Abstract
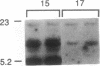
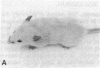
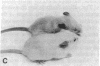
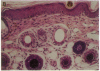
Genetically albino mouse eggs were injected with an inducible transgene comprising the wild-type tyrosinase (monophenol, L-dopa: oxygen oxidoreductase, EC 1.14.18.1) cDNA and the metallothionein gene promoter in the expectation that the transgene would be expressed to different extents in the various developing pigment cell clones of at least some individuals, thereby leading to patterned coats. This proved to be the case. Five transgenic mice had lightly pigmented patterns of transverse stripes visualizing melanoblast proliferation and migration dorsoventrally on each side. Similar patterns have been seen in genetically mosaic mouse models produced from conjoined blastomeres of different color genotypes and in many naturally patterned genotypes of mice. Four of the transgenics had heritable patterns and autosomal transgene integration. Their homozygous descendants were darker than hemizygotes and transmitted the basic pattern through many generations. Eyes were also pigmented, with clonal patches of melanized cells in the retinal pigment epithelium. The skin was dark due to many pigmented dermal melanocytes, whereas relatively few were in the hair follicles. This "inversion" is attributable to precocious maturation and migratory arrest of many melanoblasts during passage through the dermis en route to the hair bulbs. Patterning in these mice is considered in light of the view, previously proposed, that phenotypically different clones, or phenoclones, may exist in virtually all mammalian cell types and that many genes may be associated with cis-acting control regions causing variations in their expression that are mitotically perpetuated. We point out that mosaic expression has many implications for development as well as neoplasia. In the latter case, the potential for tumor susceptibility may be affected by clonal variation without further gene mutations or deletions. Thus, mice with variegating transgenes can provide molecular access to gene control mechanisms and to their consequences in development and disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beermann F., Ruppert S., Hummler E., Bosch F. X., Müller G., Rüther U., Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990 Sep;9(9):2819–2826. doi: 10.1002/j.1460-2075.1990.tb07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M., Larue L., Mintz B. Clonal coat color variation due to a transforming gene expressed in melanocytes of transgenic mice. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6447–6451. doi: 10.1073/pnas.88.15.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M. Position effect variegation in the mouse. Genet Res. 1974 Jun;23(3):291–306. doi: 10.1017/s0016672300014932. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Mintz B. Early postimplantation embryo lethality due to DNA rearrangements in a transgenic mouse strain. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6020–6024. doi: 10.1073/pnas.83.16.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S. K., McMaster M. T., Dey S. K., Andrews G. K. Cell-specific metallothionein gene expression in mouse decidua and placentae. Development. 1989 Nov;107(3):611–621. doi: 10.1242/dev.107.3.611. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Jackson I. J., Bennett D. C. Identification of the albino mutation of mouse tyrosinase by analysis of an in vitro revertant. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7010–7014. doi: 10.1073/pnas.87.18.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Larue L., Mintz B. Pigmented cell lines of mouse albino melanocytes containing a tyrosinase cDNA with an inducible promoter. Somat Cell Mol Genet. 1990 Jul;16(4):361–368. doi: 10.1007/BF01232464. [DOI] [PubMed] [Google Scholar]
- Mayer T. C. The migratory pathway of neural crest cells into the skin of mouse embryos. Dev Biol. 1973 Sep;34(1):39–46. doi: 10.1016/0012-1606(73)90337-0. [DOI] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian differentiation. Annu Rev Genet. 1974;8:411–470. doi: 10.1146/annurev.ge.08.120174.002211. [DOI] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. Histocompatibility antigens on melanoblasts and hair follicle cells. Cell-localized homograft rejection in allophenic skin grafts. Transplantation. 1970 May;9(5):497–505. doi: 10.1097/00007890-197005000-00009. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrick W. M., Maher S. E., Bridgett M. M., Bothwell A. L. A recombination event in the 5' flanking region of the Ly-6C gene correlates with impaired expression in the NOD, NZB and ST strains of mice. EMBO J. 1990 Aug;9(8):2485–2492. doi: 10.1002/j.1460-2075.1990.tb07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S., Mintz B. Multiple alternatively spliced transcripts of the mouse tyrosinase-encoding gene. Gene. 1991 Jan 15;97(2):277–282. doi: 10.1016/0378-1119(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Ragg S., Heckl-Ostreicher B., Held M., Loos U., Call K., Glaser T., Housman D., Saunders G., Zabel B. Direct pulsed field gel electrophoresis of Wilms' tumors shows that DNA deletions in 11p13 are rare. Genes Chromosomes Cancer. 1991 Mar;3(2):89–100. doi: 10.1002/gcc.2870030203. [DOI] [PubMed] [Google Scholar]
- Rubin D. C., Ong D. E., Gordon J. I. Cellular differentiation in the emerging fetal rat small intestinal epithelium: mosaic patterns of gene expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1278–1282. doi: 10.1073/pnas.86.4.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyman S., Weaver S. Chromosomal rearrangements associated with LINE elements in the mouse genome. Nucleic Acids Res. 1985 Jul 25;13(14):5085–5093. doi: 10.1093/nar/13.14.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Yamamoto H., Takeuchi S., Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990 Feb;108(2):223–227. doi: 10.1242/dev.108.2.223. [DOI] [PubMed] [Google Scholar]
- Tartof K. D., Bishop C., Jones M., Hobbs C. A., Locke J. Towards an understanding of position effect variegation. Dev Genet. 1989;10(3):162–176. doi: 10.1002/dvg.1020100306. [DOI] [PubMed] [Google Scholar]
- Terao M., Tabe L., Garattini E., Sartori D., Studer M., Mintz B. Isolation and characterization of variant cDNAs encoding mouse tyrosinase. Biochem Biophys Res Commun. 1989 Mar 15;159(2):848–853. doi: 10.1016/0006-291x(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Stewart T. A., Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T., Silversides D. W., Waymire K. G., Kwon B. S., Takeuchi T., Overbeek P. A. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990 Dec 25;18(24):7293–7298. doi: 10.1093/nar/18.24.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]