ABSTRACT
Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae generally cannot be treated with penicillins and cephalosporins. However, some later-generation cephalosporins, including cefepime, are poorly hydrolyzed by specific ESBL enzymes, and certain strains demonstrate in vitro susceptibility to these agents, potentially affording additional treatment opportunities. Moreover, the ability to adjust both the dose and dosing interval of beta-lactam agents allows the treatment of strains with elevated MICs that were formerly classified in the intermediate range. The ability to treat strains with elevated cefepime MICs is codified in new susceptible dose-dependent (SDD) breakpoints promulgated by the Clinical and Laboratory Standards Institute. In the interest of validating and implementing new cefepime SDD criteria, we evaluated the performances of Vitek 2, disk diffusion, and a MicroScan panel compared to that of reference broth microdilution (BMD) during the testing of 64 strains enriched for presumptive ESBL phenotype (based on nonsusceptibility to ceftriaxone). Surprisingly, categorical agreement with BMD was only 47.6%, 57.1%, and 44.6% for the three methods, respectively. Given these findings, we tested the performance of the HP D300 inkjet-assisted broth microdilution digital dispensing method (DDM), which was previously described by our group as an at-will testing alternative. In contrast to commercial methods, DDM results correlated well with the reference method, with 86% categorical agreement, 91.1% evaluable essential agreement, and no major or very major errors. The reproducibility and accuracy of MIC determinations were statistically equivalent to BMD. Our results provide support for the use of the DDM as a BMD equivalent methodology that will enable hospital-based clinical laboratories to support cefepime MIC-based dosing strategies.
KEYWORDS: ESBL, antimicrobial susceptibility testing, broth microdilution, cefepime, digital dispensing, extended-spectrum beta-lactamase producer, inkjet, susceptibility testing, susceptible dose dependent, verification
INTRODUCTION
Few treatment options remain for extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) (1). Therefore, the rapidly increasing worldwide prevalence of these strains is of great concern (2–8). Notably, in China, ESBL-E comprise over half of all Escherichia coli bloodstream infections (9), and colonization rates of intensive care unit patients in India have been reported to reach 90% (10). In the United States, prevalence has been rising, especially in certain vulnerable populations, such as patients in adult and pediatric intensive care units, patients with prolonged hospital stays, and patients with exposure to multiple antibiotics (11–15).
ESBL-E harbor serine beta-lactamase enzymes that are, at minimum, capable of hydrolyzing penicillins, at least one of the oxyimino-cephalosporins (commonly referred to as third-generation cephalosporins), and/or aztreonam (16). Of note, ESBL enzymes are both structurally and functionally diverse and potentially expressed at various levels, allowing variable phenotypic resistance, which in some cases can include all cephalosporins (17–20). Previously, Clinical Laboratory and Standards Institute (CLSI) guidance recommended routine ESBL confirmatory testing and classification of positive isolates as resistant (R) to all cephalosporins regardless of MIC data, even if the MIC of the isolate was in the range that would otherwise have been classified as susceptible (S) for a particular agent (21).
Notably, however, cefepime, a fourth-generation cephalosporin, often demonstrates more in vitro activity than earlier generation cephalosporins due to greater stability to ESBL hydrolysis (22, 23). This in vitro observation correlates with clinical outcome studies, which indicate that cefepime, when administered at higher-than-standard doses, may retain activity against some ESBL-E (24–26). These findings, combined with the observation that certain ESBL enzymes are not active against all third-generation cephalosporins (27), prompted the revisitation of CLSI guidelines, resulting in the lowering of interpretive breakpoints and the elimination of routine ESBL testing in favor of purely MIC-based cephalosporin antimicrobial susceptibility testing (AST) interpretations (26).
The opportunity to treat some presumptive ESBL-E was further codified in the current susceptible dose-dependent (SDD) interpretive category for cefepime (28). Specifically, SDD replaces the “intermediate” category for this drug and, depending on the exact MIC, recommends either an increased dose (MIC = 4 μg ml−1) or an increased dose and frequency (MIC = 8 μg ml−1). Clearly, such specific guidance is predicated on accurate MIC determination.
Unfortunately, these new SDD breakpoints have not yet been cleared by the U.S. Food and Drug Administration (FDA) and adopted by commercial antimicrobial susceptibility testing manufacturers. Therefore, in the interest of validating and implementing the new SDD criteria recommended by the CLSI, we evaluated the performances of several commercial methods (Vitek 2, disk diffusion [DD], and MicroScan panels) that were otherwise approved for testing of cefepime using FDA-cleared breakpoint criteria and a broth microdilution digital dispensing platform recently described by our laboratory (29). Importantly, in order to more informatively gauge accuracy, the strains selected for testing were enriched for presumptive ESBL isolates with MICs often bordering SDD breakpoints, and all methods were compared to reference manual broth microdilution.
RESULTS
The performances of Vitek 2, disk diffusion (DD), MicroScan, and the digital dispensing method (DDM) were compared to the performance of reference broth microdilution (BMD) using a collection of Enterobacteriaceae enriched for presumptive ESBL-E on the basis of ceftriaxone resistance by Vitek 2. Notably, we selected for strains that were designated by Vitek 2, our primary clinical platform, as having cefepime MICs in the CLSI susceptible (90.6%; n = 58) or SDD (9.4%; n = 6) ranges to test whether isolates with presumptive low cefepime MICs were being classified accurately. However, BMD indicated that 49.2%, 25.4%, and 25.4% of strains in this set were susceptible, SDD, and resistant, respectively (Table 1), suggesting a significant skew of Vitek 2 toward a more susceptible categorical interpretation. Notably, 47.6% of strains had a BMD MIC that fell on an interpretive breakpoint.
TABLE 1.
MIC distribution of the strain set
| Genus | No. of strains with a cefepime MICa (μg ml−1) of: |
|||||
|---|---|---|---|---|---|---|
| ≤1 | 2 | 4 | 8 | 16 | >16 | |
| Escherichia | 11 | 4 | 4 | 11 | 4 | 7 |
| Klebsiella | 4 | 2 | 0 | 2 | 1 | 2 |
| Otherb | 9 | 1 | 0 | 0 | 1 | 1 |
| Total no. (%) | 24 (37.5) | 7 (10.9) | 4 (6.3) | 13 (20.3) | 6 (9.4) | 10 (15.6) |
The modal consensus value is from at least three BMD measurements.
Other consists of Enterobacter cloacae (n = 5), Citrobacter freundii (3), Citrobacter amalonaticus (1), Enterobacter aerogenes (1), Proteus hauseri (1), and Proteus vulgaris (1).
Cefepime data from Vitek 2, DD, and MicroScan showed categorical agreement of 47.6%, 57.1%, and 44.6%, respectively, with BMD (Table 2); very major errors (VMEs) were 11.1%, 7.1%, and 0%; and major errors (MEs) were 0%, 1.8%, and 23.4%, respectively. The evaluable essential agreement (EA) of Vitek 2 was 21.7% (95% confidence interval [CI] = 9.2% to 40.5%) and overall EA was 52.3% (95% CI = 40.3% to 64.2%). EA for MicroScan was not evaluated because only three results were on scale. Given the significant discrepancies between clinical methods and BMD, we concluded that SDD CLSI breakpoints could not be reliably implemented with these methods and sought an alternative solution.
TABLE 2.
Comparison of commercial methods to reference BMD
| Method | % agreement (95% CI) |
% error category (95% CI) |
||||
|---|---|---|---|---|---|---|
| Evaluable essential | Overall essential | Categorical | Minor error | Major error | Very major error | |
| Vitek 2 | 20.8 (9.2–40.5) | 52.3 (40.3–64.2) | 47.6 (35.7–59.7) | 41.3 (29.9–53.5) | 0 | 11.1 (5.4–21.2) |
| Disk diffusion | NAa | NAa | 57.2 (44.1–69.2) | 33.9 (22.9–47) | 1.8 (0.3–9.4) | 7.1 (2.8–17.0) |
| MicroScan | NAb | NAb | 44.7 (31.4–58.8) | 31.9 (20.4–46.2) | 23.4 (13.6–37.2) | 0 |
NA, not applicable as disk diffusion is not an MIC-based method.
NA due to the limited number (n = 3) of on-scale results.
Our laboratory previously described the use of an HP D300-based digital dispensing method (DDM) to prepare a BMD equivalent in 384-well plate format with automated spectrophotometric plate reading and susceptibility calls (29). Here, we applied the same technology to generate an antimicrobial doubling dilution series in the standard 96-well plate format recommended for BMD. We first compared the reproducibility of the DDM and reference BMD through repeated measurements of all isolates in our collection. For this analysis, only on-scale measurements were used for calculations, as off-scale results are not log2 transformable. The majority of reference (78.9%) and DDM (80.7%) results were on scale. To compare within-method reproducibility, the log2 difference of each on-scale measurement from the within-method modal MIC was calculated and shown as a distribution in Fig. 1. Each measurement was further classified as “in range” (±1 twofold dilution from the modal MIC) or “out of range” based on the accepted 2-fold variability of reference BMD. We found that 95.5% (95% CI = 91.1% to 97.8%) of DDM and 92.6% (95% CI = 88.5% to 96.1%) of reference BMD measurements were in range, a nonsignificant difference (Fisher's exact test, P = 0.36).
FIG 1.
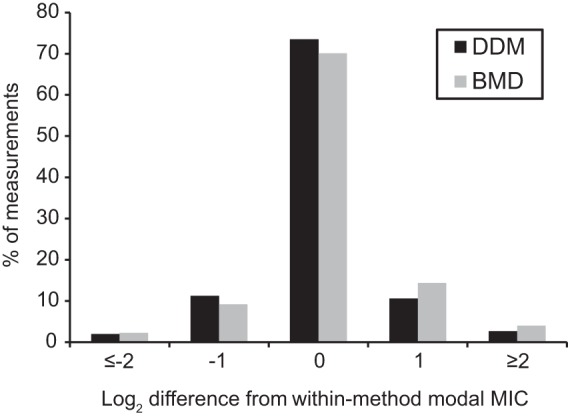
Log2 variance from modal MIC. The log2 differences shown represent the number of 2-fold dilutions away from the modal MIC. A total of 95.5% of DDM (n = 150) and 93.2% of BMD measurements (n = 163) were within ± 1 twofold dilution of the modal MIC, respectively.
We next investigated the accuracy of the DDM compared to BMD (Table 3). Evaluable EA, overall EA, and categorical agreement (CA) were 91.1% (95% CI = 85.8% to 94.7%), 88.9% (95% CI = 83.9% to 92.5%), and 86.0% (95% CI = 80.6% to 90.0%), respectively. There were no major or very major errors. There were 14% minor errors (MinEs) (n = 29 for the 207 total DDM tests performed), and the MICs for 75.9% (n = 22) of the minor errors were in evaluable EA. When SDD was evaluated as two separate categories based on an MIC of 4 or 8 μg ml−1, the DDM showed a CA of 82.6% (95% CI = 76.9% to 87.2%).
TABLE 3.
Comparison of the DDM to reference BMD
| Genus (no.) | No. of measurements with log2 difference from reference MICa |
% agreement (95% CI) |
% error category (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 3 | Evaluable essential | Overall essential | Categorical | Minor error | Major error | Very major error | |
| Escherichia (41) | 0 | 6 | 53 | 40 | 9 | 1 | 90.8 (83.9–94.9) | 88.6 (82.1–93.0) | 84.1 (76.9–89.4) | 15.9 (10.6–23.1) | 0 | 0 |
| Klebsiella (11) | 1 | 4 | 14 | 10 | 3 | 0 | 87.5 (71.9–95) | 89.1 (75.3–95.7) | 94.5 (82.3–95.5) | 5.4 (1.5–17.8) | 0 | 0 |
| Other (12) | 0 | 3 | 5 | 10 | 0 | 0 | 100 (82.4–100) | 89.4 (75.7–95.8) | 84.2 (69.6–92.6) | 15.7 (7.4–30.0) | 0 | 0 |
| Total no. (%, CI)b | 1 (0.6) | 13 (8.2) | 72 (45.3) | 60 (37.8) | 12 (7.5) | 1 (0.6) | 145 (91.1, 85.8–94.7) | 184 (88.9, 83.9–92.5) | 178 (86.0, 80.6–90.1) | 29 (14.0, 9.9–19.4) | 0 | 0 |
Includes only comparisons in which both the DDM result and the reference MIC were on scale (within the range of dilutions tested).
Numerical tabulations include comparison of all individual DDM measurements as applicable, performed in replicate for each strain as described in the Materials and Methods section, to the reference MIC (leading to a total of 207 comparisons for determination of categorical agreement and minor errors).
Notably, the percentage of in-range results for both the DDM and BMD were ∼4% lower than that previously observed in a more diverse set of Enterobacteriaceae (i.e., not enriched for ESBL phenotype) (29). This suggested a modestly increased intrinsic biological variability for cefepime response in the current strain set that would lower EA irrespective of intrinsic method accuracy. Moreover, nearly half the strains had an MIC that fell on a breakpoint, leading to an expectation of lower CA.
Therefore, to quantify for comparative purposes the variability of the reference standard, we also determined the within-method agreement of BMD. For each strain, individual BMD MIC measurements were compared to the modal BMD MIC for a within-method evaluable EA of 93.8% (95% CI = 89.2% to 96.5%), an overall EA of 93.6% (95% CI = 89.5% to 96.1%), and a CA of 90.6% (95% CI = 86% to 93.8%), results not significantly different from the DDM (P = 0.5, P = 0.1, and P = 0.2, respectively). In addition, when SDD was evaluated as two categories based on an MIC of 4 or 8 μg ml−1, within-method BMD showed a CA of 87.4% (95% CI = 82.5% to 91.2%), results not statistically different from the DDM (P = 0.2).
DISCUSSION
The new CLSI interpretive criteria for cefepime present a challenge for clinical microbiology laboratories. Updated breakpoint changes must be validated for use with FDA-cleared methods. Importantly, the new SDD category for cefepime recommends distinct dosing regimens predicated on a specific MIC result (i.e., ≤2, 4, 8, ≥16 μg ml−1). The underlying premise for use of SDD is that available AST methods provide highly accurate MIC values across this range.
Accordingly, prior to implementation of SDD breakpoints, we investigated the clinical performance of the Vitek 2, disk diffusion, and a manual MicroScan panel in interrogation of a strain set enriched for cefepime MICs across the SDD breakpoint range. Strains chosen for analysis were enriched for the presumptive ESBL phenotype (ceftriaxone resistant) and demonstrated by a screening method (Vitek 2) to show a susceptible or SDD phenotype for cefepime. These are the precise types of strains for which SDD is thought to provide utility: strains harboring presumptive enzymes that hydrolyze cefepime relatively poorly and therefore are amenable to treatment with more intense cefepime exposure.
The commercial methods tested performed poorly as assessed by metrics such as EA and CA. These findings were similar to reports prior to SDD introduction and the lowering of cefepime breakpoint susceptibility cutoffs in which Vitek 2 demonstrated a high false-susceptibility rate and a low EA for ESBL-producing organisms (30). Low cefepime CA was also previously observed for ESBL-E strains using other commercial methods, including MicroScan and disk diffusion (31).
We therefore considered an alternative use of the DDM technology that we previously validated in a 384-well plate format with automated interpretation as a BMD equivalent (29). In the present case, we adapted DDM technology to a 96-well plate format with visual reading of MICs in order to replicate exactly classic BMD, with the sole exception that antimicrobial doubling dilutions were created directly through the addition of precise droplet sizes from a stock solution rather than through serial dilution. Importantly, the DDM is technically simple, as it only requires a single pipetting step to create a total of 24 cefepime dilution series in the format described. Therefore, the DDM can be practically implemented in a clinical laboratory in contrast to laborious manual BMD testing.
Unlike commercial methods, the DDM was highly correlated with the BMD reference method. The modestly lower CA and EA observed compared to our previous DDM study (29) was expected due to the observed biological variability of the strain set for cefepime. Furthermore, the 14% minor error rate, although higher than the general 10% cutoff cited by Cumitech 31A, was completely expected. Specifically, Cumitech guidelines acknowledge the potential for and acceptability of a >10% minor error rate for strain sets containing a significant number of isolates with MICs on an interpretive breakpoint (32). The MICs of 47.6% of our strain set were on an interpretative breakpoint, a reasonable and compelling explanation for a somewhat elevated minor error rate. The accuracy of the DDM was further supported by the observation that DDM EA and CA were statistically indistinguishable from within-method BMD comparisons.
Notably, the ability of at-will dispensing technology to generate customized dilution series allows for more informative quality control (QC). MicroScan panels and other commercial methods, such as Vitek 2, determine or extrapolate MIC values from a narrow range of doubling dilutions surrounding breakpoints. In the MicroScan NM38 panel examined, the lowest panel concentration of cefepime (2 μg ml−1) is 16-fold above the quality control range for the QC strains recommended by the manufacturer and CLSI guidelines. Similarly, in the Vitek 2 GN73 panel, an MIC of ≤1 μg ml−1 is the lowest value reported. As such, either degradation or unintended misapplication of an antimicrobial by the manufacturer will be undetectable, leaving the end user in total reliance on the manufacturer to ensure adequate performance.
We note for MicroScan that cefepime MICs were consistently high compared to BMD and the DDM, apparent in the large number of observed major errors, and most consistent with low effective cefepime concentrations. Importantly, in this work, we prepared our DDM and BMD quality control dilution series to encompass the QC range of E. coli ATCC 25922 MICs, thereby allowing on-scale assessment of QC fidelity, which is of particular importance for relatively labile antibiotics such as cefepime.
Taken together, this study demonstrates that the clinical methods examined do not support the use of SDD breakpoints when tested against ESBL-E strains representative of those for which the SDD category was created. Based on our data, we would recommend that clinical laboratories verify their methodology prior to implementing these or future SDD breakpoints with a strain set whose MIC values span the SDD range and use a highly accurate reference dilution method as a comparator. If satisfactory performance cannot be ensured, SDD criteria should not be implemented. In this case, it is probably prudent for clinical laboratories to mark cefepime as resistant unless ceftriaxone resistance can be otherwise confidently ascribed to derepressed chromosomal ampC enzymes, which hydrolyze cefepime inefficiently (33). We also recommend that the CLSI revisit the suitability of disk diffusion for cefepime antimicrobial susceptibility determination in ESBL-E phenotype strains.
In contrast, we found that the DDM, as a true MIC-based methodology, was as accurate and reproducible as reference BMD. Moreover, the DDM has the ability to facilely create any required dilution series of any antimicrobial on demand. This flexibility will enable clinical labs, should they choose to validate this method as a laboratory-developed test, to tailor MIC panels to address this or future SDD implementation. Further, dilution series can be designed to encompass breakpoints for any relevant QC strain, to implement any future breakpoint updates, and to provide subdoubling dilution resolution near breakpoints of interest. Importantly, the DDM is performed with commercially available equipment and reagents. This includes the HP D300 digital dispenser (<$40,000), disposable HP T8+ Dispensehead cassettes (with up to 24 cefepime broth microdilution assays set up per channel at a cost of <$9 per channel), and standard microplates. Disposable and reagent costs per DDM cefepime microdilution assay were <$1. Therefore, we believe that the DDM will provide clinical laboratories with an important and accessible new tool to support strategies, such as SDD, that are designed to help address the emerging antimicrobial resistance threat.
MATERIALS AND METHODS
Bacterial strains and antimicrobials.
Sixty-four deidentified Enterobacteriaceae clinical isolates were collected at our institution under Institutional Review Board (IRB)-approved protocols between June and September 2016. All strains were colony purified prior to storage at −80°C in tryptic soy broth (BD Diagnostics, Franklin Lakes, NJ) containing 50% glycerol (Sigma-Aldrich, St. Louis, MO). Escherichia coli ATCC 25922 was obtained from the American Type Culture Collection (Manassas, VA). Cefepime powder was purchased from Chem-Impex International (Wood Dale, IL). Cefepime stock solution (12.8 mg ml−1) for reference BMD testing was prepared according to CLSI guidelines (34). Cefepime stock solution (30 mg ml−1) for use in digital dispensing was prepared in sterile 0.3% polysorbate 20 (Sigma-Aldrich, St. Louis, MO) as required for liquid handling by the HP D300 (HP, Inc., Palo Alto, CA). Polysorbate 20 was diluted to negligible final levels in a doubling dilution series (ranging from a maximum of 0.0006% to a minimum of 1.3 × 10−6%). All stock solutions were stored as aliquots at −20°C and discarded after a single use.
Reference broth microdilution testing.
BMD panels were prepared in-house according to established guidelines (34). Serial 2-fold dilutions of cefepime at double final concentration were made in sterile, round-bottom, polystyrene 96-well plates (Evergreen Scientific, Los Angeles, CA) using cation-adjusted Mueller-Hinton broth (BD Diagnostics, Franklin Lakes, NJ) in a volume of 50 μl. Panels were stored at −80°C until use (less than 3 weeks).
Susceptibility testing was performed according to CLSI guidelines using the colony suspension method (34). Organisms were grown overnight at 37°C in ambient air on tryptic soy agar containing 5% sheep's blood (Remel, Lenexa, KS). Inoculum was prepared by suspending several colonies in sterile normal saline (0.9% NaCl; Thermo Fisher Scientific, Waltham, MA) and adjusting the suspension to a 0.5 McFarland standard using a DensiChek Plus handheld colorimeter (bioMérieux, Durham, NC). The resulting suspension was diluted 1:150 in sterile, cation-adjusted Mueller-Hinton broth, and 50 μl was added to each well of a microdilution panel. This step brought the bacterial inoculum to a density of approximately 5 × 105 CFU ml−1, as confirmed by CLSI-recommended methods for inoculum validation (34), and cefepime to the final desired concentration, which ranged from 0.125 to 64 μg ml−1. E. coli ATCC 25922 was included as a quality control (QC) strain in every experiment. Inoculated panels were incubated at 37°C in ambient air for 16 to 20 h. The MIC was defined as the lowest concentration of antimicrobial resulting in complete inhibition of growth as determined visually (28). Uninoculated wells were included to ensure no contamination occurred during plate preparation.
Digital dispensing method.
The HP D300 digital dispensing system (HP, Inc., Palo Alto, CA) is a modified inkjet printer that dispenses droplets varying in size from 11 pl to 10 μl per the manufacturer's specifications (35). It can therefore create doubling dilution series directly from antimicrobial stock solutions by aliquoting the exact volume of antimicrobial desired into specific microwells without the need for serial dilution steps. Using this system, cefepime was dispensed directly from stock solutions into empty, round-bottom, untreated polystyrene 96-well plates (Evergreen Scientific, Los Angeles, CA) to final microwell concentrations ranging from 0.125 to 64 μg ml−1 in a 100-μl final assay volume. Panels were immediately frozen at −80°C until use (less than 3 weeks).
Prior to each experiment, organisms were grown overnight at 37°C in ambient air on tryptic soy agar containing 5% sheep's blood (Remel, Lenexa, KS). Bacterial suspensions with a 0.5 McFarland standard were prepared in normal saline (0.9% NaCl; Thermo Fisher Scientific, Waltham, MA) as for BMD and diluted 1:300 in sterile, cation-adjusted Mueller-Hinton broth (BD Diagnostics, Franklin Lakes, NJ) to yield a bacterial density of approximately 5 × 105 CFU ml−1 as confirmed by CLSI-recommended methods for inoculum validation (34). One-hundred microliters of this suspension was added per well and incubated at 37°C for 16 to 20 h. MIC was defined as for BMD, and E. coli ATCC 25922 was again included as a QC strain in every assay plate. Uninoculated wells were included to confirm the absence of contamination during microplate setup.
Determination of reference MIC.
To determine the reference MIC, BMD was performed in triplicate for each of the 64 strains in the collection. All replicates were performed on separate days with independent inocula. For each cefepime/organism combination, the modal MIC of triplicate measurements was considered the reference MIC. If a mode was not calculable due to variability among replicates, experiments were repeated in triplicate and the modal MIC was determined from all measurements. In the rare cases (3 out of 430 measurements) where results were bimodal after follow-up testing, the highest mode was recorded.
Clinical validation of Vitek 2, disk diffusion, and MicroScan.
Cefepime MIC results from Vitek 2 (GN73 panel), MicroScan (NM38 panel), and disk diffusion (BD BBL Sensi-Disc antimicrobial susceptibility test, 30-μg cefepime discs) zone diameters were interpreted according to current CLSI guidelines (28). Categorical agreement (CA) was evaluated in comparison to the reference BMD MIC, with results considered to be in CA if they fell in the same interpretive category as the BMD result (28). Very major errors (VMEs) were defined as a susceptible result in the test method and a resistant result in the BMD reference method. Major errors (MEs) were defined as a resistant result on the test method and a susceptible BMD result. Minor errors (MinEs) were defined as a susceptible/resistant result in the test method and an SDD result by BMD or the converse. For Vitek 2 only, evaluable essential agreement (EA) was calculated in comparison to BMD. Results were considered to be in evaluable EA if the Vitek 2 result and the BMD result were both on scale and within ±1 twofold dilution. Measurements for which there was growth at the highest concentration of cefepime tested or no growth at the lowest concentration were considered to be off scale. EA was not calculated for MicroScan due to the limited number of on-scale results (n = 3).
Verification of DDM.
The reproducibility of BMD and the DDM was compared through triplicate measurements of all 64 isolates in our collection. All replicates were performed on separate days with independent inocula. Each on-scale DDM and BMD result was log2 transformed and compared to the within-method modal MIC determined as described above for BMD. The resulting distributions were plotted using Microsoft Excel 2010 (Microsoft, Redmond, WA).
The accuracy of the DDM was determined as previously described (29). All DDM measurements, performed at least in triplicate for each strain, were evaluated in EA and CA determinations. A DDM MIC result was considered to be in evaluable EA if it fell within ±1 doubling dilution of the reference MIC and both results were on scale. A DDM result was considered to be in overall EA if the DDM MIC was either in evaluable EA with the reference MIC, if the DDM MIC and the reference MIC were both off scale in the same direction, or if either the DDM MIC or the reference MIC was at the lowest or highest evaluable MIC tested and the other was off scale in the same direction. Results were considered to be in CA if both methods yielded the same categorical interpretation (S, SDD, or R) based on current CLSI interpretive criteria for cefepime (28). CA analysis was also conducted with an SDD MIC of 4 μg ml−1 and an SDD MIC of 8 μg ml−1 considered to be two separate categories.
Statistical analysis.
For reproducibility studies, measurements that were on-scale were classified as in range (≤1 twofold dilution difference) or out of range (>1 twofold dilution difference) compared to the modal within-method MIC. Proportions of in-range to out-of-range measurements were compared using a two-tailed Fisher's exact test (significance defined as a P value of <0.05). For accuracy studies, EA and CA 95% confidence intervals were calculated as recommended by CLSI (36). JMP 12.0.1 (SAS, Cary, NC) was used for all statistical analyses.
ACKNOWLEDGMENTS
We thank Anthony Kang for critical reading of the manuscript.
The work was performed as part of a quality assurance effort in the clinical microbiology laboratory. Thea Brennan-Krohn was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health pediatric infectious diseases research training grant (T32HD055148).
The HP D300 digital dispenser and associated consumables were provided by Tecan (Morrisville, NC). Tecan had no role in study design, data collection/interpretation, manuscript preparation, or the decision to publish.
REFERENCES
- 1.Schwaber MJ, Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 60:913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 2.Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. 2016. Increasing incidence of extended-spectrum beta-lactamase-producing Escherichia coli in community hospitals throughout the southeastern United States. Infect Control Hosp Epidemiol 37:49–54. doi: 10.1017/ice.2015.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. 2016. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010-2014. Diagn Microbiol Infect Dis 85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. 2013. A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 6:1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Dynamics Economics and Policy. 2015. State of the world's antibiotics, 2015. Center for Disease Dynamics Economics and Policy, Washington, DC. [Google Scholar]
- 6.Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 13(47):pii=19044. [PubMed] [Google Scholar]
- 7.Eibach D, Campos CB, Krumkamp R, Al-Emran HM, Dekker D, Boahen KG, Kreuels B, Adu-Sarkodie Y, Aepfelbacher M, Park SE, Panzner U, Marks F, May J. 2016. Extended spectrum beta-lactamase producing Enterobacteriaceae causing bloodstream infections in rural Ghana, 2007-2012. Int J Med Microbiol 306:249–254. doi: 10.1016/j.ijmm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Lund O, Kibiki G, Aarestrup FM. 2016. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control 5:18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan J, Zhao D, Liu L, Chen Y, Zhou J, Jiang Y, Du X, Zhou Z, Akova M, Yu Y. 13 September 2016. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother doi: 10.1093/jac/dkw372. [DOI] [PubMed] [Google Scholar]
- 10.Azim A, Dwivedi M, Rao PB, Baronia AK, Singh RK, Prasad KN, Poddar B, Mishra A, Gurjar M, Dhole TN. 2010. Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum beta-lactamase- and metallo-beta-lactamase-producing Gram-negative bacteria—an Indian experience. J Med Microbiol 59:955–960. doi: 10.1099/jmm.0.018085-0. [DOI] [PubMed] [Google Scholar]
- 11.Benner KW, Prabhakaran P, Lowros AS. 2014. Epidemiology of infections due to extended-spectrum beta-lactamase-producing bacteria in a pediatric intensive care unit. J Pediatr Pharmacol Ther 19:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graffunder EM, Preston KE, Evans AM, Venezia RA. 2005. Risk factors associated with extended-spectrum beta-lactamase-producing organisms at a tertiary care hospital. J Antimicrob Chemother 56:139–145. doi: 10.1093/jac/dki180. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Segreti J. 2006. Overview of the epidemiological profile and laboratory detection of extended-spectrum beta-lactamases. Clin Infect Dis 42(Suppl):S153–S163. doi: 10.1086/500662. [DOI] [PubMed] [Google Scholar]
- 14.Zaoutis TE, Goyal M, Chu JH, Coffin SE, Bell LM, Nachamkin I, McGowan KL, Bilker WB, Lautenbach E. 2005. Risk factors for and outcomes of bloodstream infection caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in children. Pediatrics 115:942–949. doi: 10.1542/peds.2004-1289. [DOI] [PubMed] [Google Scholar]
- 15.Freeman JT, Sexton DJ, Anderson DJ. 2009. Emergence of extended-spectrum beta-lactamase-producing Escherichia coli in community hospitals throughout North Carolina: a harbinger of a wider problem in the United States? Clin Infect Dis 49:e30–e32. doi: 10.1086/600046. [DOI] [PubMed] [Google Scholar]
- 16.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM. 2008. Defining an extended-spectrum beta-lactamase. Clin Microbiol Infect 14(Suppl):S3–S10. [DOI] [PubMed] [Google Scholar]
- 19.Bush K, Jacoby GA, Medeiros AA. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 39:1211–1233. doi: 10.1128/AAC.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung-Tomc J, Dougherty TJ, DeOrio FJ, Simich-Jacobson V, Kessler RE. 1989. Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to beta-lactamase levels. Antimicrob Agents Chemother 33:498–502. doi: 10.1128/AAC.33.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Nikaido H, Liu W, Rosenberg EY. 1990. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother 34:337–342. doi: 10.1128/AAC.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones RN, Fuchs PC. 1989. Activity of cefepime (BMY-28142) and cefpirome (HR 810) against gram-negative bacilli resistant to cefotaxime or ceftazidime. J Antimicrob Chemother 23:163–165. doi: 10.1093/jac/23.1.163. [DOI] [PubMed] [Google Scholar]
- 24.Bhavnani SM, Ambrose PG, Craig WA, Dudley MN, Jones RN, SENTRY Antimicrobial Surveillance Program . 2006. Outcomes evaluation of patients with ESBL- and non-ESBL-producing Escherichia coli and Klebsiella species as defined by CLSI reference methods: report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 54:231–236. doi: 10.1016/j.diagmicrobio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, Bonomo RA, Rice LB, McCormack JG, Yu VL. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 39:2206–2212. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN, Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute. 2013. Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 27.Jones RN, Biedenbach DJ, Gales AC. 2003. Sustained activity and spectrum of selected extended-spectrum beta-lactams (carbapenems and cefepime) against Enterobacter spp. and ESBL-producing Klebsiella spp.: report from the SENTRY antimicrobial surveillance program (USA, 1997-2000). Int J Antimicrob Agents 21:1–7. doi: 10.1016/S0924-8579(02)00249-2. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Smith KP, Kirby JE. 2016. Verification of an automated, digital dispensing platform for at-will broth microdilution-based antimicrobial susceptibility testing. J Clin Microbiol 54:2288–2293. doi: 10.1128/JCM.00932-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang W, Park YJ, Park KG, Yu J. 2013. Evaluation of MicroScan WalkAway and Vitek 2 for determination of the susceptibility of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates to cefepime, cefotaxime and ceftazidime. J Antimicrob Chemother 68:2282–2285. [DOI] [PubMed] [Google Scholar]
- 31.Espinar MJ, Rocha R, Ribeiro M, Goncalves Rodrigues A, Pina-Vaz C. 2011. Extended-spectrum beta-lactamases of Escherichia coli and Klebsiella pneumoniae screened by the VITEK 2 system. J Med Microbiol 60:756–760. doi: 10.1099/jmm.0.024075-0. [DOI] [PubMed] [Google Scholar]
- 32.Clark RB, Lewinski MA, Loeffelholz MJ, Tibbetts RJ. 2009. Cumitech 31A, Verification and validation of procedures in the clinical microbiology laboratory. Coordinating ed, Sharp SE. American Society for Microbiology, Washington, DC. [Google Scholar]
- 33.Jacoby GA. 2009. AmpC β-Lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Tecan Inc. 2016. Tecan D300e digital dispenser—specification. http://ww3.tecan.com/mandant/files/doc/526/BR_Tecan_D300e_Specifications_399178_V1-0.pdf Accessed 15 October 2016.
- 36.Clinical and Laboratory Standards Institute. 2008. User protocol for evaluation of qualitative test performance. CLSI document EP12-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]


