ABSTRACT
The performance and interpretation of laboratory tests for Zika virus (ZKV) continue to be evaluated. Serology is cross-reactive, laborious, and frequently difficult to interpret, and serum was initially solely recommended for molecular diagnosis. ZKV testing was initiated in January 2016 in New York State for symptomatic patients, pregnant women, their infants, and patients with Guillain-Barré syndrome who had traveled to areas with ZKV transmission. Subsequently, eligibility was expanded to pregnant women with sexual partners with similar travel histories. Serum and urine collected within 4 weeks of symptom onset or within 6 weeks of travel were tested with real-time reverse transcription-PCR (RT-PCR) assays targeting the ZKV envelope and NS2B genes. In this review of lessons learned from the first 80 positive cases in NYS, ZKV RNA was detected in urine only in 50 patients, in serum only in 19 patients, and in both samples concurrently in 11 patients, with average viral loads in urine a log higher than those in serum. Among 93 positive samples from the 80 patients, 41 were positive on both gene assays, 52 were positive on the envelope only, and none were positive on the NS2B only. Of the 80 infected patients, test results for 74 (93%) would have defined their infection status as not detected or equivocal if the requirement for positive results from two assay targets (two-target-positive requirement) in the initial federal guidance to public health laboratories was enforced, if urine was not tested, or if the extended eligibility time for molecular testing was not implemented. These changes facilitated more extensive molecular diagnosis of ZKV, reducing reliance on time-consuming and potentially inconclusive serology.
KEYWORDS: diagnostics, emerging pathogens, molecular methods, specimen selection, viral diagnosis
INTRODUCTION
Since its original identification in 1947 (1), Zika virus (ZKV) outbreaks in humans have occurred in Africa and Asia, usually resulting in low case numbers (2). In 2007, an outbreak on the Yap Islands of Micronesia caused 185 suspected cases, and a large outbreak in French Polynesia in 2013 to 2014 (3) resulted in more than 30,000 cases (4). During the latter outbreak, in addition to symptoms of rash, fever, arthralgia, and headache, neurological complications including Guillain-Barré syndrome (GBS) (5, 6) and fetal microcephaly (7) were observed. Since early 2015 an outbreak in the Caribbean, Central and South America, has caused infections in more than 50 countries, with tens of thousands of reported ZKV infections and thousands of confirmed cases of microcephaly in Brazil alone. In addition to microcephaly (8), other severe complications have been reported (9, 10). This has elevated the public health importance of ZKV from what was previously believed to be similar to a mild version of dengue to a disease with serious and long-term public health consequences. Appropriate responses, interventions, and treatments are optimized by the availability of accurate and rapid diagnostic methods.
Despite extensive investigations, uncertainty continues regarding the utility, efficacy, performance, and interpretation of laboratory tests for ZKV. Current diagnostic methods use either the molecular detection of ZKV RNA or the detection of virus-reactive IgM antibodies. Serological methods developed thus far for ZKV antibodies are time-consuming, and the antibodies cross-react with other flavivirus antibodies. This is particularly problematic since patients who have traveled to areas with active ZKV transmission have a high likelihood of recent infection with other flaviviruses. Serum has been the recommended specimen type for molecular diagnosis although Zika viremia is unlikely to persist beyond several days post-onset of illness (11). A few reports have shown higher viral loads and longer duration of positivity in other sample types (12–16), and two articles have reported prolonged viremia in pregnant women (17, 18).
We describe the initial testing for ZKV in New York State (NYS) and the first cohort of 80 ZKV real-time reverse transcription-PCR (rRT-PCR)-positive patients detected. Specimen collection, testing, and result interpretation were significantly broader than the federal guidance recommended initially but proved beneficial in diagnosing more cases with molecular testing than would have otherwise been detected. An analysis is provided of the 80 positive patients, their demographics, and clinical and laboratory test characteristics, in addition to lessons learned.
RESULTS
Total Zika virus testing in NYS by rRT-PCR.
Through 8 July 2016, the Wadsworth Center (WC) tested a total of 6,527 samples, including 2,519 sera, 3,884 urine, 99 tissue, 19 amniotic fluid, and 8 cerebrospinal fluid (CSF) samples, from 3,746 patients using the ZKV rRT-PCR assays. Molecular testing was performed on amniotic fluid samples from pregnant women who had previously tested positive by either serum or urine samples. ZKV RNA was detected in one amniotic fluid sample and subsequently in multiple corresponding placenta and fetal tissue samples. Serum samples from symptomatic patients were initially also tested for dengue virus and chikungunya virus by rRT-PCR: of 285 patients also tested for dengue viruses, 5 (1.7%) tested positive (3 for type 1 and two for type 2), while of the 610 patients tested for chikungunya virus, 100% tested negative.
Patient demographics.
Demographic and epidemiologic characteristics of the 80 ZKV RNA-positive patients are shown in Table 1. As expected given the testing restrictions, more cases were detected in females (52/80, 65%) than in males. Only one case was demonstrated as unequivocally caused by sexual transmission.
TABLE 1.
Demographic and epidemiologic characteristics of the first 80 Zika virus rRT-PCR-positive NYS patientsa
| Parameter | Value for the parameter |
|---|---|
| No. of female patients (%) | 52 (65) |
| Median age (yr [range]) | 37 (7–73) |
| Travel history (no. patients [%]) | |
| Dominican Republic | 23 (28) |
| Puerto Rico | 9 (11) |
| Jamaica | 7 (9) |
| El Salvador | 6 (8) |
| Guyana | 6 (8) |
| Other country | 25 (31) |
| No travel/unknown | 4 (5) |
| Race (no. patients [%]) | |
| White | 24 (30) |
| Black | 2 (2) |
| Unknown | 50 (63) |
| Other | 4 (5) |
| Ethnicity (no. patients [%]) | |
| Hispanic/Latino | 24 (30) |
| Not Hispanic/Latino | 17 (21) |
| Unknown | 39 (49) |
| Clinical presentation (no. patients [%]) | |
| Symptomatic | 72 (90) |
| Asymptomatic | 5 (6) |
| Unknown | 3 (4) |
| Pregnancy statusb | |
| Pregnant, symptomatic | 3 (6) |
| Pregnant, asymptomatic | 6 (12) |
| Not pregnant | 43 (83) |
| PCR-positive specimen(s) (no. patients [%]) | |
| Urine only | 50 (62) |
| Serum only | 19 (24) |
| Serum and urine | 11 (14) |
| IgM positivity (no. patients [%])c | |
| Reactive | 47 (64) |
| Nonreactive | 22 (30) |
| Indeterminate | 4 (6) |
A total of 80 patients were rRT-PCR-positive.
A total of 9 (17%) of 52 female patients were pregnant.
A total of 73 patients were tested.
Zika IgM testing.
Among the 80 patients, 73 were tested with the CDC Zika IgM antibody capture enzyme-linked immunosorbent assay (ELISA). While a lack of availability of testing in early 2016 led to seven patients without concurrent IgM testing, of the 73 tested, 47 (64%) were reactive. The more detailed analysis of these results is addressed in another paper currently in preparation.
Zika virus rRT-PCR testing.
Among the 80 patients testing positive for ZKV by rRT-PCR, 50 patients were positive in urine only, 19 were positive in serum only, and 11 were positive in both urine and serum (Fig. 1). Convalescent-phase serum specimens were obtained for follow-up serology from 25 of the 80 patients, and molecular testing was performed on these serum samples as well as on concurrently collected urine samples. Two of these specimens, one serum and one urine, were rRT-PCR positive. From the these 80 PCR-positive patients, a total of 195 serum and urine specimens, including repeat specimens, were rRT-PCR tested, and 93 of these were ZKV RNA positive.
FIG 1.
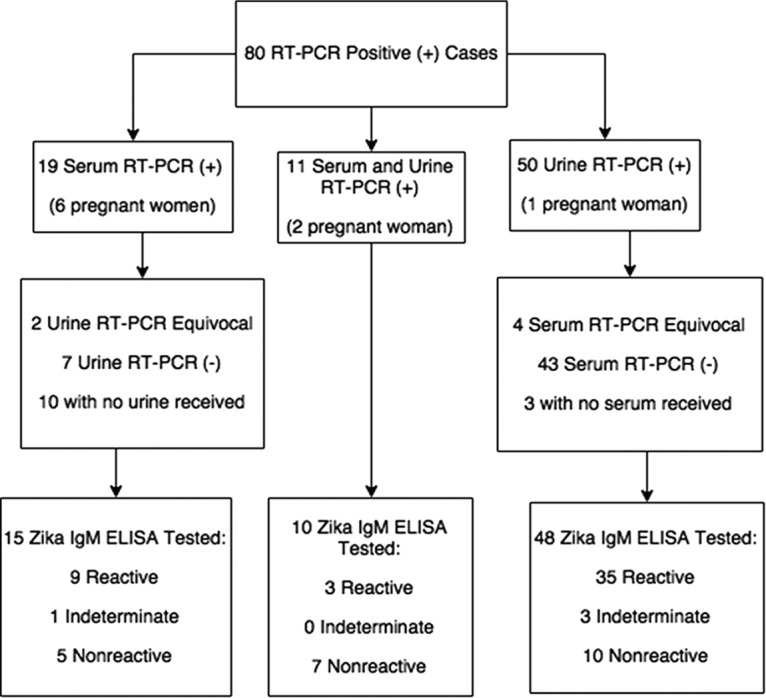
Serum and urine RT-PCR and Zika IgM ELISA results (n = 80 cases).
A total of 9 of the 80 patients were pregnant; 6 were positive by serum sample only: for 2 patients no urine was received, and 4 were negative by urine testing. Two pregnant women were positive by both urine and serum testing, and one pregnant woman was positive by urine testing only. All nine pregnant women displayed reactivity in the Zika IgM test.
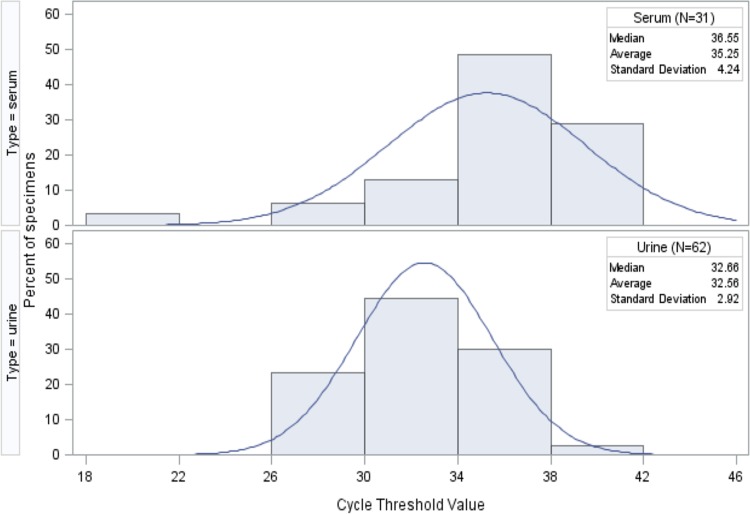
Reactivity differed in the two viral target assays. Among 31 ZKV-positive serum samples, 23 were tested with both the envelope and NS2B gene target assays. Of these 23, 14 (61%) tested positive only in the envelope assay, and 9 (39%) tested positive in both the envelope and NS2B assays. Among 62 ZKV-positive urine samples, 35 were tested with both the envelope and NS2B gene target assays. Of these 35, 3 (9%) tested positive only in the envelope assay, while 32 (91%) tested positive in both the envelope and NS2B assays. No sample from any patient tested positive in the NS2B assay and negative in the envelope assay. Viral loads, as indicated by, and inversely proportional to, cycle threshold (CT) values were a log higher in urine samples than in serum. The average serum cycle threshold value for rRT-PCR-positive specimens, including the two positive convalescent-phase samples, in the envelope target assay was 35.25, while that for urine was 32.56. Other summary statistics are provided in Fig. 2.
FIG 2.
Distribution of cycle threshold values in positive specimens of serum (n = 31) and urine (n = 62) tested in the envelope rRT-PCR assay for Zika virus RNA.
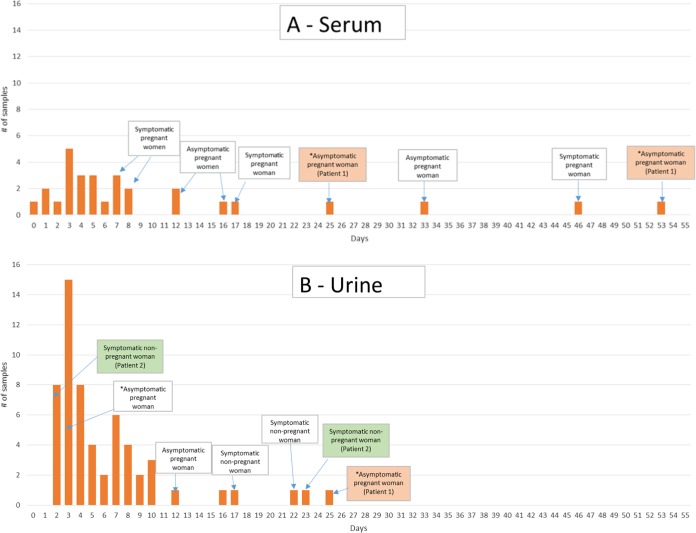
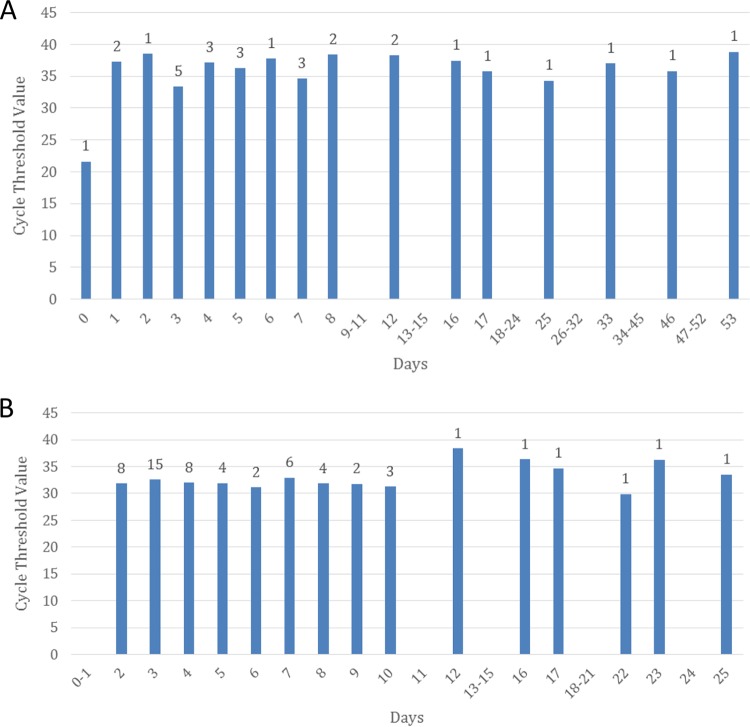
Differences were also observed in the duration of positivity between specimen types. Information on symptoms and time to positivity was available for all but five of the cases (n = 75) (Fig. 3). For asymptomatic patients, date of last travel was used to calculate time to specimen positivity. Mean time from symptom onset or last travel date to detection of viremia was 10.4 days, with a range of 0 to 53 days. Notably, the six serum samples with PCR positivity at 16, 17, 25, 33, 46, and 53 days were from pregnant women. If these instances of prolonged viremia, defined as ZKV present in the serum for 14 or more days after symptom onset date or 21 days after last exposure, in pregnant women are excluded, the mean and range are 4.9 and 0 to 12 days, respectively. Mean time from symptom onset or last travel dates to detection of viruria was 6.2 days, with a range of 2 to 25 days. Among the three patients who were positive by serum rRT-PCR within 1 day of symptom onset, one had a test result that was equivocal for ZKV RNA in a concurrently collected urine sample, and no urine samples were received from the other two patients. The asymptomatic pregnant patient who was positive by serum rRT-PCR at 25 and 53 days posttravel was also viruric at 25 days but notably negative on repeat urine testing at 53 days. Further, with the exception of a single sample received within 24 h of onset of symptoms, there was no apparent association between viral load and days post-symptom onset or -travel in either serum or urine specimen of envelope target assay-positive cases (Fig. 4). Overall, envelope target CT values for rRT-PCR-positive urine samples were significantly lower than those for rRT-PCR-positive serum samples (P value of <0.0001).
FIG 3.
Time difference in days from date of symptom onset (or date of last travel), indicated by an asterisk, to date of positive Zika rRT-PCR in serum samples (n = 29) and urine samples (n = 58). Patients with repeated positive testing are indicated as patient 1 and patient 2.
FIG 4.
Comparison of ZKV envelope target rRT-PCR-positive cycle threshold values over time from symptom onset or last date of travel in serum (A) or urine (B) specimens.
DISCUSSION
Response efforts to the ZKV outbreak have been challenging. In addition to severe clinical manifestations, including GBS and neurological damage to the fetus in pregnant women, the disease is frequently asymptomatic, and symptoms cannot be readily distinguished from those of similar syndromes caused by a number of other cocirculating arboviruses. Additionally, the combination of laboratory tests required to ensure comprehensive case detection has proven difficult and complicated to implement and interpret. A review of the test results from the first 80 ZKV cases among NYS residents, assessed as positive by the detection of ZKV RNA with rRT-PCR, provides insight into some of the disease parameters.
The viral load in serum is universally very low, and since the duration of viremia is brief and variable, the negative predictive value of molecular testing in serum may be low. Virus was more frequently detected in urine: average viral loads were a log higher than those in serum, and shedding in urine lasted longer than viremia, with the exception of prolonged viremia in pregnant women. We report the detection of additional cases of prolonged viremia in pregnant women, with viremia detected in four pregnant women (five specimens) beyond 14 days post-onset of symptoms or 21 days from last travel and recommend the use of molecular testing in these patients regardless of time since exposure. The addition of urine rRT-PCR testing identified another 50 ZKV-infected patients above those detected by serum positivity. We therefore consider the concurrent testing of urine to be highly beneficial. Data from Florida also found significant benefit in the molecular testing of concurrent urine samples (19).
It must be acknowledged that molecular testing for ZKV is a limited tool for ZKV diagnosis and that serology is relied upon for many cases. However, currently available IgM ELISAs are labor-intensive and time-consuming, and the ZKV antibodies are cross-reactive with other flaviviruses. This cross-reactivity is particularly problematic for patients who have traveled to, or lived in, countries with active ZKV transmission and have a high likelihood of immune responses to other flaviviruses. Confirmation of reactivity with plaque reduction neutralization tests requires considerably more extensive laboratory experience, and these tests are more labor-intensive than current IgM assays. Further, these tests are not able to resolve a diagnosis in all cases, even with the testing of paired acute- and convalescent-phase sera. In practice, paired acute- and convalescent-phase serum samples, collected and tested in multiple laboratory assays over the course of more than a month, can still result in an inconclusive diagnosis. The development of improved serologic assays is essential for accurate diagnosis of infections beyond the viremic or viruric period. However, diagnosis of ZKV infection with sensitive and specific viral RNA detection has advantages both from a public health perspective and for the clinical management of pregnant women. Efforts to optimize the performance and utility of rRT-PCR are therefore important.
The selection of genomic targets has been an issue in the application of molecular ZKV tests. Many assays that detect all genetic lineages of the virus target the envelope gene while others that specifically detect the Asian lineage commonly target the NS2B or prM gene. Some investigators and CDC guidance have recommended the use of assays for two targets when testing for the virus is performed, requiring both target assays to return a positive result (two-target-positive result) before considering a sample to be positive. Most laboratories have found assays targeting the envelope gene to be more sensitive than those for other genomic targets (20). With the low viral loads seen in ZKV specimens, especially in serum samples, a requirement for both targets to be positive may result in numerous equivocal results, causing ongoing clinical dilemmas particularly for pregnant patients. We considered a repeatedly positive signal in either target assay to be sufficient evidence of the presence of ZKV RNA. Further, a requirement to perform testing always on multiple targets causes considerable increases in cost and workloads and can result in major testing backlogs and delays. In this study, across 31 positive sera and 62 positive urine samples, no specimen was ever positive on the NS2B assay that was not also positive on the envelope assay. In contrast, 52 samples were positive only in the envelope assay. If a two-target-positive requirement had been in place for laboratory interpretation of a positive result, 56% of the samples in this study would have been assessed as equivocal rather than positive. It is therefore difficult to justify a requirement to perform both assays.
The utility of multiplex assays for arboviruses has also been considered. While these assays are helpful in providing multiple results with efficient laboratory testing, in practice, their utility during a major outbreak of one particular agent may be questionable. Additionally, many of the patients tested were exposed to, and at high risk for serious consequences of, ZKV infection but asymptomatic, a situation where such multiplex assays have questionable utility. In this study, initial testing of symptomatic patients revealed only five cases of dengue virus and none of chikungunya virus among several hundred patients, and this testing was therefore discontinued. In comparison, several hundred cases of ZKV infection have now been detected among NYS residents since January 2016. The multiplex arbovirus assays may prove more useful for testing symptomatic patients when they are not performed in the midst of a single-virus outbreak.
Sexual history data are well documented for only two of the six asymptomatic pregnant women included in the study. One individual reported the same date for last date of travel and last date of unprotected sexual contact with a partner who traveled to an area of active Zika virus activity (i.e., potential sexual exposure). The other individual had no potential sexual exposure. Sexual history data for exposure calculations are limited, however, for the other four asymptomatic pregnant women included in the study. For three of the four, the history of potential sexual exposure was unknown. For another individual with a known history of sexual contact with a traveler, dates of sexual contact were unknown; it is uncertain, therefore, how the history of unprotected sexual contact may have impacted the patient's infection and laboratory results.
In conclusion, we have found the molecular testing for ZKV in urine as well as serum to be highly beneficial and recommend that it be included as part of routine diagnostic testing. Allowing longer time frames post-symptom onset is beneficial for detecting additional infections, and among pregnant women the waiving of time frames should be considered, given the increasing evidence of prolonged viremia in these patients. Further, the molecular testing of one genomic target is sufficient for detection of the virus and may be an important factor in simplifying results and optimizing the use of available resources. Using rRT-PCR on urine as well as serum specimens, allowing extended time frame eligibility criteria, and classifying single-target rRT-PCR-reactive results as positive will facilitate the prompt and efficient diagnosis of more ZKV infections and reduce the reliance on time-consuming and potentially inconclusive serology. In this NYS cohort, samples for 74 of the 80 patients (93%) identified by rRT-PCR testing as ZKV infected would have been classified either as not positive or equivocal if a two-target-positive requirement was in place, if urine was not tested, and if the extended PCR testing eligibility policy had not been in place. These data support the expanded testing eligibility changes made by NYS and the CDC and raise questions for further study on appropriate molecular testing approaches.
MATERIALS AND METHODS
Patient eligibility.
In January 2016, the NYS Department of Health announced its initial response to the ZKV outbreak in the Caribbean, Central and South America, including laboratory testing performed at the Wadsworth Center (WC), at no cost to eligible residents. Initial eligibility included pregnant women who traveled to an area with active ZKV transmission while pregnant and infants with possible ZKV exposure in utero, as well as individuals who developed symptoms of ZKV disease or GBS with similar travel. In early March, following increasing evidence of sexual transmission (21, 22), eligibility was expanded to include pregnant women who had unprotected sex with a partner who had relevant travel history.
Demographics.
Data on age, sex, pregnancy status, symptoms, and travel history were obtained as part of a testing authorization process administered by all local health departments statewide. Additionally, clinical data were collected prospectively on patients with evidence of ZKV infection to assess outcomes, in accordance with routine practice for arboviral disease surveillance.
Specimen collection.
Both peripheral blood (for serum) and urine samples were collected for ZKV rRT-PCR; sera were also processed for serological testing. Initial molecular ZKV testing by the Centers for Disease Control and Prevention (CDC) for similar risk groups among residents of other states and territories was limited to serum only and to symptomatic patients presenting within 2 weeks of symptom onset (23, 24); testing was recommended for asymptomatic pregnant women only if abnormalities were detected on fetal ultrasound. However, due to the paucity of data on incubation periods and duration of viremia, inclusion criteria in NYS were rapidly changed to allow for molecular testing on samples collected up to 4 weeks post-onset of symptoms for all patients or at 6 weeks after the last date of travel for asymptomatic pregnant patients. No time limits were applied for serological testing. Later, following the report of prolonged viremia in pregnant women (17), archived samples previously collected from female patients beyond these time frames that had been processed only for serology were retrieved from −70°C storage and tested by rRT-PCR. Subsequently, in NYS time limits for molecular testing in pregnant women were discontinued. The CDC subsequently expanded recommended eligibility to include asymptomatic pregnant women, longer time frames, and the inclusion of urine rRT-PCR testing (25–27).
Laboratory methods.
Total nucleic acid was extracted from serum and urine samples on easyMAG instruments (bioMérieux, Durham, NC) using the generic protocol, with 250 μl of sample eluted to 60 μl. Extracted samples were tested with two rRT-PCR assays which target the envelope and NS2B genes of the virus, as recommended in the original CDC guidance to public health laboratories. Primer and probe sequences for the assays are shown in Table 2. Extensive validation included the optimization of primer and probe concentrations and the evaluation of several commercial master mix kits. Reactions were performed with a TaqMan Fast Virus 1-Step RT-PCR master mix (ThermoFisher Scientific, Waltham, MA) on an ABI 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA) using fast cycling conditions and 45 cycles. A spiked exogenous control and corresponding rRT-PCR assay were also performed to test for the presence of PCR inhibitors (28). Both virus target assays were performed in duplicate on all samples.
TABLE 2.
Sequences of primers and probes used in real-time RT-PCR assays
| Zika target | Primer (type) or probe | Sequencea | Reference |
|---|---|---|---|
| Envelope | 1086 (forward) | 5′-CCGCTGCCCAACACAAG-3′ | 11 |
| 1162cm (reverse)b | 5′-CCACTAAYGTTCTTTTGCAGACAT-3′ | ||
| 1107-FAM | 5′-FAM-AGCCTACCTTGACAAGCAGTCAGACACTCAA-BHQ-3′ | ||
| NS2B | 4481 (forward) | 5′-CTGTGGCATGAACCCAATAG-3′ | 29 |
| 4552c (reverse) | 5′-ATCCCATAGAGCACCACTCC-3′ | ||
| 4507-FAMc | 5′-FAM-CCACGCTCCAGCTGCAAAGG -BHQ-3′ |
FAM, 6-carboxyfluorescein; BHQ, Black Hole quencher.
Degeneracy was added to the reverse primer (indicated by boldface sequence). The modification enhanced the detection of all Zika virus lineages.
Samples were considered positive for ZKV RNA if cycle threshold values less than 40 were detected in both replicates of either assay. Samples with cycle threshold values greater than 40 or with only one replicate producing a signal were retested. Retested samples were considered positive if there were consistent positive signals in the replicate tests while those producing sporadic or intermittently positive results were considered equivocal. Negative samples demonstrating inhibition in the control assay were reported as indeterminate.
As recommended by the CDC, rRT-PCR assays for dengue and chikungunya viruses were also initially performed on sera from symptomatic patients. Chikungunya virus testing utilized a laboratory-developed test based on previously published primer and probe sequences (17) while testing for dengue viruses was performed with rRT-PCR assays for all four subtypes of the virus using the U.S. Food and Drug Administration (FDA)-approved CDC-DENV1-4 real-time RT-PCR kit.
Serological testing on serum included the CDC Zika IgM antibody capture enzyme-linked immunosorbent assay (29), and plaque reduction neutralization testing was performed on all specimens with positive screening serology results.
ACKNOWLEDGMENTS
We sincerely thank the following staff of the Virology Laboratory, Wadsworth Center, for specimen management and molecular testing: Rene Hull, Li Zeng, Michael Popowich, Meghan Fuschino, Daryl Lamson, Greg Farrell, Scott Brunt, and Patrick Bryant. We also thank the following staff of the Diagnostic Immunology Laboratory for serological testing: William Lee, Karen Kulas, Valerie Demarest, Sharon Casterlin, Tim Rem, Mary Marchewka, and Steven Bush. We also sincerely thank Local Health Department and Regional Office staff throughout New York State for their efforts in coordinating testing among eligible individuals and investigating cases of Zika virus, as well as the following staff of the Division of Epidemiology for their contributions to the NYS Zika response efforts and for helpful discussions during the preparation of the manuscript: Nina Ahmad, Valerie Haley, Shelley Zansky, Lou Ann Lance, Lynn Couey, Angie Maxted, Paula Pennell-Huth, and Phillip Kurpiel.
Kirsten St. George receives research funding support from ThermoFisher and Akonni Biosystems and has a Royalty Paying Collaborative Agreement with Zeptometrix. This report was supported in part by an appointment (ISS) to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists, funded by Centers for Disease Control and Prevention (CDC) cooperative agreement number 1U38OT000143-03. The work was also supported in part by CDC cooperative agreement number U50CK000423 (awarded to Debra Blog).
REFERENCES
- 1.Dick GW, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. 2014. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derraik JG, Slaney D. 2015. Notes on Zika virus—an emerging pathogen now present in the South Pacific. Aust N Z J Public Health 39:5–7. doi: 10.1111/1753-6405.12302. [DOI] [PubMed] [Google Scholar]
- 5.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. 2014. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 7.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 9.Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, Mekontso Dessap A. 2016. Zika virus associated with meningoencephalitis. N Engl J Med 374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- 10.Karimi O, Goorhuis A, Schinkel J, Codrington J, Vreden SG, Vermaat JS, Stijnis C, Grobusch MP. 2016. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet 387:939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 11.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. 2015. Detection of Zika virus in urine. Emerg Infect Dis 21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara K, Kutsuna S, Takasaki T, Moi ML, Ikeda M, Kotaki A, Yamamoto K, Fujiya Y, Mawatari M, Takeshita N, Hayakawa K, Kanagawa S, Kato Y, Ohmagari N. 2016. Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J Travel Med 23:tav011. doi: 10.1093/jtm/tav011. [DOI] [PubMed] [Google Scholar]
- 14.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. 2015. Detection of Zika virus in saliva. J Clin Virol 68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. 2016. Detection of Zika virus in semen. Emerg Infect Dis 22:940. doi: 10.3201/eid2205.160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. 2016. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis 16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- 17.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O. 2016. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374:2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 18.Meaney-Delman D, Oduyebo T, Polen KN, White JL, Bingham AM, Slavinski SA, Heberlein-Larson L, St George K, Rakeman JL, Hills S, Olson CK, Adamski A, Culver Barlow L, Lee EH, Likos AM, Munoz JL, Petersen EE, Dufort EM, Dean AB, Cortese MM, Santiago GA, Bhatnagar J, Powers AM, Zaki S, Petersen LR, Jamieson DJ, Honein MA, U.S. Zika Pregnancy Registry Prolonged Viremia Working Group. 2016. Prolonged detection of Zika virus RNA in pregnant women. Obstet Gynecol 128:724–730. doi: 10.1097/AOG.0000000000001625. [DOI] [PubMed] [Google Scholar]
- 19.Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, Likos A. 2016. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR Morb Mortal Wkly Rep 65:475–478. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 20.Waggoner JJ, Pinsky BA. 2016. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 54:860–867. doi: 10.1128/JCM.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Yazdanpanah Y, Leparc-Goffart I. 2016. Evidence of sexual transmission of Zika virus. N Engl J Med 374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 23.Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. 2016. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 24.Hennessey M, Fischer M, Staples JE. 2016. Zika virus spreads to new areas—region of the Americas, May 2015-January 2016. MMWR Morb Mortal Wkly Rep 65:55–58. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. 2016. Interim guidance for Zika virus testing of urine—United States, 2016. MMWR Morb Mortal Wkly Rep 65:474. doi: 10.15585/mmwr.mm6518e1. [DOI] [PubMed] [Google Scholar]
- 26.Oduyebo T, Igbinosa I, Petersen EE, Polen KN, Pillai SK, Ailes EC, Villanueva JM, Newsome K, Fischer M, Gupta PM, Powers AM, Lampe M, Hills S, Arnold KE, Rose LE, Shapiro-Mendoza CK, Beard CB, Munoz JL, Rao CY, Meaney-Delman D, Jamieson DJ, Honein MA. 2016. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure—United States, July 2016. MMWR Morb Mortal Wkly Rep 65:739–744. doi: 10.15585/mmwr.mm6529e1. [DOI] [PubMed] [Google Scholar]
- 27.Oduyebo T, Petersen EE, Rasmussen SA, Mead PS, Meaney-Delman D, Renquist CM, Ellington SR, Fischer M, Staples JE, Powers AM, Villanueva J, Galang RR, Dieke A, Munoz JL, Honein MA, Jamieson DJ. 2016. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 65:122–127. doi: 10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- 28.Tavakoli NP, Nattanmai S, Hull R, Fusco H, Dzigua L, Wang H, Dupuis M. 2007. Detection and typing of human herpesvirus 6 by molecular methods in specimens from patients diagnosed with encephalitis or meningitis. J Clin Microbiol 45:3972–3978. doi: 10.1128/JCM.01692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. 2016. Zika MAC-ELISA. Centers for Disease Control and Prevention, Atlanta, GA: www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM488044.pdf. [Google Scholar]