ABSTRACT
Using serotyping, multilocus sequence typing, and whole-genome sequencing (WGS) of selected strains, we studied the population structure of 102 group B Streptococcus (GBS) isolates prospectively sampled in 2014 from vaginal/rectal swabs of healthy pregnant women in metropolitan Toronto, Canada. We also determined the susceptibilities of each of the colonizing isolates to penicillin, erythromycin, clindamycin, tetracycline, and other antimicrobial agents. Overall, we observed a high rate of tetracycline resistance (89%) among colonizing GBS isolates. We found resistance to erythromycin in 36% of the strains, and 33% were constitutively or inducibly resistant to clindamycin. The most frequently identified serotypes were III (25%), Ia (23%), and V (19%). Serotype IV accounted for 6% of the colonizing isolates, a rate consistent with that observed among patients with invasive GBS infections in metropolitan Toronto. The majority of serotype IV isolates belonged to sequence type (ST)459, a tetracycline-, erythromycin-, and clindamycin-resistant ST first identified in Minnesota, which is considered to be the main driver of serotype IV GBS expansion in North America. WGS revealed that ST459 isolates from Canada are clonally related to colonizing and invasive ST459 organisms circulating in regions of the United States. We also used WGS to study recombination in selected colonizing strains from metropolitan Toronto, which revealed multiple episodes of capsular switching. Present and future circulating GBS organisms and their genetic diversity may influence GBS vaccine development.
KEYWORDS: group B Streptococcus, Streptococcus agalactiae, antibiotic resistance, serotype IV, colonizing GBS, serotype switching, GBS vaccine
INTRODUCTION
Group B Streptococcus (GBS) is a leading cause of neonatal sepsis and meningitis and an agent of invasive infections in pregnant and nonpregnant adults (1, 2). GBS strains can be divided into 10 distinct serotypes (Ia, Ib, and II to IX) based on a serological reaction directed against the polysaccharide capsule (3, 4) or as determined by a multiplex PCR assay (5). Multilocus sequence typing (MLST) is also used to classify GBS strains. There are more than 750 MLST sequence types (STs), although most human isolates cluster into six major clonal complexes (CCs) (6). Results from several studies have shown that some serotypes and/or STs are associated with specific disease phenotypes (7, 8). For example, serotype Ia strains belonging to ST23 and ST24, as well as ST17 serotype III strains, may have enhanced potential to cause early onset disease (EOD; onset of GBS disease from birth to 6 days old) (9, 10). Similarly, late onset disease (LOD; the onset of GBS disease in infants aged 7 to 89 days) is strongly associated with meningitis-prone ST17 serotype III strains (9, 11, 12).
GBS strains colonize the gastrointestinal and genital tracts of 10 to 30% of healthy humans (13–16). In many countries, including Canada and the United States, the screening of pregnant women for GBS colonization at 35 to 37 weeks gestation and intrapartum antibiotic prophylaxis (IAP) for colonized women have substantially reduced the burden of EOD (17, 18). Penicillin is the recommended antibiotic for IAP, although clindamycin or vancomycin may be used in patients with β-lactam allergies who are at risk for anaphylaxis (19). Erythromycin was previously recommended as an alternative antibiotic for women at high risk for anaphylaxis. However, current US and Canadian guidelines no longer recommend erythromycin due to an observed increase in macrolide resistance in GBS (19, 20). Increasing resistance to clindamycin has also been reported among colonizing and invasive GBS isolates (20–22). The most recent characterization of erythromycin and clindamycin resistance among colonizing isolates from metropolitan Toronto was published more than 15 years ago (22).
Maternal antibodies against type-specific GBS capsular polysaccharides appear to be protective (23), and immunizing pregnant women using polysaccharide conjugate vaccine formulations promises to further reduce the burden of GBS neonatal disease (24). Clinical trials have recently begun for a trivalent (serotypes Ia, Ib, and III) vaccine formulation (25, 26). However, vaccine effectiveness may be limited if non-vaccine serotypes predominate among colonizing GBS isolates. For example, earlier reports from Minnesota described the isolation of serotype IV GBS strains belonging to different STs among isolates of both colonizing and neonatal invasive GBS in the United States (27, 28). We and others recently described an increase in the frequency of isolation of serotype IV strains among invasive GBS infections in Canada (29–31). In addition, as exemplified by polyvalent conjugate pneumococcal vaccination schemes (32), vaccinating with the GBS trivalent formulation may select for vaccine escape recombinants through capsular switching. Thus, a detailed understanding of the circulating population of GBS colonizing strains is important to foresee the potential effects of vaccine introduction in a defined geographical area.
Here, we report high rates of antibiotic resistance and many examples of capsular switching among colonizing GBS isolates recovered from pregnant women in metropolitan Toronto, Canada. We also report a relatively high (6%) frequency of non-vaccine serotype IV isolates and show that they belonged, for the most part, to ST459. Using whole-genome sequencing (WGS) and an extended collection of colonizing and invasive serotype IV ST459 organisms from the United States, we report that ST459 strains circulating in areas of both countries are members of a highly clonal tetracycline-, clindamycin-, and erythromycin-resistant group of organisms.
RESULTS
Serotype and MLST distribution of colonizing isolates from metropolitan Toronto.
Most of the 102 colonizing GBS isolates from metropolitan Toronto were serotype III, Ia, or V (25%, 23%, and 19%, respectively) (Fig. 1). The next most common serotypes were serotypes II (13%) and Ib (9%). Five isolates were typed as serotype IV (5%), and we identified a single serotype VI isolate (1%). We did not find isolates of serotypes VII, VIII, or IX in the sample. We assigned six strains that were nontypeable by serological methods (probably because they do not express capsules) to serotypes Ia, Ib, IV, and V using a capsular-typing PCR scheme (see Table S1 in the supplemental material). Using WGS, we identified mutations predicted to result in abolishment of capsule expression in one or more genes of the cps locus of each of the six NT strains, including multiple instances of point mutations and short insertions/deletions (see Table S3). We next investigated the genetic diversity of the colonizing GBS isolates using MLST. The colonizing isolates belonged to 23 different STs that grouped into the six major human CCs that Da Cunha et al. recently identified as predominant among human GBS infections (6). The most frequently identified ST was ST1, which was particularly associated with serotype V strains. The next most common ST was ST23, which was found exclusively among serotype Ia strains. ST8 and ST12 were the most common STs among serotype Ib strains. Among serotype II strains, ST22 and ST28 predominated. Of the 26 serotype III colonizing isolates in our collection, 15 (58%) were ST17 (see Table S1). All colonizing ST17 strains possessed the hvgA gene, encoding the surface protein HvgA, a major virulence factor thought to be key for the development of meningitis in neonates. We screened for hvgA among samples of invasive GBS causing LOD in metropolitan Toronto between 2009 and 2014 and found that 89% of the serotype III strains (70/79) were hvgA positive (our unpublished data). Among our collection of colonizing isolates, 58% of the serotype III strains (15/26) were hvgA positive. Thus, the rate of hvgA-positive serotype III isolates was significantly lower among colonizing isolates than among isolates causing invasive disease in neonates (P = 0.0014, chi-square test). All but one of the remaining serotype III colonizing isolates belonged to ST19 or STs grouped in CC19 (Fig. 1).
FIG 1.
Serotype and MLST distribution of colonizing group B Streptococcus isolates from metropolitan Toronto. Bars indicate the numbers of isolates per serotype in the collection of 102 isolates. Labels within the bars indicate the ST, and the colored blocks indicate the clonal complex to which the ST belongs. NT, nontypeable.
Serotype IV colonizing isolates from metropolitan Toronto are mainly ST459 strains clonally related to colonizing and invasive ST459 organisms circulating in several regions of the United States.
Among our colonizing GBS isolates, serotyping identified five serotype IV strains, and one additional serotype IV strain was identified by PCR typing (total of 6/102 [6%]). Four of the serotype IV colonizing strains belonged to ST459, one belonged to the closely related ST196, and one belonged to ST452 (see Table S1). This ST distribution parallels the results for invasive serotype IV GBS reported in Toronto and elsewhere in Canada (29, 30). To understand the genetic relationship between colonizing and invasive ST459 strains circulating in Canada and the United States, we sequenced the genomes of all four colonizing serotype IV ST459 strains in our collection. We had previously analyzed the genomes of 96 invasive ST459 strains from Canada and Europe and identified a highly clonal population structure (1,563 unique nonredundant single nucleotide polymorphism [SNP] loci among all 96 invasive ST459 strains) (29). To compare our colonizing strains, we chose 18 genomes from this previous data set based on temporal and geographical matching (see Table S4). We also included 19 colonizing and 9 invasive serotype IV ST459 strains from the United States. Phylogenetic relationships were established using genome-wide SNP data (Fig. 2). Consistent with the hypothesis of recent emergence, and despite the extended geographical range of the ST459 isolates that were compared (Ontario [Toronto], Minnesota, Pennsylvania, Georgia, and Texas), there were only 949 nonredundant polymorphic loci in the ST459 collection analyzed here relative to the core genome of the reference strain. The four ST459 colonizing isolates from metropolitan Toronto differed from the reference strain, on average, by only 72 SNPs. The 19 colonizing isolates from Pennsylvania, Georgia, and Texas, and the nine invasive strains from Minnesota differed from the reference, on average, by 56 and 57 SNPs, respectively. Except for some invasive strains from Minnesota and from metropolitan Toronto, there was minimal clustering, and in general, colonizing ST459 strains were interspersed with the invasive isolates in the phylogenetic tree (Fig. 2).
FIG 2.
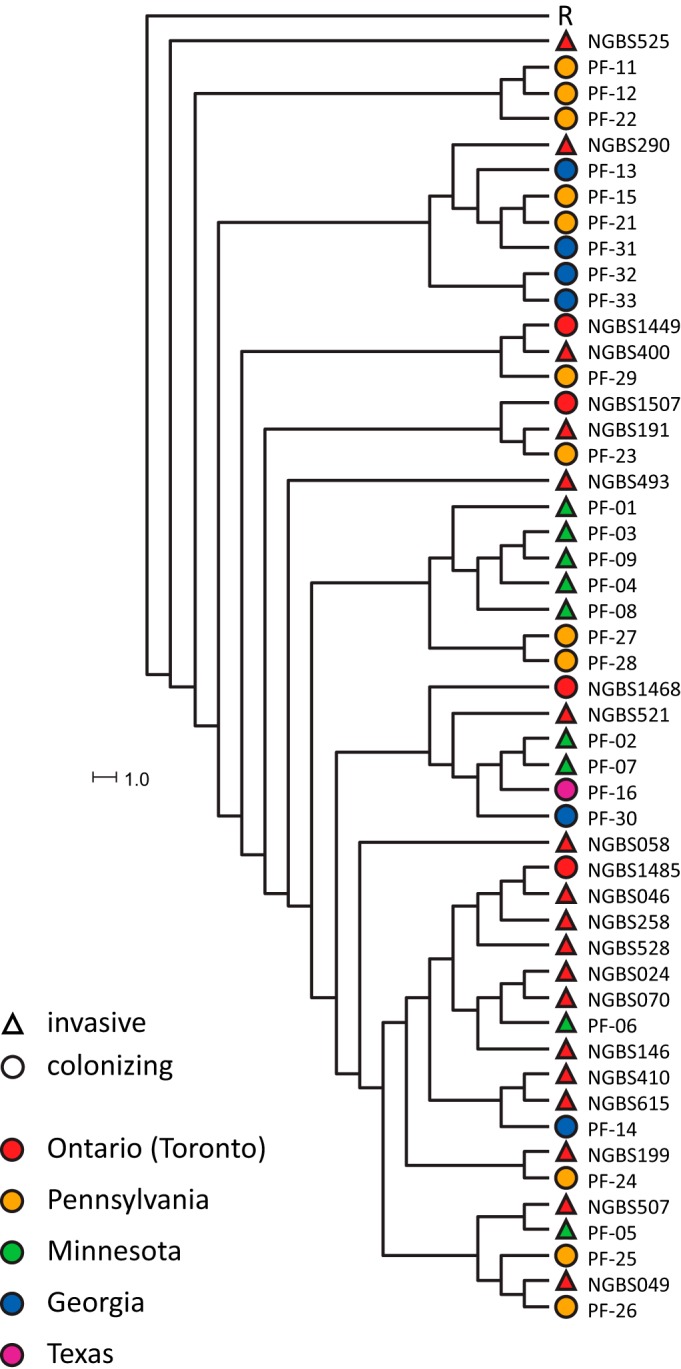
Phylogenetic relationships of colonizing and invasive serotype IV ST459 group B Streptococcus isolates from metropolitan Toronto and regions of the United States. Neighbor-joining phylogenetic tree based on 949 nonredundant SNP loci identified among all ST459 strains included in this study relative to the reference core genome of strain NGBS061 (R) as well as among 18 previously reported invasive serotype IV ST459 organisms isolated from patients with invasive disease in Canada. Triangles indicate invasive strains, circles represent colonizing isolates, and colors represent the geographical location of origin.
Multiple episodes of recombination leading to capsule switching among colonizing GBS isolates from metropolitan Toronto.
Among our collection of colonizing GBS isolates from metropolitan Toronto, some serotypes were clearly associated with specific CCs (Fig. 1). However, we observed several exceptions to these patterns. We hypothesized that those “mismatches” resulted from recombination leading to capsular switching. To investigate this hypothesis in more detail, we examined at the whole-genome level six examples (see Table S2) of unusual serotype/MLST genotype associations.
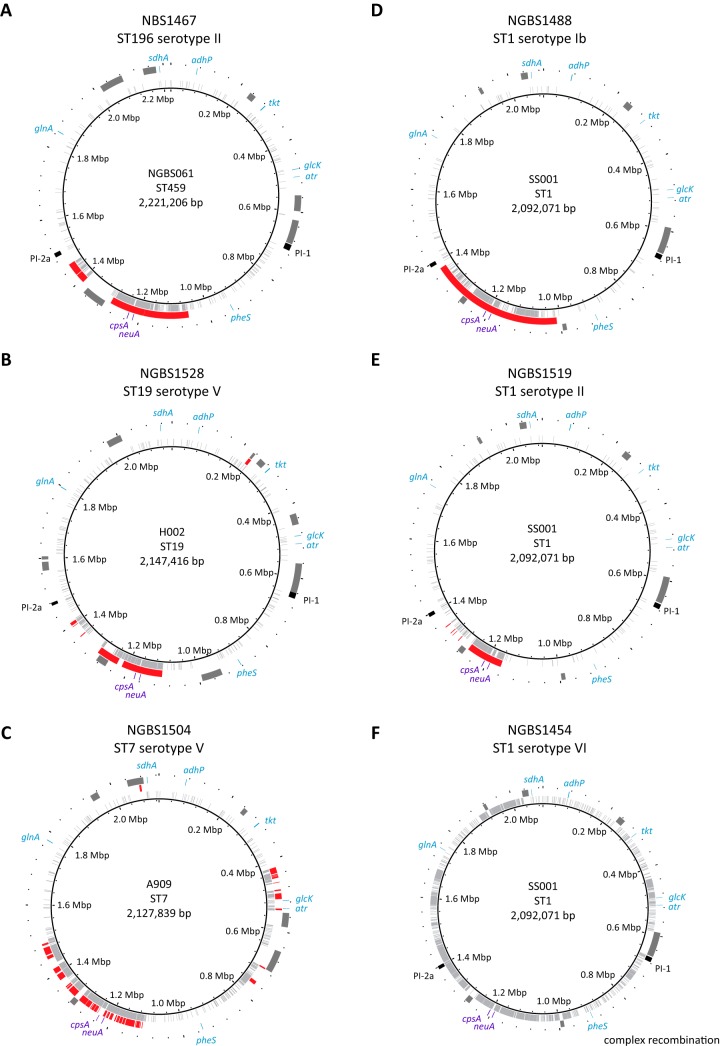
One of the isolates was serotype II and belonged to ST196 (CC1), which is commonly associated with serotype IV (27, 29). When we plotted polymorphisms identified in the genome of this isolate relative to reference strain NGBS061 (ST459, a single locus variant of ST196), we observed a nonrandom distribution of polymorphisms, with an overabundance of polymorphisms clustering in an area of the genome surrounding the cps locus (Fig. 3A). Thus, the genome of this strain is, for the most part, highly similar to that of serotype IV ST196 strains but differs at the capsular locus. The strain possessed a cpsII locus, which likely was acquired from a serotype II donor strain by recombination. To precisely define the recombination area, we used BratNextGen, which identified a region of 294,875 bp that spans across the cps locus (see Table S5). Similarly, we identified one serotype V strain belonging to ST19. Strains of ST19 are commonly associated with serotype III GBS (6). When we examined polymorphisms identified in the genome of this strain relative to the genome of reference strain H002 (an ST19 serotype III vaginal isolate from China) (33), we again found a distinct region of recombination around the capsular locus (Fig. 3B). One ST7 strain was found to be serotype V. This strain also had a distinct region of recombination, which included the cps locus, when polymorphism data were plotted against serotype Ia ST7 reference strain A909 (Fig. 3C). BratNextGen was used to define precisely the extent of recombination (see Table S5). Most of our colonizing ST1 strains from metropolitan Toronto were serotype V, but we identified one strain each of serotypes Ib, II, and VI among the ST1 organisms. Genomic analysis showed that strains of serotypes Ib and II acquired these capsule types by means of a single recombination event resulting in capsular switch (Fig. 3D and E; see also Table S5). However, the serotype VI ST1 strain showed a much more complex genomic organization (Fig. 3F). We are investigating in more detail the events leading to these complex arrangements using a collection of invasive and colonizing serotype VI strains (Neemuchwala et al., manuscript in preparation).
FIG 3.
Recombination leading to serotype switching in colonizing group B Streptococcus strains. Genome-wide polymorphisms identified relative to appropriate reference genomes are shown for six colonizing strains (A to F). For each of the panels, reference genomes are noted within the circles. Colonizing strain names, sequence types, and serotypes are given above each circle. Polymorphisms are plotted in light gray in the inner circles. Recombination events relative to the reference strain are shown in red in the second circles. MLST genes are indicated in blue in the outermost circles. Mobile genetic elements defined in the reference strains are shown in dark gray. Polymorphisms identified within these areas were excluded from the analysis. Pilus island loci are marked in black. The cps loci, bounded by genes neuA and cpsA, are marked in purple.
Antibiotic susceptibility of colonizing GBS isolates.
All of the 102 colonizing GBS strains from metropolitan Toronto were sensitive to penicillin, ampicillin, and vancomycin. None of the strains displayed high-level gentamicin resistance (HLGR). On the other hand, only 11 strains were sensitive to tetracycline, erythromycin, and clindamycin (Table 1; see also Tables S6 and S7 for antimicrobial resistance profiles by serotype and ST, respectively). With the exception of serotype VI, we observed resistance to tetracycline in each of the serotypes. A total of 89% of the colonizing isolates were resistant to tetracycline. Resistance to erythromycin and clindamycin was observed in 36% and 25% of the isolates, respectively. Among the isolates that were sensitive to clindamycin, nine had inducible clindamycin resistance (Table 1), for an overall rate of 33% constitutive or inducible resistance to clindamycin. Erythromycin resistance was found in all of the clonal complexes, but especially among ST1 and ST459. Consistently, all serotype IV ST459 isolates from the United States, including the invasive strains from Minnesota, were resistant to erythromycin and clindamycin (see Table S8).
TABLE 1.
Antibiotic susceptibility profiles of GBS colonizing isolates from metropolitan Toronto
| Antimicrobial drug | No. of isolates (%)a |
MIC (μg/ml)b |
|||
|---|---|---|---|---|---|
| Resistant | Susceptible | MIC50 | MIC90 | Range | |
| Tetracycline | 91 (89) | 11 (11) | 32 | 64 | 0.25 to >64 |
| Erythromycin | 37 (36) | 65 (64) | 0.12 | >8 | <0.06 to >8 |
| Clindamycin (constitutive) | 25 (25) | 77 (75) | 0.12 | >8 | <0.06 to >8 |
| Clindamycin (inducible) | 34 (33) | 68 (67) | — | — | — |
| Penicillin | 0 (0) | 102 (100) | 0.12 | 0.25 | <0.06–0.25 |
| Ampicillin | 0 (0) | 102 (100) | 0.06 | 0.12 | 0.03–0.12 |
| Vancomycin | 0 (0) | 102 (100) | 0.5 | 0.5 | 0.25–0.5 |
Cutoff values of susceptibility and resistance as per CLSI guidelines. No strains of intermediate susceptibility were identified.
MIC50, MIC of 50% of the strains; MIC90, MIC of 90% of the strains. —, not available.
DISCUSSION
Colonizing GBS isolates may act as reservoirs of serotype diversity, virulence factors, and antibiotic resistance. Thus, defining the population structure of colonizing isolates is key for understanding GBS disease. Using a combination of traditional typing tools and whole-genome sequencing, we examined a collection of colonizing GBS isolates recovered from pregnant women in metropolitan Toronto. Overall, the serotype distribution of GBS colonizing isolates closely mirrored the previously reported serotype distribution of invasive GBS diseases in the area (34), although serotypes VII, VIII, and IX were absent from the colonizing collection. The three most commonly identified serotypes among the colonizing isolates were III, Ia, and V. A similar predominance of serotypes III, Ia, and V has been observed among colonizing isolates in the United States (15) and among invasive GBS strains in other Canadian geographies (34, 35). The genetic diversity was relatively high. We identified more than 20 STs among the colonizing isolates from metropolitan Toronto.
We identified resistance to several commonly used antimicrobials in a substantial number of the GBS colonizing strains. The rates of GBS resistance to erythromycin and clindamycin have been increasing in Canada and elsewhere in the world (22, 36). The rate of macrolide resistance in colonizing GBS isolates in metropolitan Toronto was most recently characterized in 1999, when 18% and 8% of isolates were resistant to erythromycin and clindamycin, respectively (22). In our collection from 2014, resistance to erythromycin was significantly higher at 36% (P = 0.004, chi-square test). The rate of clindamycin resistance among the colonizing isolates was also significantly greater in 2014 (25%) (P = 0.001, chi-square test). The increasing resistances to macrolide and lincosamide antibiotics compared to those in the same population in the 1990s reflects the global trend of increasing resistance to these antibiotics (6, 22). On the other hand, we did not observe decreased susceptibility to penicillin in any of the metropolitan Toronto colonizing isolates. We also did not observe resistance to vancomycin or HLGR among isolates. GBS isolates resistant to these two antimicrobials have recently been reported in other geographies (37, 38).
Six GBS colonizing isolates were nontypeable by serology. Although these strains may represent new serotypes, in most cases, the absence of reactions with capsular antisera has been ascribed to mutations in the cps locus leading to the inactivation of enzymes involved in capsular polysaccharide biosynthesis (39–41). In accordance with this hypothesis, WGS analysis of nontypeable strains revealed the presence of mutations in one or more genes of their respective cps loci. The identified mutations are predicted in most cases to result in abolishment of capsule expression. Encapsulation is critical for invasive GBS disease but may be less important for carriage. In fact, it has been suggested that unencapsulation may favor GBS colonization (42). During laboratory routine typing and surveillance from 2009 to 2014, we typed 948 invasive GBS isolates collected in metropolitan Toronto and identified 10 (1%) that were nontypeable by serological methods (34, and our unpublished data). Thus, there is a significantly higher percentage of nontypeable strains among colonizing isolates than among invasive isolates in metropolitan Toronto (P = 0.002, chi-square test). We speculate that during long periods of persistent colonization, GBS isolates may be more prone to exchange DNA laterally and to accumulate mutations resulting in the loss of capsule expression.
It has long been described that among LOD isolates there is a disproportionate number of “hypervirulent” serotype III ST17 strains (8, 11, 43). ST17 strains normally express HvgA, a factor associated with increased adherence to epithelial cells and with enhanced ability to cross the blood-brain barrier (44, 45). Our recent investigation of invasive GBS in metropolitan Toronto identified that the majority (89%) of LOD serotype III isolates were hvgA gene positive (34). In contrast, a lower proportion (58%) of the serotype III colonizing strains belonged to ST17. However, all of the colonizing ST17 serotype III isolates that we identified possessed the hvgA gene. Interestingly, this gene has recently been described among serotype IV ST459 invasive GBS isolates from Alberta, Canada (31). The emergence of serotype IV GBS as an agent of invasive disease, particularly among adults, was first reported in the United States (27, 46) and later in Canada (31, 34). Frequencies of less than 1% were common in the 1990s (46) but have increased to 6 to 12% in populations studied between 2008 and 2015 (27, 29, 30, 47). The emergence of serotype IV GBS among colonizing isolates has also been noticed in the United States (28) and Europe (48). In this study, we found that serotype IV strains accounted for 6% of the colonizing GBS isolates recovered in metropolitan Toronto. This frequency of isolation is higher than the one described before 2000 in Canada (35) and is consistent with the rate of isolation of serotype IV strains from invasive GBS disease in metropolitan Toronto between 2009 and 2012 (34). It is also similar to the frequencies of isolation of serotype IV GBS from invasive disease cases submitted to our laboratories between 2013 and 2015 (our unpublished data). Most serotype IV carriage strains belonged to ST459, a relatively recently described ST originally identified in the United States (27, 28). Genome-wide SNP phylogenies demonstrated that colonizing and invasive ST459 serotype IV GBS strains from metropolitan Toronto and the United States are members of the same genetic pool and constitute a genetically highly homogeneous group of organisms that continues to expand across North America. However, unlike reports from Alberta, Canada (31), none of the ST459 organisms included in our extended collection of colonizing and invasive ST459 serotype IV GBS isolates from metropolitan Toronto and regions of the United States were hvgA positive.
Capsular switching has been described in group B Streptococcus (7, 34, 47, 49) and extensively studied in S. pneumoniae (50). In our collection, we observed five clear examples of single recombinatorial events leading to capsule switching. Interestingly, capsular switching occurred across multiple serotypes and among strains with dissimilar genomic backgrounds. We speculate that multiserotype colonization of the urogenital tract (51) and the persistence of colonization over many years in some patients (P. Ferrieri, unpublished observations) may facilitate capsular switching by lateral exchange of DNA among GBS colonizing strains. Implementing GBS vaccination using the trivalent conjugate formulation may have profound effects on the community of colonizing GBS, as has been exemplified by the emergence of vaccine escape variants after pneumococcal conjugate vaccines were introduced (32, 52). As shown here, capsular switching occurred relatively frequently among the colonizing isolates. The additional immunogenic pressure induced by vaccination may further expand non-vaccine serotypes and/or select recombinant vaccine escape variants in both colonizing and invasive GBS. Therefore, active surveillance of GBS vaginal/rectal colonization and invasive disease and identification of serotypes is critical in the pre- and postvaccine era.
MATERIALS AND METHODS
Collection, growth conditions, and typing of GBS colonizing isolates.
Nine out of 13 hospital members of the Toronto Invasive Bacterial Diseases Network (53) recovered GBS isolates from consecutive vaginal/rectal swabs from pregnant women in metropolitan Toronto and Peel region (54). These 9 hospitals provide approximately 80% of obstetrical services in the geographical area. Samples were collected between 1 November and 15 December 2014. Nineteen colonizing serotype IV ST459 isolates and nine invasive serotype IV ST459 isolates from the United States collected between 2005 and 2014 were also included in this investigation (see Table S1 in the supplemental material). Strains were cultured on Columbia agar plates containing 5% sheep blood and grown at 37°C with 5% CO2. Liquid cultures were grown overnight in Todd-Hewitt broth supplemented with 0.2% yeast extract. DNA was prepared from 10 ml of overnight liquid cultures using the Qiagen DNA minikit, following the manufacturers' instructions for Gram-positive organisms (Qiagen, Toronto, Canada). PCR amplification of a 234-bp region of the monocopy regulatory gene dltR, which is specific to GBS (55), was used to confirm the species identification. Serotyping was performed at the National Microbiology Laboratory in Winnipeg, Manitoba, by latex agglutination, as previously described (56). Serotyping of the isolates from the United States was performed as previously described, using monospecific rabbit antisera prepared in-house (57). PCR capsular typing of nontypeable strains was performed using a multiplex PCR scheme (5). PCR amplification of the CC17-associated virulence gene hvgA and MLST determinations were carried out as previously described (34).
Antibiotic susceptibility testing.
Susceptibility to ampicillin, penicillin, vancomycin, tetracycline, clindamycin, and erythromycin was tested by agar dilution according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (58). To determine inducible clindamycin resistance, clindamycin disks were placed on plates at 12 and 22 mm from a central erythromycin (15 μg) disk and incubated overnight at 37°C in 5% CO2. Inducible resistance was indicated by the blunting of growth between clindamycin and erythromycin disks and a lack of inhibition around the clindamycin disk. We defined constitutive resistance as growth inhibition zones of ≤15 mm around both the clindamycin and erythromycin disks (58). HLGR has recently been reported in GBS (37). In the absence of CLSI guidelines for GBS, we followed the agar-dilution procedures described by Sendi et al. (37).
Whole-genome sequencing and bioinformatics analysis.
Genomic libraries of selected strains were prepared using Nextera XT kits (Illumina, San Diego, CA) and sequenced as paired-end reads (of 101 bp or 150 bp) on Illumina HiSeq 2500 or MiSeq instruments (Illumina). In all cases, quality scores, as determined using Illumina onboard software, were >30 (data not shown). Onboard software was also used to parse the multiplexed sequencing reads and remove barcode information (data not shown). The average read depth across the genomes was 197× (see Table S2, which also lists Sequence Read Archive accession numbers and assembly quality metrics for all genomes sequenced here). The genome sequences of the following strains were used as references for genomic comparisons: strain NGBS061 (serotype IV, ST459, isolated in 2010 from a case of invasive GBS disease in Toronto and the only available circularized ST459 genome), strain A909 (serotype Ia, ST7), strain H002 (serotype III, ST19) and strain SGBS001 (serotype V, ST1) (GenBank accession numbers CP007631.2, CP000114.1, CP011329.1, and CP010867.1, respectively). Polymorphisms were identified relative to the reference genomes using the variant ascertainment algorithm VAAL (59). Whole-genome SNP-based phylogenies were established as follows. A matrix file containing the genotype of all strains at each polymorphic locus was created from the VAAL polymorphism output data using a custom script. For each individual strain, SNPs were then concatenated in order of occurrence relative to the genome of the reference strain and converted to a multiFASTA sequence. Neighbor-joining phylogenetic trees (1,000 bootstrap replications) were then generated with SplitsTree4 (60). The A5 pipeline (61) run with default parameters was used for de novo assembly of Illumina sequenced GBS strains. Recombination was assessed with BratNextGen (62) using 20 replicates and 100 iterations with a significance cutoff of 0.05. Genome visualizations were created using the BLAST ring image generator (BRIG) (63) and edited using Adobe Illustrator.
Accession number(s).
The sequences described were submitted to the Sequence Read Archive under the accession numbers SRR4414138 to SRR4414182.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01615-16.
REFERENCES
- 1.Farley MM. 2001. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 33:556–561. doi: 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- 2.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ, Active Bacterial Core surveillance/Emerging Infections Program Network. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 3.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE. 2005. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect Immun 73:3096–3103. doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL. 2007. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol 45:2929–2936. doi: 10.1128/JCM.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao K, Poulsen K, Maione D, Rinaudo CD, Baldassarri L, Telford JL, Sorensen UB, Members of the DEVANI Study Group, Kilian M. 2013. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J Clin Microbiol 51:503–507. doi: 10.1128/JCM.02417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Cunha V, Davies MR, Douarre PE, Rosinski-Chupin I, Margarit I, Spinali S, Perkins T, Lechat P, Dmytruk N, Sauvage E, Ma L, Romi B, Tichit M, Lopez-Sanchez MJ, Descorps-Declere S, Souche E, Buchrieser C, Trieu-Cuot P, Moszer I, Clermont D, Maione D, Bouchier C, McMillan DJ, Parkhill J, Telford JL, Dougan G, Walker MJ, DEVANI Consortium, Holden MT, Poyart C, Glaser P. 2014. Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nature Commun 5:4544. doi: 10.1038/ncomms5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan SL, Granlund M, Sellin M, Lagergard T, Spratt BG, Norgren M. 2005. Multilocus sequence typing of Swedish invasive group B Streptococcus isolates indicates a neonatally associated genetic lineage and capsule switching. J Clin Microbiol 43:3727–3733. doi: 10.1128/JCM.43.8.3727-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones N, Oliver KA, Barry J, Harding RM, Bisharat N, Spratt BG, Peto T, Crook DW, Oxford Group BSC. 2006. Enhanced invasiveness of bovine-derived neonatal sequence type 17 group B Streptococcus is independent of capsular serotype. Clin Infect Dis 42:915–924. doi: 10.1086/500324. [DOI] [PubMed] [Google Scholar]
- 9.Martins ER, Pessanha MA, Ramirez M, Melo-Cristino J, Portuguese Group for the Study of Streptococcal I. 2007. Analysis of group B streptococcal isolates from infants and pregnant women in Portugal revealing two lineages with enhanced invasiveness. J Clin Microbiol 45:3224–3229. doi: 10.1128/JCM.01182-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyart C, Reglier-Poupet H, Tazi A, Billoet A, Dmytruk N, Bidet P, Bingen E, Raymond J, Trieu-Cuot P. 2008. Invasive group B streptococcal infections in infants, France. Emerg Infect Dis 14:1647–1649. doi: 10.3201/eid1410.080185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musser JM, Mattingly SJ, Quentin R, Goudeau A, Selander RK. 1989. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B Streptococcus) causing invasive neonatal disease. Proc Natl Acad Sci U S A 86:4731–4735. doi: 10.1073/pnas.86.12.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin FY, Whiting A, Adderson E, Takahashi S, Dunn DM, Weiss R, Azimi PH, Philips JB III, Weisman LE, Regan J, Clark P, Rhoads GG, Frasch CE, Troendle J, Moyer P, Bohnsack JF. 2006. Phylogenetic lineages of invasive and colonizing strains of serotype III group B Streptococci from neonates: a multicenter prospective study. J Clin Microbiol 44:1257–1261. doi: 10.1128/JCM.44.4.1257-1261.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuchat A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner C, Turner P, Po L, Maner N, De Zoysa A, Afshar B, Efstratiou A, Heath PT, Nosten F. 2012. Group B streptococcal carriage, serotype distribution and antibiotic susceptibilities in pregnant women at the time of delivery in a refugee population on the Thai-Myanmar border. BMC Infect Dis 12:34. doi: 10.1186/1471-2334-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ippolito DL, James WA, Tinnemore D, Huang RR, Dehart MJ, Williams J, Wingerd MA, Demons ST. 2010. Group B Streptococcus serotype prevalence in reproductive-age women at a tertiary care military medical center relative to global serotype distribution. BMC Infect Dis 10:336. doi: 10.1186/1471-2334-10-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. 2000. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol 96:498–503. doi: 10.1016/S0029-7844(00)00977-7. [DOI] [PubMed] [Google Scholar]
- 17.Puopolo KM, Madoff LC, Eichenwald EC. 2005. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 115:1240–1246. doi: 10.1542/peds.2004-2275. [DOI] [PubMed] [Google Scholar]
- 18.Money DM, Dobson S, Canadian Paediatric Society, Infectious Diseases Committee. 2004. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can 26:826–840. doi: 10.1016/S1701-2163(16)30157-8. [DOI] [PubMed] [Google Scholar]
- 19.Money D, Allen VM, Society of Obstetrician and Gynaecologists of Canada. 2013. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can 35:939–951. doi: 10.1016/S1701-2163(15)30818-5. [DOI] [PubMed] [Google Scholar]
- 20.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). 2010. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 59:1–36. [PubMed] [Google Scholar]
- 21.Bland ML, Vermillion ST, Soper DE, Austin M. 2001. Antibiotic resistance patterns of group B streptococci in late third-trimester rectovaginal cultures. Am J Obstet Gynecol 184:1125–1126. doi: 10.1067/mob.2001.115478. [DOI] [PubMed] [Google Scholar]
- 22.de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A. 2001. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B Streptococcus isolates from Ontario, Canada. Antimicrob Agents Chemother 45:3504–3508. doi: 10.1128/AAC.45.12.3504-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker CJ, Kasper DL. 1976. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 24.Edwards MS, Gonik B. 2013. Preventing the broad spectrum of perinatal morbidity and mortality through group B streptococcal vaccination. Vaccine 31(Suppl4:D66–D71. doi: 10.1016/j.vaccine.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Heyderman RS, Madhi SA, French N, Cutland C, Ngwira B, Kayambo D, Mboizi R, Koen A, Jose L, Olugbosi M, Wittke F, Slobod K, Dull PM. 2016. Group B Streptococcus vaccination in pregnant women with or without HIV in Africa: a non-randomised phase 2, open-label, multicentre trial. Lancet Infect Dis 16:546–555. doi: 10.1016/S1473-3099(15)00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leroux-Roels G, Maes C, Willekens J, De Boever F, de Rooij R, Martell L, Bedell L, Wittke F, Slobod K, Dull P. 2016. A randomized, observer-blind phase Ib study to identify formulations and vaccine schedules of a trivalent group B Streptococcus vaccine for use in non-pregnant and pregnant women. Vaccine 34:1786–1791. doi: 10.1016/j.vaccine.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 27.Ferrieri P, Lynfield R, Creti R, Flores AE. 2013. Serotype IV and invasive group B Streptococcus disease in neonates, Minnesota, USA, 2000-2010. Emerg Infect Dis 19:551–558. doi: 10.3201/eid1904.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diedrick MJ, Flores AE, Hillier SL, Creti R, Ferrieri P. 2010. Clonal analysis of colonizing group B Streptococcus, serotype IV, an emerging pathogen in the United States. J Clin Microbiol 48:3100–3104. doi: 10.1128/JCM.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teatero S, McGeer A, Li A, Gomes J, Seah C, Demczuk W, Martin I, Wasserscheid J, Dewar K, Melano RG, Fittipaldi N. 2015. Population structure and antimicrobial resistance of invasive serotype IV group B Streptococcus, Toronto, Ontario, Canada. Emerg Infect Dis 21:585–591. doi: 10.3201/eid2014.140759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teatero S, Athey TB, Van Caeseele P, Horsman G, Alexander DC, Melano RG, Li A, Flores AR, Shelburne SA, McGeer A, Demczuk W, Martin I, Fittipaldi N. 2015. Emergence of serotype IV group B Streptococcus adult invasive disease in Manitoba and Saskatchewan, Canada, is driven by clonal sequence type 459 Strains. J Clin Microbiol 53:2919–2926. doi: 10.1128/JCM.01128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alhhazmi A, Hurteau D, Tyrrell GJ. 2016. Epidemiology of invasive group B streptococcal disease in Alberta, Canada, from 2003 to 2013. J Clin Microbiol 54:1774–1781. doi: 10.1128/JCM.00355-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brueggemann AB, Pai R, Crook DW, Beall B. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 3:e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Li L, Luo F, Liang W, Gan X, Chen M. 2015. Genome sequence of Streptococcus agalactiae strain H002, serotype III, isolated in China from a pregnant woman. Genome Announc 3:e01109-15. doi: 10.1128/genomeA.01109-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teatero S, McGeer A, Low DE, Li A, Demczuk W, Martin I, Fittipaldi N. 2014. Characterization of invasive group B Streptococcus strains from the greater Toronto area, Canada. J Clin Microbiol 52:1441–1447. doi: 10.1128/JCM.03554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyrrell GJ, Senzilet LD, Spika JS, Kertesz DA, Alagaratnam M, Lovgren M, Talbot JA. 2000. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study–1996. Sentinel Health Unit Surveillance System Site Coordinators. J Infect Dis 182:168–173. doi: 10.1086/315699. [DOI] [PubMed] [Google Scholar]
- 36.Compain F, Hays C, Touak G, Dmytruk N, Trieu-Cuot P, Joubrel C, Poyart C. 2014. Molecular characterization of Streptococcus agalactiae isolates harboring small erm(T)-carrying plasmids. Antimicrob Agents Chemother 58:6928–6930. doi: 10.1128/AAC.03855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sendi P, Furitsch M, Mauerer S, Florindo C, Kahl BC, Shabayek S, Berner R, Spellerberg B. 2016. Chromosomally and extrachromosomally mediated high-level gentamicin resistance in Streptococcus agalactiae. Antimicrob Agents Chemother 60:1702–1707. doi: 10.1128/AAC.01933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park C, Nichols M, Schrag SJ. 2014. Two cases of invasive vancomycin-resistant group B Streptococcus infection. N Engl J Med 370:885–886. doi: 10.1056/NEJMc1308504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramaswamy SV, Ferrieri P, Flores AE, Paoletti LC. 2006. Molecular characterization of nontypeable group B Streptococcus. J Clin Microbiol 44:2398–2403. doi: 10.1128/JCM.02236-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosini R, Campisi E, De Chiara M, Tettelin H, Rinaudo D, Toniolo C, Metruccio M, Guidotti S, Sorensen UB, Kilian M, DEVANI Consortium, Ramirez M, Janulczyk R, Donati C, Grandi G, Margarit I. 2015. Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS One 10:e0125985. doi: 10.1371/journal.pone.0125985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswamy SV, Ferrieri P, Madoff LC, Flores AE, Kumar N, Tettelin H, Paoletti LC. 2006. Identification of novel cps locus polymorphisms in nontypable group B Streptococcus. J Med Microbiol 55:775–783. doi: 10.1099/jmm.0.46253-0. [DOI] [PubMed] [Google Scholar]
- 42.Rubens CE, Wessels MR, Heggen LM, Kasper DL. 1987. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A 84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bisharat N, Crook DW, Leigh J, Harding RM, Ward PN, Coffey TJ, Maiden MC, Peto T, Jones N. 2004. Hyperinvasive neonatal group B Streptococcus has arisen from a bovine ancestor. J Clin Microbiol 42:2161–2167. doi: 10.1128/JCM.42.5.2161-2167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tazi A, Disson O, Bellais S, Bouaboud A, Dmytruk N, Dramsi S, Mistou MY, Khun H, Mechler C, Tardieux I, Trieu-Cuot P, Lecuit M, Poyart C. 2010. The surface protein HvgA mediates group B Streptococcus hypervirulence and meningeal tropism in neonates. J Exp Med 207:2313–2322. doi: 10.1084/jem.20092594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tazi A, Bellais S, Tardieux I, Dramsi S, Trieu-Cuot P, Poyart C. 2012. Group B Streptococcus surface proteins as major determinants for meningeal tropism. Curr Opin Microbiol 15:44–49. doi: 10.1016/j.mib.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis 49:85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 47.Meehan M, Cunney R, Cafferkey M. 2014. Molecular epidemiology of group B streptococci in Ireland reveals a diverse population with evidence of capsular switching. Eur J Clin Microbiol Infect Dis 33:1155–1162. doi: 10.1007/s10096-014-2055-5. [DOI] [PubMed] [Google Scholar]
- 48.Florindo C, Damiao V, Silvestre I, Farinha C, Rodrigues F, Nogueira F, Martins-Pereira F, Castro R, Borrego M, Santos-Sanches I, the Group for the Prevention of Neonatal GBS Infection. 2014. Epidemiological surveillance of colonising group B Streptococcus epidemiology in the Lisbon and Tagus Valley regions, Portugal (2005 to 2012): emergence of a new epidemic type IV/clonal complex 17 clone. Euro Surveill 19:pii=20825 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20825. [DOI] [PubMed] [Google Scholar]
- 49.Martins ER, Melo-Cristino J, Ramirez M. 2010. Evidence for rare capsular switching in Streptococcus agalactiae. J Bacteriol 192:1361–1369. doi: 10.1128/JB.01130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyres KL, Lambertsen LM, Croucher NJ, McGee L, von Gottberg A, Linares J, Jacobs MR, Kristinsson KG, Beall BW, Klugman KP, Parkhill J, Hakenbeck R, Bentley SD, Brueggemann AB. 2013. Pneumococcal capsular switching: a historical perspective. J Infect Dis 207:439–449. doi: 10.1093/infdis/jis703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrieri P, Hillier SL, Krohn MA, Moore D, Paoletti LC, Flores AE. 2004. Characterization of vaginal and rectal colonization with multiple serotypes of group B streptococci using multiple colony picks. Indian J Med Res 119(Suppl):208–212. [PubMed] [Google Scholar]
- 52.Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331:430–434. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies HD, Adair CE, Schuchat A, Low DE, Sauve RS, McGeer A, Alberta Neonatal Group B Streptococcal Network, the Toronto Invasive Bacterial Diseases Network. 2001. Physicians' prevention practices and incidence of neonatal group B streptococcal disease in 2 Canadian regions. CMAJ 164:479–485. http://www.cmaj.ca/content/164/4/479.long. [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen SM, Sorensen UB. 2003. Method for quantitative detection and presumptive identification of group B streptococci on primary plating. J Clin Microbiol 41:1399–1403. doi: 10.1128/JCM.41.4.1399-1403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poyart C, Lamy MC, Boumaila C, Fiedler F, Trieu-Cuot P. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J Bacteriol 183:6324–6334. doi: 10.1128/JB.183.21.6324-6334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slotved HC, Elliott J, Thompson T, Konradsen HB. 2003. Latex assay for serotyping of group B Streptococcus isolates. J Clin Microbiol 41:4445–4447. doi: 10.1128/JCM.41.9.4445-4447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson DR, Ferrieri P. 1984. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol 19:506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing, 23rd informational supplement M100–S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 59.Nusbaum C, Ohsumi TK, Gomez J, Aquadro J, Victor TC, Warren RM, Hung DT, Birren BW, Lander ES, Jaffe DB. 2009. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat Methods 6:67–69. doi: 10.1038/nmeth.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 61.Tritt A, Eisen JA, Facciotti MT, Darling AE. 2012. An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7:e42304. doi: 10.1371/journal.pone.0042304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marttinen P, Hanage WP, Croucher NJ, Connor TR, Harris SR, Bentley SD, Corander J. 2012. Detection of recombination events in bacterial genomes from large population samples. Nucleic Acids Res 40:e6. doi: 10.1093/nar/gkr928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.