ABSTRACT
Phenotypic variants of Staphylococcus aureus that display small colonies, reduced pigmentation, and decreased hemolysis and/or coagulase activity are periodically isolated by the clinical laboratory. Antimicrobial susceptibility testing (AST) of these isolates is complicated, because many do not grow on routine AST media, including Mueller-Hinton agar (MHA) and cation-adjusted Mueller-Hinton broth. This multicenter study evaluated cefoxitin disk diffusion for 37 atypical S. aureus isolates (156 readings) with MHA supplemented with 5% sheep's blood (BMHA), using mecA PCR as the reference standard. The correlation of two commercial PBP2a assays with mecA PCR was also assessed. Ten isolates were negative and 27 positive for mecA. No major errors for cefoxitin were observed, but 19.5% very major errors (VMEs) were observed at 24 h of incubation, and 17.2% VMEs were observed at 48 h. The proportions of VMEs ranged from 14.7 to 23.0% at 24 h, and from 13.3 to 17.6% at 48 h, across three testing laboratories. PBP2a tests were performed from growth on BMHA and blood agar plates (BAP), with and without cefoxitin disk induction. The Alere PBP2a SA culture colony test sensitivities for mecA were 90.0% with uninduced growth and 97.4% with induced growth from BMHA. On BAP, sensitivity was 96.0% with induced growth. The sensitivities of the Oxoid PBP2′ latex agglutination test were 85.7% with uninduced growth and 93.9% with induced growth from BMHA and 95.9% with induced growth on BAP. On the basis of these data, we recommend that laboratories perform only mecA PCR and/or PBP2a tests when requested to perform AST on atypical isolates of S. aureus.
KEYWORDS: Staphylococcus aureus, cefoxitin, mecA, small-colony variants, susceptibility testing
INTRODUCTION
Phenotypic variants of bacteria can arise spontaneously within microbial populations in response to environmental stresses, including exposure to antimicrobial agents. Populations of Staphylococcus aureus are particularly susceptible to generating such phenotypic variants, the best described of which are the small-colony variants (SCV). SCV are characterized by colonies 1/10 the size of those produced by wild-type S. aureus; they may also display reduced pigmentation and decreased hemolysis and/or coagulase activity (1). Isolates with normal-sized colonies but with one or more of the other features associated with SCV are also periodically observed growing in clinical cultures. The SCV phenotype itself has been documented as being caused by various metabolic deficits, including menadione, hemin, thymidine, or CO2 auxotrophy; however, the exact cause cannot be defined for all isolates (1). SCV and other atypical S. aureus colonies may or may not be coisolated with parent strains and often have an unstable colony phenotype, reverting to the wild type upon serial subculture (1). Atypical S. aureus has been isolated in cases of prosthetic joint infection, osteomyelitis (2), and soft tissue infection. These variants are most commonly observed in respiratory tract cultures from cystic fibrosis (CF) patients, a population among whom the prevalence of SCV may reach 33% (3). While S. aureus SCV are thought to have reduced virulence, they are, interestingly, better able to survive inside the host than are wild-type S. aureus strains, resulting in persistent infection (4), worse respiratory outcomes for CF patients (5), and a longer duration of symptoms for prosthetic joint infections (2).
The antibiogram of an atypical S. aureus isolate should not be assumed to be identical to that of its wild-type parent strain (6). Aminoglycosides, for example, have no activity against SCV with menadione or hemin auxotrophy (7), and antifolate agents are inactive against thymidine-dependent SCV (1). Similarly, some vancomycin-intermediate S. aureus isolates can present with an SCV phenotype (8). In contrast, wild-type parental strains may be susceptible to these antibiotics. Antimicrobial susceptibility testing (AST) of the atypical S. aureus variant is therefore necessary but poses a significant challenge to clinical laboratories, since many atypical S. aureus strains will not grow in commercial, automated AST systems. Similarly, many strains will not grow on Mueller-Hinton agar (MHA) or in cation-adjusted Mueller-Hinton broth (CA-MHB), which are recommended by the Clinical and Laboratory Standards Institute (CLSI) for the testing of typical S. aureus isolates (9, 10). Some clinical and research laboratories have evaluated AST performed on alternative test media that support the growth of SCV, based on supplementation for the typical auxotrophies observed (9, 10). However, determination of the accuracy of such testing is a challenge, because no gold-standard AST method exists for atypical S. aureus, and genotypic resistance mechanisms are not fully defined for all antimicrobials that might be considered for the treatment of these infections. Nonetheless, clinical laboratories are commonly asked by the clinicians they serve to test atypical S. aureus isolates, particularly for oxacillin susceptibility, and may attempt to do so using these alternative media. Thus, the Methods Working Group of the CLSI AST Subcommittee investigated whether an alternative AST medium for the testing of atypical S. aureus could be defined. This study documents the findings of the ad hoc working group for disk diffusion testing of cefoxitin on atypical S. aureus with MHA supplemented with 5% sheep's blood (BMHA), using mecA PCR as the reference standard. The correlation of two commercial PBP2a assays with mecA PCR for the atypical S. aureus isolates was also assessed.
RESULTS
Description of isolate morphologies and testing for mecA.
One to seven colony morphologies per isolate were observed at each laboratory. However, the number and description of morphotypes for each isolate differed between the laboratories (not shown). mecA PCR results were the same across all morphotypes of a given isolate. Ten of the isolates were mecA negative, and 27 were mecA positive.
Selection of media for testing of atypical S. aureus.
Ten isolates, which yielded 20 unique colony morphologies (mode, 2 colony morphologies per isolate; range, 1 to 3 morphologies per isolate), were chosen for the evaluation of the abilities of BMHA, MHA, brain heart infusion broth (BHI), and CA-MHB supplemented with 2.5% lysed horsed blood (LHB) to support growth after 24 h of incubation at 35°C under 5% CO2. Of these 20 morphotypes, 2 (10.0%) grew on MHA (i.e., reverted to the wild type) and were not tested further. None of the isolates grew in CA-MHB plus LHB (0.0%); 15 (83.3%) grew in BHI; and 18 (100%) grew on BMHA. A subcollection of 5 isolates from the 10 evaluated initially (2 mecA-negative and 3 mecA-positive isolates) was then evaluated at two laboratories, where cefoxitin disk diffusion was performed on BMHA from three manufacturers (BD, Remel, and Hardy). Both laboratories noted very poor growth of the isolates on the BMHAs from Remel and Hardy (Fig. 1). Compared to the mecA results, 1 very major error (VME) was observed, for a morphotype tested on Hardy BMHA, and 2 major errors (MEs) were observed, one on Hardy BMHA and one on Remel BMHA. Due to these observations, and the difficulty of assessing zone sizes because of faint growth on Remel and Hardy BMHAs, BBL BMHA from BD was used as the sole medium for the remainder of the study.
FIG 1.
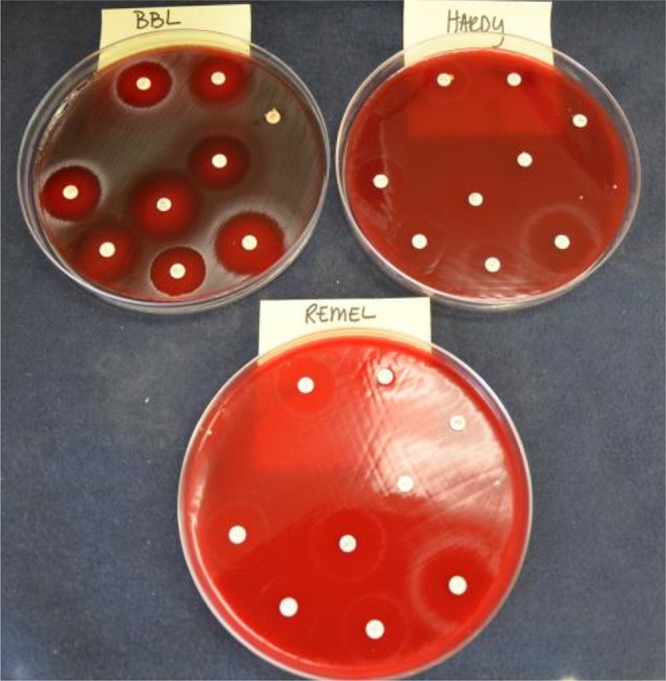
Growth of an atypical S. aureus isolate on BMHAs from three vendors: BD, Hardy, and Remel.
Cefoxitin disk diffusion.
Cefoxitin disk diffusion on BMHA was performed at three laboratories using all morphotypes observed for the 37 study isolates. This testing yielded 156 readings after 24 h of incubation. Despite promising results in the preliminary studies described above, cefoxitin disk diffusion results correlated poorly with mecA PCR results (Fig. 2; Table 1). By use of current CLSI breakpoints for S. aureus, 24 VMEs were observed among 123 readings for mecA-positive isolates (false susceptibility, 19.5%) after 24 h of incubation. Similarly, 21 VMEs among 122 readings for mecA-positive isolates (17.2%) were observed after 48 h of incubation. The difference in the numbers of VMEs was due in part to one morphotype of isolate 14-11-57 that could not be evaluated because the disk fell off the plate (Table 1, lab 2). The remaining differences were due to one morphotype of isolate 14-11-28 for which the zone size was read as 23 mm at 24 h and 20 mm at 48 h by lab 2, a morphotype of isolate 14-11-59 that had a zone size of 23 mm at 24 h and 21 mm at 48 h in lab 3, and a morphotype of isolate 14-11-57 that had a zone size of 35 mm at 24 h but 12 mm at 48 h in lab 3, due to reversion to the wild-type morphology at the later time point (Fig. 3). In contrast, one of the morphotypes for isolate 14-11-51 had a zone of 21 mm at 24 h but 24 mm at 48 h in lab 1, yielding an additional VME at 48 h for this isolate. In this case, and in others where larger zone sizes were observed at 48 h (shown in Table 1), zones were clearer at 48 h, leading to slight changes in zone size readings. VMEs are detailed in Table 1. In all cases, the mecA PCR result was confirmed in duplicate. No MEs (false resistance) were observed among 33 readings for 10 mecA-negative isolates at 24 h or among 30 readings after 48 h of incubation. The overall categorical agreement of cefoxitin disk diffusion results on BMHA with mecA PCR results was 84.5% after 24 h of incubation and 86.4% after 48 h of incubation. All quality control (QC) readings for S. aureus ATCC 25923 and Streptococcus pneumoniae ATCC 49619 were within the QC ranges of CLSI supplement M100S, 26th edition (not shown).
FIG 2.
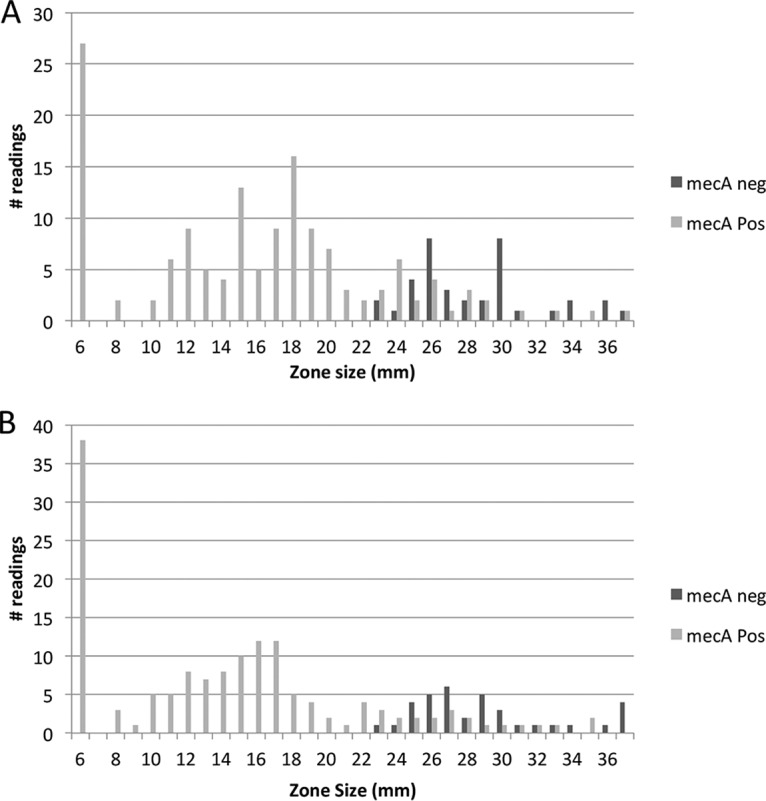
Distribution of cefoxitin zone sizes after 24 h (A) or 48 h (B) of incubation at 35°C under 5% CO2. Testing was performed at 3 laboratories for 37 isolates, with 1 to 7 morphologies per isolate.
TABLE 1.
Very major errors observed for cefoxitin disk diffusion on BMHA from a collection of 27 mecA-positive atypical S. aureus isolates
| Isolate | No. of colony morphologies observeda | Cefoxitin zone (mm)b or no. of VME/no. of mecA-positive morphotypes (%) |
|||||
|---|---|---|---|---|---|---|---|
| 24 h of incubation under CO2 |
48 h of incubation under CO2 |
||||||
| Lab 1 | Lab 2 | Lab 3 | Lab 1 | Lab 2 | Lab 3 | ||
| 14-11-28 | 3 | 15 | 24 | 21 | 15 | 22 | 14 |
| 13 | 23 | 20 | 8 | 20 | 20 | ||
| — | — | 20 | — | — | 18 | ||
| 14-11-34 | 2 | 24 | 26 | 27 | 26 | 25 | 27 |
| 26 | 25 | 28 | 27 | 26 | 28 | ||
| 14-11-47 | 2 | — | 24 | 11 | — | 23 | 6 |
| — | — | 6 | — | — | 6 | ||
| 14-11-48 | 1 | 6 | 19 | 29 | 6 | 17 | 29 |
| 14-11-50 | 2 | — | — | 22 | — | — | 22 |
| — | 21 | 22 | — | 19 | 22 | ||
| 14-11-51 | 3 | 21 | 20 | 18 | 24 | 19 | 17 |
| 11 | 26 | 25 | 10 | 25 | 23 | ||
| 14 | 19 | 18 | 15 | 19 | 8 | ||
| 14-11-57 | 2 | 26 | 37 | 28 | 35 | 32 | 35 |
| 26 | 33 | 35 | 35 | — | 12 | ||
| 14-11-59 | 2 | 19 | — | 24 | 15 | — | 24 |
| 24 | 24 | 23 | 23 | 22 | 21 | ||
| No. of VME/no. of mecA-positive morphotypes (%) | 5/34 (14.7) | 9/39 (23.0) | 10/50 (20.0) | 6/34 (17.6) | 7/38 (18.4) | 8/50 (16.0) | |
| Overall no. of VME/no. of mecA-positive morphotypes (%) | 24/123 (19.5) | 21/122 (17.2) | |||||
In any one row, the results may not be from the same morphotype in all 3 labs (e.g., a hemolytic strain may have been labeled morphotype 1 in one lab and morphotype 3 in another lab).
Boldface indicates a VME. —, the laboratory did not isolate the morphotype.
FIG 3.
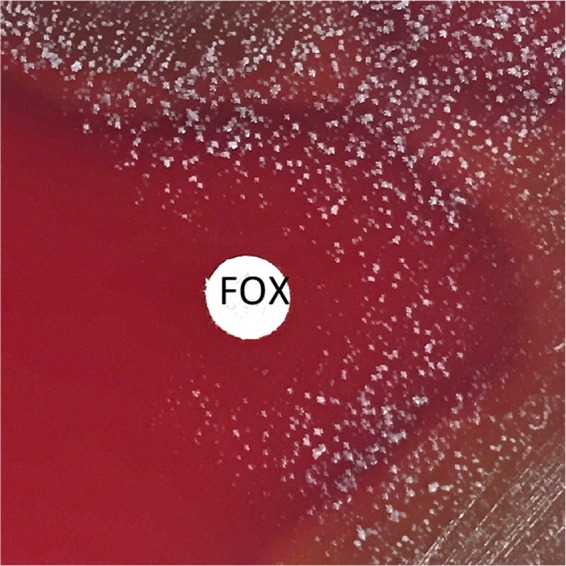
Growth of S. aureus 14-11-57 after 48 h. Note the growth of colonies that have reverted to the wild type surrounding the cefoxitin (FOX) disk. This isolate harbored the mecA gene but had a cefoxitin zone of 35 mm at 24 h of incubation at 35°C under 5% CO2.
Considering that all morphotypes of a given isolate had concordant mecA PCR results, we reanalyzed the data such that each isolate would be considered resistant if one or more morphotypes tested resistant. In this case, 11 VMEs across 3 laboratories (81 readings for 27 mecA-positive isolates [13.5% VMEs]) were observed at 24 h of incubation and 8 VMEs (9.9%) at 48 h of incubation.
PBP2a tests.
PBP2a tests were performed in two laboratories: one that used the Alere SA PBP2a colony culture test and one that used the Oxoid PBP2′ latex agglutination test. Testing was performed on all morphotypes, using uninduced colonies from blood agar plates (BAP) and cefoxitin-induced growth from both BMHA and BAP. Induced growth on BMHA and on BAP was tested on different days in each laboratory, and thus, the numbers of morphotypes tested on these two media differed (Tables 2 and 3). For uninduced growth, the Alere PBP2a test was 90.0% sensitive and 100% specific relative to mecA PCR (Table 2). BMHA with cefoxitin induction improved sensitivity to 97.4%, and BAP with cefoxitin induction improved sensitivity to 96.0% (Table 2).
TABLE 2.
Comparison of the performance of the Alere PBP2a SA culture colony test with that of mecA PCR for atypical S. aureusa
| Test condition (medium) and result | No. of isolates with the following mecA PCR result: |
Sensitivity (%) (95% CI)b | Specificity (%) (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| Uninduced growth (BAP) | 90.0 (75.4–96.7) | 100 (73.2–100) | ||
| PBP2a+ | 36 | 0 | ||
| PBP2a− | 4 | 14 | ||
| Induced growth (BMHA) | 97.4 (84.9–99.0) | 100 (73.2–100) | ||
| PBP2a+ | 38 | 0 | ||
| PBP2a− | 1c | 14 | ||
| Induced growth (BAP) | 96.0 (85.1–99.2) | 100 (78.1–100) | ||
| PBP2a+ | 48 | 0 | ||
| PBP2a− | 2 | 18 | ||
Induced growth on BMHA and BAP was tested on different days in a single laboratory. Subculture of 37 isolates yielded 54 colony morphotypes to be tested with cefoxitin induction on BMHA and 68 morphotypes to be tested with cefoxitin induction on BAP.
CI, confidence interval.
One morphotype could not be tested induced due to insufficient cells surrounding the cefoxitin disk.
TABLE 3.
Comparison of the performance of the Oxoid PBP2′ latex agglutination test with that of mecA PCR for 37 isolatesa of atypical S. aureus
| Test condition (medium) and result | No. of isolates with the following mecA PCR result: |
Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| Uninduced growth (BAP) | 85.7 (69–94.6) | 87.5 (46.7–99.3) | ||
| PBP2a+ | 30 | 1 | ||
| PBP2a− | 5 | 7 | ||
| Induced growth (BMHA) | 93.9 (78.3–98.9) | 100 (56.2–100) | ||
| PBP2a+ | 31 | 0b | ||
| PBP2a− | 2c | 7 | ||
| Induced growth (BAP) | 95.9 (84.8–99.2) | 100 (77.1–100) | ||
| PBP2a+ | 47 | 0 | ||
| PBP2a− | 2 | 17 | ||
Induced growth on BMHA and BAP was tested on different days in a single laboratory. Subculture of 37 isolates yielded 43 colony morphotypes to be tested with cefoxitin induction on BMHA and 66 morphotypes to be tested with cefoxitin induction on BAP.
One morphotype could not be tested induced due to insufficient growth surrounding the cefoxitin disk.
Two morphotypes could not be tested induced due to insufficient growth surrounding the cefoxitin disk.
The sensitivity of the Oxoid PBP2′ latex agglutination test performed on uninduced growth was 85.7%, and its specificity was 87.5%. The use of cefoxitin-induced growth on BMHA increased sensitivity to 93.9%, and the use of induced growth on a BAP increased sensitivity to 95.9% (Table 3). The increase in sensitivity was observed because some isolates that yielded false-negative results when uninduced could not be tested with induced growth due to insufficient growth around the cefoxitin disk (Table 3). Specificity was similarly increased, because the morphotype that yielded the sole false-positive result could not be tested from induced growth, due to insufficient growth (Table 3). PBP2a test errors are summarized in Table 4.
TABLE 4.
Summary of PBP2a test errors
| Isolate | Morphotype no. | Resulta by: |
Error | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mecA PCR | Alere PBP2a SA culture colony test |
Oxoid PBP2′ latex agglutination test |
|||||||
| Uninduced growth on BAP | Induced growth on: |
Uninduced growth on BAP | Induced growth on: |
||||||
| BMHA | BAP | BMHA | BAP | ||||||
| 14-11-15 | 1 | + | − | + | + | − | + | + | Alere and Oxoid tests required induction |
| 2 | + | + | + | + | NA | NA | + | No error | |
| 14-11-34 | 1 | + | + | + | + | − | − | + | False-negative results on uninduced growth, and on induced growth in BMHA, by the Oxoid test |
| 2 | + | + | + | + | − | − | + | False-negative result in BMHA by the Oxoid test | |
| 14-11-48 | 1 | + | − | + | + | − | + | + | Alere and Oxoid tests required induction |
| 2 | + | + | + | + | NA | NA | + | No error | |
| 14-11-52 | 1 | − | − | − | − | + | ND | − | False-positive result on uninduced growth by the Oxoid test |
| 2 | − | − | − | − | NA | NA | − | No error | |
| 14-11-57 | 1 | + | − | − | − | − | ND | − | False-negative results by the Alere and Oxoid tests |
| 2 | + | − | ND | − | + | ND | − | False-negative results by the Alere and Oxoid tests; true-positive result on uninduced growth by the Oxoid test | |
ND, not done due to insufficient growth surrounding the cefoxitin disk; NA, not applicable, because the morphotype was not recovered on the day of testing.
DISCUSSION
The altered growth of atypical S. aureus makes the performance of AST a significant challenge. Often, laboratories attempt to perform such testing using test media or conditions that differ from those recommended for typical S. aureus by the CLSI (11). However, in light of the findings of this study, we strongly discourage such practices. While we did identify a medium (BBL BMHA from BD) that supported the growth of atypical S. aureus better than other available brands, the use of this medium for cefoxitin disk diffusion resulted in an unacceptable number of VMEs relative to mecA PCR results. Since no MEs were observed (when BBL BMHA from BD was used), laboratories may consider performing cefoxitin disk testing on BD BMHA if only resistant results are reported. Susceptible results would have to be confirmed using either mecA PCR or PBP2a tests.
BMHA is the CLSI-recommended medium for disk diffusion testing of many fastidious bacteria, including Streptococcus pneumoniae, beta-hemolytic and viridans group streptococci, and Neisseria meningitidis (standard M100S) (11), as well as several other organisms (M45 guideline) (12), but has not been evaluated previously for testing of atypical S. aureus. Cefoxitin disk testing was chosen for this study due to the availability of a clear, genotype-based reference method against which to compare results (i.e., mecA PCR). While we did not formally evaluate other antimicrobial agents that might be considered for treatment of S. aureus infections, we anticipate that the poor performance observed for cefoxitin testing may also extend to other agents. As a result of this study, in the forthcoming 27th edition of standard M100S, the CLSI will recommend that atypical S. aureus be evaluated for the presence of mecA or, alternatively, PBP2a, using cefoxitin-induced growth to determine oxacillin susceptibility, and that testing for susceptibility to other agents should not be attempted due to the risk of false-susceptible results. Laboratories may consider reporting atypical S. aureus with the SCV phenotype recovered from cystic fibrosis patients as resistant to aminoglycosides and trimethoprim-sulfamethoxazole, given that the most common auxotrophs observed are to menadione and hemin, which confer resistance to the former, and thymidine, which confers resistance to the latter (1).
Our data on the performance of PBP2a tests contrast with those of Kipp and colleagues, who evaluated 11 mecA-positive SCV isolates, among which only 4 were correctly identified as PBP2a positive by the PBP2′ latex agglutination test when performed according to the manufacturer's instructions. Those investigators did observe that the use of an increased inoculum improved the detection of PBP2a to 100% among these 11 isolates (10). Similarly, we noted that several isolates could not be tested by the latex test using induced growth, because insufficient cell mass was present surrounding the cefoxitin disk (Table 3). Of note, while the Alere SA PBP2a test does include an induction option in the FDA-cleared product, this is with a 1-μg oxacillin disk from growth on MHA. Similarly, the Oxoid PBP2′ test is cleared for use with induced growth for coagulase-negative staphylococci only, again using a 1-μg oxacillin disk. As such, laboratories would need to verify the performance of the test off-label with the use of a cefoxitin disk on BAP or BMHA prior to using this option for testing of patient isolates.
While our goal was to evaluate media currently in use by clinical laboratories, thereby avoiding special orders and supplemental quality control testing by the laboratory, others have suggested that a novel medium be developed for AST of atypical S. aureus (9). In order for such a medium to achieve widespread utility for clinical laboratories, it should (i) support the growth of all (or nearly all) atypical S. aureus isolates, (ii) be commercially available, (iii) generate accurate results compared to a reference method (e.g., mecA PCR), and (iv) have good lot-to-lot and manufacturer-to-manufacturer reproducibility. Even with these requirements, such a medium would be best suited for use by laboratories that serve large CF patient populations, orthopedic surgical centers, or large reference laboratories, since most clinical laboratories encounter atypical S. aureus isolates infrequently. Precit and colleagues developed an MHA supplemented with menadione, hemin, and thymidine for the testing of SCV (9), which yielded AST results similar to those observed for isogenic wild-type isolates (9). However, this medium requires further, multicenter evaluation, including evaluation of its performance when it is produced by more than one manufacturer. The latter point is particularly important, given the significant manufacturer-to-manufacturer differences in the ability of BMHA to support the growth of atypical S. aureus in our study. All three BMHAs used in this study were from manufacturers who adhered to CLSI standards for controlling the content of MHA (13). In addition, sheep's blood contains high concentrations of thymidine, so one would expect BMHA to support the growth of thymidine auxotrophs. However, we postulate that hemin levels may differ based on the age of the erythrocytes (and degree of lysing) in these media, accounting for the differences in performance. Similar manufacturer-to-manufacturer differences in the ability of BHI agar to support SCV growth have been observed (9). Furthermore, the medium developed by Precit and colleagues was designed to support the growth of SCV isolated from CF patients, since about one-third of CF isolates are thymidine dependent, and two-thirds are menadione and/or hemin dependent (3, 14, 15). However, isolates from other sources are less well characterized and may include variants not supported by these supplements (2). Furthermore, a major challenge in evaluating such media is the lack of availability of a gold standard against which to compare results, since resistance mechanisms may not be well defined for all antimicrobials and S. aureus.
A limitation of this study was our inability to ensure that all three testing laboratories evaluated the same subclones of each isolate. Given the heterogeneous nature of atypical S. aureus, this was not possible. As such, we may have underestimated the number of VMEs, since not all laboratories observed VMEs for each isolate (Table 1). Nonetheless, the number of VMEs was far above the acceptable level (<3%) for clinical testing. The assumption that clinicians would treat the patient for methicillin-resistant S. aureus (MRSA) if just one morphotype tested resistant did not improve performance, since all morphotypes for two mecA-positive isolates tested susceptible to cefoxitin at all three laboratories (Table 1). Similarly, for several mecA-positive isolates, individual laboratories observed false-susceptible cefoxitin results for all morphotypes (Table 1).
In summary, we observed poor performance of cefoxitin disk diffusion performed on BMHA, compared to mecA PCR, for atypical S. aureus isolates. In contrast, PBP2a tests, in particular those performed from induced growth, yielded good correlation with mecA PCR. Thus, we recommend that laboratories perform only mecA PCR and/or PBP2a tests on cefoxitin-induced growth (following laboratory verification) when requested to perform AST on atypical isolates of S. aureus that fail to grow on the media required for the CLSI standardized method. We discourage laboratories from attempting to test additional agents on alternative media unless a validation study has demonstrated that such testing yields accurate AST results. In the future, methods such as whole-genome sequencing may yield actionable data regarding the susceptibility of these isolates. In addition, we discourage laboratories from subculturing atypical S. aureus isolates until they revert to the wild-type phenotype, since the antimicrobial susceptibility of the atypical isolates cannot be assumed to be the same as that of the wild-type parent.
MATERIALS AND METHODS
Bacterial isolates.
Atypical S. aureus isolates were defined in this study as those that did not grow on MHA or CA-MHB at the time of standard-of-care testing. Thirty-seven deidentified single-patient isolates were included in this study and were obtained from six submitting clinical laboratories, in Los Angeles, CA, Seattle, WA, Portland, OR, Cleveland, OH, Rochester, MN, and Atlanta, GA. The majority of the isolates were recovered from respiratory specimens from CF patients (n = 21). Six isolates were from infected prosthetic joints, five from respiratory secretions of patients without CF, one from an exudate, one from blood, and three from undocumented sources. After minimal subculturing by the submitting laboratory, a sweep of growth from sheep's blood agar plates (BAP) (BBL medium; BD, Sparks, MD) was collected on a swab and was sent to the coordinating laboratory (UCLA) (see Fig. S1 in the supplemental material). Upon receipt, the swab was inoculated onto a BAP and was incubated for 48 h at 35°C under 5% CO2. A sweep of this growth was transferred to Brucella broth with 15% glycerol (Hardy Diagnostics, Santa Maria, CA) for the storage of isolates at −70°C. Isolates were subsequently shipped frozen to the other two testing laboratories. Atypical S. aureus isolates often display multiple colony morphologies upon subculture from the freezer (see Fig. S2 in the supplemental material). Thus, after subculture to BAP and incubation for 48 h at 35°C under 5% CO2, each isolate was carefully evaluated for the number and types of colony morphologies present, which were documented by each testing laboratory using specific descriptors (i.e., size, pigment, and hemolysis pattern). Each unique colony morphology was confirmed to be S. aureus by use of the Vitek MS system (bioMérieux, Durham, NC) by the testing laboratories, and each morphotype was subcultured to MHA (BD, Sparks, MD), BMHA (BD), and BAP (BD or Remel [Lenexa, KS]) and was incubated at 35°C under 5% CO2 for 24 h. If no growth was apparent at 24 h, the plates were reincubated for an additional 24 h. Colony morphotypes that grew on BMHA but not on MHA, or demonstrated very poor growth on MHA (only pinpoint colonies) relative to that on BMHA, were tested further. If multiple colony morphologies were again observed on the second subculture of a single colony of a given morphotype (filial generation 2 [F2] from frozen stock), a sweep of the colonies from the F2 plate was used for further testing (Fig. S1). If the colony morphotypes observed at F1 bred true to the F2 plate, these were tested independently. Each colony morphotype was evaluated for the presence of mecA by PCR in one laboratory, using a method described previously (16).
Pilot study to evaluate growth on different media.
A subset of 10 atypical S. aureus isolates was evaluated at a single laboratory in order to determine whether commercial media used routinely by clinical laboratories for AST could support the growth of atypical S. aureus isolates. The media evaluated included CA-MHB supplemented with 2.5% lysed horsed blood (LHB), brain heart infusion broth (BHI), and BMHA. Subsequently, three brands of BMHA (BD, Hardy, and Remel [Lenexa, KS]) were evaluated at all three laboratories for their abilities to support the growth of five atypical S. aureus isolates.
Cefoxitin disk diffusion tests.
Cefoxitin disk diffusion was performed at three laboratories using 30-μg disks and BMHA purchased from BD. Each laboratory prepared an isolate suspension equivalent to a 0.5 McFarland standard for each colony morphology observed at F2, and this suspension was used to inoculate BMHA according to CLSI standard M2 (13). Cefoxitin disks were applied, and plates were incubated at 35°C under 5% CO2. The diameter of growth inhibition surrounding the cefoxitin disk was read after 24 h of incubation, and again after 48 h of incubation, according to the guidelines for disk diffusion on enriched media in CLSI standard M2 (13). Quality control (QC) for each lot of disks was carried out by testing S. aureus ATCC 25923 on unsupplemented MHA with incubation in ambient air. QC for each lot of BMHA was carried out by testing S. pneumoniae ATCC 49619 on this medium with a ceftaroline disk, which was incubated under CO2, in the absence of published QC ranges for this organism and cefoxitin.
PBP2a tests.
Two commercially available, FDA-cleared PBP2a detection tests were evaluated in this study. The Alere PBP2a SA culture colony test (Alere, San Diego, CA), which uses a lateral-flow immunochromatography method, was performed in one laboratory, and the Oxoid PBP2′ latex agglutination test (Thermo Scientific, Waltham, MA) was performed in a second laboratory. In both cases, PBP2a tests were performed according to the manufacturer's instructions, from the F2 colonies on BAP. An “induced” PBP2a test was performed by inoculating a suspension equivalent to a 0.5 McFarland standard into BMHA or a BAP and applying a cefoxitin disk. The cells from the periphery of the cefoxitin zone of growth inhibition were harvested after 24 h of incubation at 35°C under 5% CO2 and were used for PBP2a testing. Quality control of PBP2a tests was performed according to the manufacturers' recommendations.
Data analysis.
Phenotypically different colony morphologies of atypical S. aureus may display different antimicrobial susceptibility profiles, and therefore, each reading (i.e., the result for each morphotype at each laboratory) was evaluated as an independent data point.
Cefoxitin disk diffusion was interpreted according to the breakpoints for S. aureus (≤21 mm for resistance and ≥22 mm for susceptibility) in CLSI supplement M100S (26th edition) (11). Cefoxitin and PBP2a results were compared to the results of mecA PCR. Very major errors (VMEs) were defined as mecA-positive morphotypes with a susceptible cefoxitin zone; the percentage of VMEs was calculated by using the number of mecA-positive morphotypes as the denominator. Major errors (MEs) were defined as mecA-negative morphotypes that were cefoxitin resistant by disk diffusion; the percentage of MEs was calculated by using the number of mecA-negative morphotypes as the denominator. The sensitivity and specificity of PBP2a tests were calculated relative to the mecA PCR results. If errors were observed between mecA PCR, PBP2a tests, and cefoxitin disk diffusion for all morphotypes at all laboratories, mecA testing and PBP2a testing were repeated in parallel at a single laboratory, with careful separation of each colony morphotype per isolate and independent testing. If errors were resolved, they were not counted in the data analysis.
Supplementary Material
ACKNOWLEDGMENTS
S.R. has received research funding from bioMérieux and BD Diagnostics. Financial support for this project was provided by internal funds at participating laboratories, medium support from BD, and PBP2a tests from Thermo Fisher.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02211-16.
REFERENCES
- 1.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 2.Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. 2014. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 5:e01910-14. doi: 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis 177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 4.Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis 20:95–102. doi: 10.1093/clinids/20.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, Houston LS, Rogers GB, Marsh P, Prehar K, Pope CE, Blackledge M, Deziel E, Bruce KD, Ramsey BW, Gibson RL, Burns JL, Hoffman LR. 2013. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 57:384–391. doi: 10.1093/cid/cit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, Tulkens PM, Van Bambeke F. 2013. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother 68:1455–1464. doi: 10.1093/jac/dkt072. [DOI] [PubMed] [Google Scholar]
- 7.Norstrom T, Lannergard J, Hughes D. 2007. Genetic and phenotypic identification of fusidic acid-resistant mutants with the small-colony-variant phenotype in Staphylococcus aureus. Antimicrob Agents Chemother 51:4438–4446. doi: 10.1128/AAC.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Precit MR, Wolter DJ, Griffith A, Emerson J, Burns JL, Hoffman LR. 2016. Optimized in vitro antibiotic susceptibility testing method for small-colony variant Staphylococcus aureus. Antimicrob Agents Chemother 60:1725–1735. doi: 10.1128/AAC.02330-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipp F, Becker K, Peters G, von Eiff C. 2004. Evaluation of different methods to detect methicillin resistance in small-colony variants of Staphylococcus aureus. J Clin Microbiol 42:1277–1279. doi: 10.1128/JCM.42.3.1277-1279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed CLSI Supplement M100S. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.CLSI. 2015. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed, vol M45 A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.CLSI. 2015. Performance standards for antimicrobial disk susceptibility tests; approved standard, 12th ed, vol M02-A12 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol 25:1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besier S, Smaczny C, von Mallinckrodt C, Krahl A, Ackermann H, Brade V, Wichelhaus TA. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol 45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MT, Burnham CA, Westblade LF, Dien Bard J, Lawhon SD, Wallace MA, Stanley T, Burd E, Hindler J, Humphries RM. 2016. Evaluation of oxacillin and cefoxitin disk and MIC breakpoints for prediction of methicillin resistance in human and veterinary isolates of Staphylococcus intermedius group. J Clin Microbiol 54:535–542. doi: 10.1128/JCM.02864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


