouse phenotypes may be attributable to their expression patterns in vivo.
The profiles of glycans attached to Wnt5b were determined by ESI‐MS/MS. N93 and N305 of Wnt5b, which correspond to N114 and N326 of Wnt5a,57 were modified with high‐mannose‐type glycans. At least one of these asparagine residues is conserved in 17 Wnt members, including Wnt3a and Wnt11,47 among 19 human Wnts. In addition, Wnt5b was modified with a hybrid‐type glycan at N291, which corresponds to N312 of Wnt5a. Although the glycosylation sites are unique in Wnt5a and Wnt5b, the role of glycosylation at asparagine residues (N291 of Wnt5b and N312 of Wnt5a) remains to be clarified. Wnt5b was also modified with palmitoleic acid at S223, along with serine residues at the same positions of Wnt3a, Wnt5a, and Wnt11.46, 47, 57 The lipidation of Wnt5b could allow it to be inserted into the groove of the extracellular domain of Frizzled.58 Consistent with the results of other Wnts, Wnt5b NQ and Wnt5b S223A, indeed, failed to be secreted.
Evidence has accumulated that cancer cells, along with epithelial cells and neurons, secrete exosomes that originate from MVBs in vitro and in vivo.49 As tumor‐derived exosomes, it has been proposed that they exhibit anti‐immune effects to promote tumor aggressiveness and antitumor responses,59 and to facilitate cancer cell migration to future metastatic sites by educating bone marrow progenitors.60 Such diverse roles of exosomes could depend on their composition in terms of proteins, lipids, and nucleic acids. Some Wnts were associated with exosomes that are secreted from human and Drosophila cell lines.40, 41 Fibroblast‐derived exosomes are associated with Wnt11 in BCCs and exosome‐associated Wnt11 promotes cancer cell metastatic activity through a β‐catenin‐independent pathway.42
Although a definitive protocol to isolate exosomes has not yet been established, one of the acceptable methods is sedimentation by ultracentrifugation at 100 000 g after successive centrifugations at increasing speeds (2000 and 10 000 g). Using this method, we found that the association of Wnts with exosomes varies among cell types, and that Wnt5b is secreted with exosomes from PANC‐1 and Caco‐2 cells. The association of Wnt5b with exosomes was confirmed by immunoelectron microscopy.
To examine whether Wnt5b‐associated exosomes are active, we developed a reporter assay system for measuring Wnt5a and Wnt5b signaling using CHO cells stably expressing a Ror2‐LRP6 chimera and a TOP‐flash gene. In this system, when Wnt5a or Wnt5b binds to the extracellular domain of Ror2 of the chimera with endogenous Fz, the signal induces the expression of luciferase through the intracellular domain of LRP6. As PANC‐1 cells express high levels of Wnt5b relative to other Wnt ligands, the luciferase activity induced by exosomes could be derived from Wnt5b. Wnt5b‐associated exosomes from Caco‐2/Wnt5b cells also increased the luciferase activity. However, exosomes prepared from a large amount (50 mL) of CM from WNT5B KO PANC‐1 cells still showed slight luciferase activity. Thus, the possibility that other Wnt ligands, which bind to endogenous LRP6 in CHO cells, also activate luciferase expression cannot be excluded. The presence of active Wnt5b‐associated exosomes from PANC‐1 and Caco‐2 cells was also supported by results showing that the exosomes promote cell migration and proliferation of A549 cells. The results also suggest that Wnt5b‐associated exosomes act in a paracrine manner. However, exosomes from PANC‐1 and Caco‐2 cells did not affect their migration and proliferation in an autocrine manner. This might be due to the difference of the sensitivity for Wnt5b‐associated exosomes.
Almost all Wnt5b secreted from Caco‐2 cells was associated with exosomes. Wnt5b secretion and Wnt5b‐dependent proliferation was suppressed by TSG101 knockdown. TSG101 belongs to endosomal sorting complex required for transport components and is required for the secretion of Wnt3a with exosomes in HEK293 cells.40 However, as no individual protein or class of proteins has been shown to be universally required for exosome production in all systems,59 the trafficking steps that target Wnt to exosomes may vary among cancer cell lines. Further studies are necessary for a full understanding of the actions of Wnt5b and Wnt5b‐associated exosome in cancer cells.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Characterization of Wnt5b protein.
Fig. S2. Wnt secretion in MDCK cells and expression of Wnt5b in cancer cell lines.
Fig. S3. Wnt5b is not involved in Caco‐2 cell migration.
Fig. S4. TSG101 knockdown suppresses the secretion of Wnt5b‐associated exosomes.
Table S1. Forward and reverse primers for real‐time RT‐PCR used in this study.
Table S2. Small interfering RNA used in this study.
Data S1. Supplementary materials and methods.
Acknowledgments
We deeply appreciate Drs. T. Yano and S. Tsukita for immunoelectron microscopic analysis. This work was supported by Grants‐in‐Aid for Scientific Research to A.K. (2013–2015, no. 25250018; 2016, no. 16K11501), for Scientific Research on Innovative Areas to A.K. (2011–2015, no. 23112004), and for Scientific Research (2011–2013, no. 23590333; 2014–2016, no. 26460365) to H.Y. from the Ministry of Education, Science, and Culture of Japan, and by grants from the Uehara Memorial Foundation (2014).
Cancer Sci 108 (2017) 42–52
Funding Information
Ministry of Education, Science, and Culture of Japan (25250018, 16K11501, 23112004, 23590333, 26460365); Uehara Memorial Foundation
References
- 1. Clevers H, Nusse R. Wnt/β‐catenin signaling and disease. Cell 2012; 149: 1192–205. [DOI] [PubMed] [Google Scholar]
- 2. Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 2009; 19: 119–29. [DOI] [PubMed] [Google Scholar]
- 3. Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 2003; 94: 225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the β‐catenin‐independent pathway of Wnt signaling. Cancer Sci 2008; 99: 202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kikuchi A, Yamamoto H, Sato A et al Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012; 204: 17–33. [DOI] [PubMed] [Google Scholar]
- 6. Sato A, Kayama H, Shojima K et al The Wnt5a‐Ror2 axis promotes the signaling circuit between interleukin‐12 and interferon‐gamma in colitis. Sci Rep 2015; 5: 10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saitoh T, Katoh M. Molecular cloning and characterization of human WNT5B on chromosome 12p13.3 region. Int J Oncol 2001; 19: 347–51. [DOI] [PubMed] [Google Scholar]
- 8. Yoshikawa S, McKinnon RD, Kokel M et al Wnt‐mediated axon guidance via the Drosophila Derailed receptor. Nature 2003; 422: 583–8. [DOI] [PubMed] [Google Scholar]
- 9. Kilian B, Mansukoski H, Barbosa FC et al The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev 2003; 120: 467–76. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi TP, Bradley A, McMahon AP et al A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126: 1211–23. [DOI] [PubMed] [Google Scholar]
- 11. van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet 2006; 22: 678–89. [DOI] [PubMed] [Google Scholar]
- 12. Yang Y, Topol L, Lee H et al Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 2003; 130: 1003–15. [DOI] [PubMed] [Google Scholar]
- 13. Wu BT, Wen SH, Hwang SP et al Control of Wnt5b secretion by Wntless modulates chondrogenic cell proliferation through fine‐tuning fgf3 expression. J Cell Sci 2015; 128: 2328–39. [DOI] [PubMed] [Google Scholar]
- 14. van Tienen FH, Laeremans H, van der Kallen CJ et al Wnt5b stimulates adipogenesis by activating PPARγ, and inhibiting the β‐catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun 2009; 387: 207–11. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Jansson PA, Nagaev I et al Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 2004; 317: 1045–51. [DOI] [PubMed] [Google Scholar]
- 16. Kurayoshi M, Oue N, Yamamoto H et al Expression of Wnt‐5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–48. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto H, Kitadai Y, Yamamoto H et al Laminin γ2 mediates Wnt5a‐induced invasion of gastric cancer cells. Gastroenterology 2009; 137: 242–52. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto H, Oue N, Sato A et al Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 2010; 29: 2036–46. [DOI] [PubMed] [Google Scholar]
- 19. Hanaki H, Yamamoto H, Sakane H et al An anti‐Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor‐mediated endocytosis. Mol Cancer Ther 2012; 11: 298–307. [DOI] [PubMed] [Google Scholar]
- 20. Shojima K, Sato A, Hanaki H et al Wnt5a promotes cancer cell invasion and proliferation by receptor‐mediated endocytosis‐dependent and ‐independent mechanisms, respectively. Sci Rep 2015; 5: 8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hatano H, Kudo Y, Ogawa I et al IFN‐induced transmembrane protein 1 promotes invasion at early stage of head and neck cancer progression. Clin Cancer Res 2008; 14: 6097–105. [DOI] [PubMed] [Google Scholar]
- 22. Deraz EM, Kudo Y, Yoshida M et al MMP‐10/stromelysin‐2 promotes invasion of head and neck cancer. PLoS ONE 2011; 6: e25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klemm F, Bleckmann A, Siam L et al β‐Catenin‐independent WNT signaling in basal‐like breast cancer and brain metastasis. Carcinogenesis 2011; 32: 434–42. [DOI] [PubMed] [Google Scholar]
- 24. Kessenbrock K, Dijkgraaf GJ, Lawson DA et al A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell 2013; 13: 300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato S, Hayakawa Y, Sakurai H et al Mesenchymal‐transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci 2014; 105: 281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kikuchi A, Yamamoto H, Sato A et al New insights into the mechanism of wnt signaling pathway activation. Int Rev Cell Mol Biol 2011; 291: 21–71. [DOI] [PubMed] [Google Scholar]
- 27. Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev Dyn 2010; 239: 184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakane H, Yamamoto H, Matsumoto S et al Localization of glypican‐4 in different membrane microdomains is involved in the regulation of Wnt signaling. J Cell Sci 2012; 125: 449–60. [DOI] [PubMed] [Google Scholar]
- 29. Panakova D, Sprong H, Marois E et al Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 2005; 435: 58–65. [DOI] [PubMed] [Google Scholar]
- 30. Neumann S, Coudreuse DY, van der Westhuyzen DR et al Mammalian Wnt3a is released on lipoprotein particles. Traffic 2009; 10: 334–43. [DOI] [PubMed] [Google Scholar]
- 31. Mulligan KA, Fuerer C, Ching W et al Secreted Wingless‐interacting molecule (Swim) promotes long‐range signaling by maintaining Wingless solubility. Proc Natl Acad Sci USA 2012; 109: 370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mihara E, Hirai H, Yamamoto H et al Active and water‐soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α‐albumin. Elife 2016; 5: e11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gross JC, Boutros M. Secretion and extracellular space travel of Wnt proteins. Curr Opin Genet Dev 2013; 23: 385–90. [DOI] [PubMed] [Google Scholar]
- 34. Zhang L, Wrana JL. The emerging role of exosomes in Wnt secretion and transport. Curr Opin Genet Dev 2014; 27: 14–9. [DOI] [PubMed] [Google Scholar]
- 35. Luga V, Wrana JL. Tumor‐stroma interaction: revealing fibroblast‐secreted exosomes as potent regulators of Wnt‐planar cell polarity signaling in cancer metastasis. Cancer Res 2013; 73: 6843–7. [DOI] [PubMed] [Google Scholar]
- 36. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–93. [DOI] [PubMed] [Google Scholar]
- 37. Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol 2009; 19: 43–51. [DOI] [PubMed] [Google Scholar]
- 38. Korkut C, Ataman B, Ramachandran P et al Trans‐synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 2009; 139: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koles K, Nunnari J, Korkut C et al Mechanism of evenness interrupted (Evi)‐exosome release at synaptic boutons. J Biol Chem 2012; 287: 16820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gross JC, Chaudhary V, Bartscherer K et al Active Wnt proteins are secreted on exosomes. Nat Cell Biol 2012; 14: 1036–45. [DOI] [PubMed] [Google Scholar]
- 41. Beckett K, Monier S, Palmer L et al Drosophila S2 cells secrete wingless on exosome‐like vesicles but the wingless gradient forms independently of exosomes. Traffic 2013; 14: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luga V, Zhang L, Viloria‐Petit AM et al Exosomes mediate stromal mobilization of autocrine Wnt‐PCP signaling in breast cancer cell migration. Cell 2012; 151: 1542–56. [DOI] [PubMed] [Google Scholar]
- 43. Thery C, Amigorena S, Raposo G et al Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3: Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 44. Khatua AK, Taylor HE, Hildreth JE et al Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol 2009; 83: 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hino S, Tanji C, Nakayama KI et al Phosphorylation of β‐catenin by cyclic AMP‐dependent protein kinase stabilizes β‐catenin through inhibition of its ubiquitination. Mol Cell Biol 2005; 25: 9063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takada R, Satomi Y, Kurata T et al Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 2006; 11: 791–801. [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto H, Awada C, Hanaki H et al The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J Cell Sci 2013; 126: 2931–43. [DOI] [PubMed] [Google Scholar]
- 48. Colombo M, Moita C, van Niel G et al Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126: 5553–65. [DOI] [PubMed] [Google Scholar]
- 49. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30: 255–89. [DOI] [PubMed] [Google Scholar]
- 50. Matsumoto S, Fumoto K, Okamoto T et al Binding of APC and dishevelled mediates Wnt5a‐regulated focal adhesion dynamics in migrating cells. EMBO J 2010; 29: 1192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caradec J, Kharmate G, Hosseini‐Beheshti E et al Reproducibility and efficiency of serum‐derived exosome extraction methods. Clin Biochem 2014; 47: 1286–92. [DOI] [PubMed] [Google Scholar]
- 52. Fevrier B, Raposo G. Exosomes: endosomal‐derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004; 16: 415–21. [DOI] [PubMed] [Google Scholar]
- 53. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014; 29: 116–25. [DOI] [PubMed] [Google Scholar]
- 54. Grumolato L, Liu G, Mong P et al Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 2010; 24: 2517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116: 2627–34. [DOI] [PubMed] [Google Scholar]
- 56. Farin HF, Jordens I, Mosa MH et al Visualization of a short‐range Wnt gradient in the intestinal stem‐cell niche. Nature 2016; 530: 340–3. [DOI] [PubMed] [Google Scholar]
- 57. Yamamoto H, Awada C, Matsumoto S et al Basolateral secretion of Wnt5a in polarized epithelial cells is required for apical lumen formation. J Cell Sci 2015; 128: 1015–63. [DOI] [PubMed] [Google Scholar]
- 58. Janda CY, Waghray D, Levin AM et al Structural basis of Wnt recognition by Frizzled. Science 2012; 337: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bobrie A, Colombo M, Raposo G et al Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 2011; 12: 1659–68. [DOI] [PubMed] [Google Scholar]
- 60. Peinado H, Aleckovic M, Lavotshkin S et al Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med 2012; 18: 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Characterization of Wnt5b protein.
Fig. S2. Wnt secretion in MDCK cells and expression of Wnt5b in cancer cell lines.
Fig. S3. Wnt5b is not involved in Caco‐2 cell migration.
Fig. S4. TSG101 knockdown suppresses the secretion of Wnt5b‐associated exosomes.
Table S1. Forward and reverse primers for real‐time RT‐PCR used in this study.
Table S2. Small interfering RNA used in this study.
Data S1. Supplementary materials and methods.


