Short abstract
Rich countries should follow the lead of poor countries and adopt a more systematic way of controlling the cost of drugs
Industrialised countries, faced with increasing demands for quality health care by ageing populations and ever increasing costs of medicines, can learn from low income countries how to respond to pharmaceutical policy issues in a comprehensive way.
Since the 1970s many developing countries have started national programmes for essential drugs to promote the availability, accessibility, affordability, quality, and rational use of medicines. The cornerstones of such programmes are the careful selection of essential medicines for public supply and reimbursement, based on a systematic review of comparative efficacy, safety, and value for money; evidence based national clinical guidelines as the basis for training and rational prescribing; and a national medicines policy to balance conflicting policy objectives and to express government commitment to a common goal. Industrialised countries would do well to consider and adopt these approaches, which have been so beneficial to developing countries.
The concept of essential medicines
The concept of essential medicines was launched in 1977 with the publication of the first World Health Organization's Model List of Essential Medicines. Since then the list has been revised every two years. Both its content and the process by which it is updated are intended as a model for developing countries. Twenty five years later the original concept is seen as a breakthrough in international public health.w1 By the turn of the century, 156 mostly developing countries have a national list of essential medicines, two thirds of which have been updated in the past five years. Lists of essential medicines are also used by Unicef, the United Nations high commissioner for refugees, and many non-governmental organisations.
Selection is a two step process
Within a country, the selection of essential medicines is a two step process. Regulatory approval is usually based on a review of efficacy, safety, and quality without comparison with other medicines. From these registered products, essential medicines within a therapeutic class are then selected on the basis of comparative efficacy, safety, and cost (“value for money”). National lists of essential medicines are used to guide the procurement and supply of medicines in the public sector, reimbursement schemes, medicine donations, and local production of medicine; they also help define the training of health workers. In short, lists of essential medicines provide the scientific and public health basis for focus and expenditure in the pharmaceutical sector.
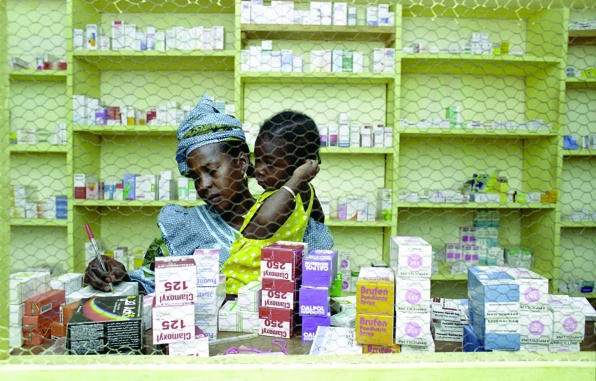
Figure 1.
Essential medicines are not second rate medicines for poor people
Credit: CRISPIN HUGHES/PANOS
Important changes to WHO Model List
In 2002, WHO completed a rigorous overhaul of the process to update the Model List.1 An important change was that affordability changed from a precondition into a consequence of the selection. For example, before 2002, effective but expensive medicines, such as single dose azithromycin for trachoma, were not listed because of their price. Under the new definition (box 1), 12 antiretroviral medicines for HIV/AIDS were listed, irrespective of high cost. Their listing now implies that these medicines should become affordable to all patients who need them.
Advantages of clinical guidelines and lists
Good evidence shows that clinical guidelines and lists of essential medicines, when properly developed, introduced, and supported, improve prescribing quality and lead to better health outcomes.3-6 But there is also an economic argument. Firstly, in developing countries pharmaceuticals are the second biggest budget line in the health system, after salaries. Secondly, new essential medicines are expensive. For example, even with good differential pricing, lumefantrine-artemisinine is 25 times more expensive than chloroquine, the first line antimalarial it is supposed to replace; atovaquone-proguanil is about 400 times as expensive. Life saving antiretroviral combinations cost £83-£138 ($150-$250; €119-€199) per year whereas 38 countries have less than £1 per person per year available for all medicines.7 The selection of new essential medicines for public supply, subsidy, or reimbursement has enormous financial implications for developing countries.
The advantages of limited lists are therefore both medical and economical. From a medical point of view they lead to better quality of care and better health outcomes and help focus quality control, drug information, prescriber training, and medical audit. Economically they lead to better value for money, to lower costs through economies of scale, and to simplified systems of procurement, supply, distribution, and reimbursement.
Box 1: Definition of essential medicines (WHO, 2002)2
Essential medicines are those that satisfy the priority health care needs of the population. They are selected with due regard to disease prevalence, evidence on efficacy and safety, and comparative cost effectiveness. Essential medicines are intended to be available within the context of functioning health systems at all times, in adequate amounts, in the appropriate dosage forms, with assured quality, and at a price the individual and the community can afford. The implementation of the concept of essential medicines is intended to be flexible and adaptable to many different situations; exactly which medicines are regarded as essential remains a national responsibility
In many countries it has taken several years and several editions of treatment guidelines and lists of essential medicines to develop a more or less stable product accepted by most prescribers and used for training, procurement, and supply. Although time consuming, the wide involvement of a large number of prescribers, academic departments, health facilities, and professional organisations is crucial. It is also important to stress that essential medicines are not second rate medicines for poor people, but that they represent the most cost effective treatments for a given condition. Over time, prescribers increasingly recognise and trust the value of the clinical guidelines.
Is the concept of essential medicines relevant for rich countries?
Problems of increasing demand and rising medicine costs are not limited to developing countries. Pharmaceutical expenditure in the United States rose by 18% in 1999, 16% in 2000, and 17% in 2001. This rise is due to an ageing population, direct to consumer advertising, and, especially, the increased average cost of medicines (volume rose with only 5-6% per year over the same period). In Canada the average cost per prescription rose by 93% between 1987 and 1993. One third of this rise was due to price increases of existing medicines and 15% to increased quantities per prescription, but 55% was the result of use of new medicines.8
As most of the cost increases seem linked to the introduction of new medicines, systematic selection becomes important for industrialised countries as well. The decision is easy when new medicines are better and cheaper; but when they are only slightly more effective and much more expensive, the perceived advantages should be balanced against the extra cost. As most supply or reimbursement schemes operate within capped budgets, providing an expensive new medicine to one patient may imply that a clinically equivalent but cheaper medicine can not be given to several others.
The challenge to get the best value for money was common in developing countries, but is increasingly obvious in middle income situations as well. For example, there were acute problems in the supply of medicines in the Commonwealth of Independent States after the collapse of the former Soviet Union, and two years ago in Argentina. But even developed countries are increasingly following the same approach. Australia has become very strict about the selection of pharmaceuticals for reimbursement in their Pharmaceutical Benefits Scheme9; in the United States most health management organisations use a restricted list of pharmaceuticals for reimbursement.10,11 What started in countries such as Cuba (1963),w2 Tanzania (1970),w3 and Peru (1972)w4 and was initially (in 1978) called “desert island drugs”,12 is now relevant for us all.
The link with national clinical guidelines
These first essential medicines' lists of the 1970s were often just commonsense stock lists for supply systems for the public sector. Over the years the selection criteria have become more systematic, and currently medicines are only listed when they feature in a clinical guideline. The evidence is then linked to the treatment, not to the medicine. For example, azithromycin is now on the model list for single dose treatment of genital Chlamydia trachomatis and trachoma only and not as a general antibiotic, for which its advantages are much less clear. By the turn of the century, 135 countries had developed national clinical guidelines, mostly linked to national lists of essential medicines. Good examples are Zimbabwe,13 South Africa14 and, more recently, Delhi State Capital Territory.15
It has long been thought that national clinical guidelines were only relevant and, indeed, only possible in developing countries (perhaps with the exception of the antibiotic guidelines of Australiaw5). But in the early 1990s, discrepancies in the quality of care between the various districts and hospitals in Scotland led the Department of Health and the Royal Colleges to start the Scottish Intercollegiate Guidelines Network (SIGN). This network has now prepared over 70 guidelines for disorders where treatments showed large differences despite the availability of good clinical evidence. In other developed countries the number of clinical guidelines is also growing rapidly. Unfortunately their scientific evidence base and management of potential conflicts of interests are not always transparent. This has led to international groups, such as the AGREE (Appraisal of Guidelines Research and Evaluation) collaboration to standardise the guideline development process, GRADE (the Working Group on Grading Harmonization) to standardise the grading of evidence, and GIN (the Guidelines International Network) to exchange evidence tables. What started in New Guinea (1974)w6 and Mozambique (1981)w7 is now happening in industrialised countries.
National medicine policies
Different objectives of a national pharmaceutical programme are often contradictory.w8 For example, reimbursement restrictions may lead to irrational alternative prescribing, and preference for the national pharmaceutical industry may result in higher prices for medicines. A national medicines' policy, when developed in a consultative way, helps to bring out and resolve such diverging interests. The policy then becomes the expression of government commitment to a common goal and a framework for action.16
In 1982, Bangladesh was the first country with a national drug policy, focusing on promoting the national drug industry; India followed soon after. The 1988 policy of the Philippinesw9 focused on generic prescribing and was widely opposed by the international pharmaceutical industry and the medical profession. The 1996 policy of South Africaw10 focuses on equity. By the turn of the century, 109 developing countries had developed a national medicines' policy.
In industrialised countries the picture is different. Components of a pharmaceutical policy are often in place but are rarely dealt with systematically; from a public health point of view the end result is rarely satisfactory. For example, in the United States over 40 million people are not covered by health insurance, but efforts to create a national health service stranded in opposition by various stakeholders. In the United Kingdom, recommendations by the National Institute for Clinical Excellence may result in the reimbursement of new therapies; but this has an impact on district budgets for which NICE is not responsible. A suggestion in this journal to develop a national drug policy for the United Kingdom has not been taken up.17
Box 2: Components of a national medicine policy potentially relevant for developed countries
Additional criteria for market approval (comparison with best available treatment, comparative cost effectiveness, price, regional harmonisation)
Evidence based national clinical guidelines (for training, prescribing, and audit)
Insurance and reimbursement (systematic evidence based selection of treatments for reimbursement; type and level of reimbursement focused on essential medicines)
State subsidies (direct supply, subsidy or subsidised insurance for poor and disadvantaged people; access to essential medicines as part of human rights; sex equity)
Price controls (taxes and margins on essential medicines, dispensing fees, reference pricing)
Local production versus importation
Patent policies (balance between innovation and equitable access to essential medicines)
Quality (quality control, counterfeits, quality of the distribution chain)
Rational prescribing (training; financial incentives, separated prescribing and dispensing; audit)
Rational use by the public (public education; medicine information)
Promotion (regulation, monitoring, conflict of interest in prescribing and research)
Human resources (number of pharmacists, technicians, and dispensers needed over time)
Research and development (public support for public health priorities)
Summary points
Most industrialised countries respond in a fragmented way to the increasing costs of medicines and the demands for quality health care by ageing populations
Evidence shows that clinical guidelines and lists of essential medicines improve quality of care and lead to better health outcomes
Essential medicines are not cheap medicines for poor people in rural areas in developing countries; they are the most cost effective treatment for a given condition
Lists of essential medicines provide the scientific and public health basis for focus and expenditure in the pharmaceutical sector
The long experience of developing countries with essential medicines, clinical guidelines, and national medicine policies is useful for middle income and industrialised countries
Comprehensive national pharmaceutical policies may therefore also be helpful in industrialised countries (box 2). Australia was in such a situation, with four good but separate programmes for improving the availability, quality, and quality use of medicines and the viability of the national pharmaceutical industry. In 2000 the National Drug Policy of Australia was launched, bringing these successful components together into one government paper.18 Many observers think that this was partly due to the political pressure by national experts and non-governmental organisations that had assisted developing countries with the establishment of such policies.
Conclusion
The selection of essential medicines based on sound scientific review and public health grounds, the development of evidence based national clinical guidelines and a national medicines' policy are the cornerstones of any essential medicines' programme. Although some of these components may be in place, industrialised countries would do well to consider in a more systematic way these comprehensive approaches that have proved so beneficial to developing countries.
Supplementary Material
 References w1-w10 are on bmj.com
References w1-w10 are on bmj.com
I thank Kath Hurst and Shalini Jayasekar for support and Richard Laing and James Tumwine for their comments. Important WHO references to support the selection of essential medicines are: 13th model list of essential medicines (www.who.int/medicines); WHO model formulary 2004 (mednet3.who.int/eml/modelFormulary.asp); and the WHO essential medicines library (mednet3.who.int/eml/).
Contributors: HVH is the sole contributor to this article.
Funding: Department of Essential Drugs and Medicines Policy, WHO.
Competing interests: None declared
References
- 1.World Health Organization. The selection and use of essential medicines. Report of the WHO Expert Committee, 2002 (including the 12th Model List of Essential Medicines). Technical Report Series No 914. Geneva: WHO, 2003. [PubMed]
- 2.World Health Organization. The selection and use of essential medicines. Report of the WHO Expert Committee, 2002 (including the 12th Model List of Essential Medicines). Technical Report Series No 914. Geneva: WHO, 2003: 15. [PubMed]
- 3.Grimshaw J, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993;342: 1317-22. [DOI] [PubMed] [Google Scholar]
- 4.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations and harms of clinical guidelines. BMJ 1999;318: 527-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kafuko J, Bagenda D. Impact of national standard treatment guidelines on rational drug use in Uganda health facilities. Kampala: Unicef/Uganda, 1994.
- 6.Laing RO, Hogerzeil HV, Ross-Degnan D. Ten recommendations to improve use of medicines in developing countries. Health Policy Plann 2001;16: 13-20. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO medicines strategy 2004-2007. Geneva: WHO, 2004.
- 8.SCRIP 1994;No 1974: 21.
- 9.Drummond MF. Basing prescription drug payment on economic analysis: the case of Australia. Health Affairs 1992;11: 191-6. [DOI] [PubMed] [Google Scholar]
- 10.Lipton HL, Gross DJ, Stebbins MR, Syed LH. Managing the pharmacy benefit in Medicare HMOs: what do we really know? Health Affairs 2000;19: 42-58. [DOI] [PubMed] [Google Scholar]
- 11.Gold MR, Hurley R, Lake T, Ensor T, Berenson R. A national survey of the arrangements managed-care plans made with physicians. N Engl J Med 1995;333: 1678-83. [DOI] [PubMed] [Google Scholar]
- 12.Desert-island drugs [editorial]. Lancet 1978;i: 977-8. [Google Scholar]
- 13.Ministry of Health and Child Welfare. EDLIZ: 4th essential drugs list and standard treatment guidelines for Zimbabwe. Harare: Ministry of Health and Child Welfare, 2000.
- 14.Department of Health. Standard treatment guidelines and essential drugs list. Primary health care. Pretoria, South Africa: Department of Health, 2003.
- 15.Sharma S, Sethi GR, Gulati RK. Standard treatment guidelines. New Delhi: Delhi Society for Promotion of Rational Use of Drugs, 2002.
- 16.How to establish and implement a national drug policy. Geneva: WHO, 2001.
- 17.Walley T, Earl-Slater A, Haycox A, Bagust A. An integrated national pharmaceutical policy for the United Kingdom? BMJ 2000;321: 1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commonwealth Department of Health and Aged Care. National medicines policy. Canberra: Commonwealth Department of Health and Aged Care, 2000.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.