ABSTRACT
The objective of this study was to evaluate the clinical characteristics and outcomes of hospitalized patients tested for Clostridium difficile and determine the correlation between pretest probability for C. difficile infection (CDI) and assay results. Patients with testing ordered for C. difficile were enrolled and assigned a high, medium, or low pretest probability of CDI based on clinical evaluation, laboratory, and imaging results. Stool was tested for C. difficile by toxin enzyme immunoassay (EIA) and toxigenic culture (TC). Chi-square analyses and the log rank test were utilized. Among the 111 patients enrolled, stool samples from nine were TC positive and four were EIA positive. Sixty-one (55%) patients had clinically significant diarrhea, 19 (17%) patients did not, and clinically significant diarrhea could not be determined for 31 (28%) patients. Seventy-two (65%) patients were assessed as having a low pretest probability of having CDI, 34 (31%) as having a medium probability, and 5 (5%) as having a high probability. None of the patients with low pretest probabilities had a positive EIA, but four were TC positive. None of the seven patients with a positive TC but a negative index EIA developed CDI within 30 days after the index test or died within 90 days after the index toxin EIA date. Pretest probability for CDI should be considered prior to ordering C. difficile testing and must be taken into account when interpreting test results. CDI is a clinical diagnosis supported by laboratory data, and the detection of toxigenic C. difficile in stool does not necessarily confirm the diagnosis of CDI.
KEYWORDS: Clostridium difficile, Clostridium difficile infection, enzyme immunoassay
INTRODUCTION
Clostridium difficile infection (CDI) is a common and serious health care-associated infection; the Centers for Disease Control and Prevention estimates that there were 453,000 CDI cases and 29,300 associated deaths in the United States in 2011 (1). One of the challenges health care facilities encounter is the complexity of accurately diagnosing CDI (2, 3). A confounding variable that contributes to this diagnostic challenge is that asymptomatic carriage of C. difficile is common (4). Currently, available assays can detect the presence of C. difficile and/or its toxins, but there are no available assays to diagnose CDI. The diagnosis of CDI requires the presence of appropriate clinical signs and symptoms in combination with a positive test for toxigenic C. difficile or its toxins (5). Therefore, CDI is a clinical diagnosis that is supported by laboratory data and/or endoscopic findings (2, 3).
While there is no true “gold standard” assay for the diagnosis of CDI, toxigenic culture (TC) is the gold standard for detection of toxin-producing C. difficile in stool and the cell culture cytotoxicity assay (CCCA) is the gold standard for detecting free C. difficile toxin(s) in stool (6). Because these methods have a prolonged turnaround time, are labor-intensive, and require specialized expertise, TC and the CCCA are not currently used as routine diagnostic methods in the United States. Commercially available assays include enzyme immunoassays (EIAs) for toxins A and B, EIAs for glutamate dehydrogenase, and nucleic acid amplification tests (NAATs). NAAT sensitivity approaches that of TC, and this methodology is now the most commonly used in the United States to detect C. difficile in stool (7).
There is increasing recognition that NAATs detect asymptomatically colonized patients and have poor specificity for CDI and that careful patient selection for C. difficile testing would decrease false-positive tests for CDI (2, 3, 8). However, data on the impact of clinical characteristics and pretest probability of CDI on CDI diagnosis are scarce. Thus, the objective of this study was to evaluate the clinical characteristics and outcomes of hospitalized patients tested for C. difficile and determine the correlation between pretest probability for CDI and assay results.
RESULTS
Demographics.
Among the 111 patients enrolled in the study, stool samples from four (3.6%) were positive by toxin EIA (EIA+) and samples from nine (8.1%) were TC positive (TC+). In this sample, there were 2 EIA+/TC+, 7 EIA−/TC+, 2 EIA+/TC−, and 100 EIA−/TC− patients. Patient demographics and clinical characteristics are shown in Table 1. Potential reasons for diarrhea, other than CDI, were identified and included 22 (20%) patients with recent chemotherapy, 18 (16%) that received a laxative within the 24 h prior to the test request, and 15 (14%) that were receiving tube feeds. The presence of clinically significant diarrhea (CSD) could not be assessed in 31 (28%) patients. Among those for which it was possible to determine the severity of diarrhea (n = 80), 61 (76%) patients experienced CSD. Among those with CSD (n = 61), potential non-CDI reasons for diarrhea included 14 (23%) patients undergoing chemotherapy and 9 (15%) who had received a laxative in the previous 24 h (Table 2). There were significant differences in recent chemotherapy or tube feeds by CSD status (Table 2).
TABLE 1.
Demographics and clinical characteristics of study populationa
| Variable | No. (%) |
|---|---|
| Demographics | |
| Female | 53 (48) |
| Nonwhite race | 29 (26) |
| Age | |
| ≤40 | 18 (16) |
| 41–65 | 56 (51) |
| >65 | 37 (33) |
| Admitted from | |
| Home | 78 (70) |
| Long-term care facility | 11 (10) |
| Outside hospital | 19 (17) |
| Unknown | 3 (3) |
| History of CDI in the 12 weeks before admission | 5 (5) |
| Stool characteristics at the time of stool collection | |
| No. of bowel movements a day | |
| <1 | 1 (1) |
| 1 | 5 (5) |
| 2 | 10 (9) |
| 3–9 | 54 (49) |
| ≥10 | 6 (5) |
| Ostomy/tube | 4 (4) |
| Unable to determine | 31 (28) |
| Bristol stool scale | |
| 1–3 | 4 (4) |
| 4–5 | 6 (5) |
| 6–7 | 86 (78) |
| Unable to obtain | 15 (14) |
| Clinical data | |
| Clinically significant diarrheab | |
| Yes | 61 (55) |
| No | 19 (17) |
| Unable to determine | 31 (28) |
| Temperature of ≥38°C | 18 (16) |
| White blood cell counts within 24 h of stool collection | |
| Low (<4.0) | 30 (27) |
| Normal (4.0 to <12.0) | 43 (39) |
| High (12.0 to <20.0) | 23 (21) |
| Very high (≥20.0) | 7 (6) |
| Not available | 8 (7) |
| Abdomen/pelvis computed tomography scan within 7 days of testc | 34 (31) |
| Colitis | 7 (6) |
| Colonic wall thickening | 7 (6) |
| Pericolonic stranding | 6 (5) |
| Other | 34 (31) |
| Abdominal pain scale (0–10) | |
| 0 | 47 (42) |
| 1–3 | 15 (14) |
| 4–6 | 15 (14) |
| 7–10 | 14 (13) |
| Unable to obtain | 20 (18) |
| Abdominal tenderness on exam | |
| None | 71 (64) |
| Mild | 24 (22) |
| Moderate | 4 (4) |
| Severe | 0 (0) |
| Unable to obtain | 12 (11) |
| Abdominal distension on exam | |
| None | 82 (74) |
| Mild | 23 (21) |
| Moderate | 6 (5) |
| Severe | 0 (0) |
| Rebound tenderness | |
| None | 91 (82) |
| Mild | 7 (6) |
| Moderate | 1 (1) |
| Severe | 0 (0) |
| Unable to obtain | 12 (11) |
| Other explanations for diarrhea | |
| Laxative within 24 h of test | 18 (16) |
| Inflammatory bowel disease | 7 (6) |
| Abdominal surgery in past 7 days | 3 (3) |
| Tube feeds | 15 (14) |
| Current chemotherapy | 22 (20) |
| Gastrointestinal graft vs host disease | 5 (5) |
| Other gastrointestinal infection | 3 (3) |
| Short gut syndrome | 1 (1) |
The study population included 111 participants.
Clinically significant diarrhea was defined as ≥3 stools per day of Bristol stool chart type 6 or 7 or abdominal pain plus Bristol stool chart type 6 or 7 stools.
Patients could have more than one finding.
TABLE 2.
Other reasons for diarrhea, stratified by the presence of clinically significant diarrhea
| Variablea | No. (%) with no clinically significant diarrhea (n = 19) | No. (%) with clinically significant diarrhea (n = 61) | No. (%) unable to determine (n = 31) |
|---|---|---|---|
| Female* | 9 (47) | 35 (57) | 9 (29) |
| Age | |||
| ≤40 | 2 (11) | 13 (21) | 3 (10) |
| 41–65 | 7 (37) | 32 (53) | 17 (55) |
| >65 | 10 (53) | 16 (26) | 11 (36) |
| Nonwhite race | 2 (11) | 16 (26) | 11 (36) |
| Admission location* | |||
| Home | 13 (68) | 49 (80) | 16 (52) |
| Long-term care facility | 0 (0) | 3 (5) | 8 (26) |
| Outside hospital | 6 (32) | 8 (13) | 5 (16) |
| Unknown | 0 (0) | 1 (2) | 2 (7) |
| History of CDI in previous 12 weeks | 0 (0) | 3 (5) | 2 (7) |
| Other reasons for diarrhea | |||
| Laxative within 24 h | 2 (11) | 9 (15) | 7 (23) |
| Inflammatory bowel disease | 1 (5) | 3 (5) | 3 (10) |
| Tube feeds* | 0 (0) | 3 (5) | 12 (39) |
| Chemotherapy* | 7 (37) | 14 (23) | 1 (3) |
| Gastrointestinal graft vs host disease | 1 (5) | 2 (3) | 2 (7) |
| Bowel prepn with polyethylene glycol | 0 (0) | 1 (2) | 0 (0) |
| Other gastrointestinal infection | 1 (5) | 2 (3) | 0 (0) |
| Other infection | 0 (0) | 1 (2) | 0 (0) |
An asterisk indicates a P value of <0.05.
Pretest probability for CDI.
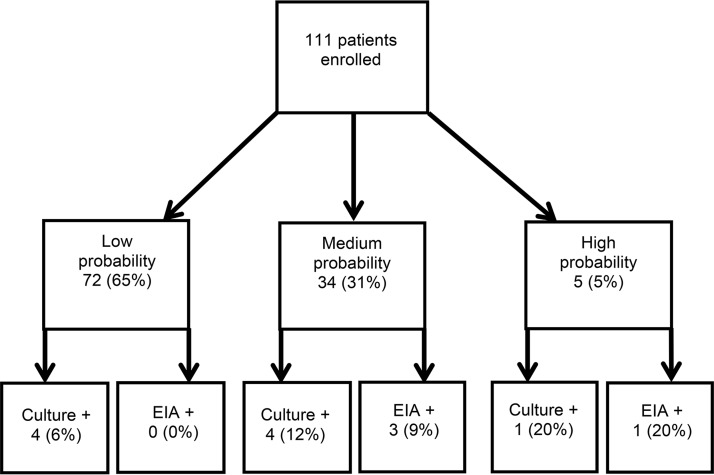
Overall, 72 (65%) patients were assessed as having low pretest probability for CDI, 34 (31%) as having a medium probability, and 5 (5%) as having a high probability (Fig. 1). There were significant differences (P < 0.05) among patients by pretest classification for the following characteristics: empiric CDI treatment, abdominal tenderness, leukocytosis, and toxin EIA results (Table 3). Among the four patients (3.6%) with positive toxin EIA results, three had been rated as having a medium probability for CDI and one had been rated as having a high probability. The characteristics of these patients are given in Table 4. The median 90-day survival was 90 days for the low and medium groups and 76 days for the high pretest probability of CDI (log rank, P < 0.01).
FIG 1.
Assigned probability of CDI and toxin EIA results.
TABLE 3.
Selected clinical characteristics and outcomes, stratified by pretest probability of Clostridium difficile infection
| Variable | Pretest probability (no. [%]) |
||
|---|---|---|---|
| Low (n = 72) | Medium (n = 34) | High (n = 5) | |
| Laxatives within 24 h | 12 (17) | 6 (18) | 0 (0) |
| Empiric CDI treatment | 9 (13) | 6 (18) | 3 (60) |
| Able to determine presence of clinically significant diarrhea (n = 80) | 53 (74) | 25 (74) | 2 (40) |
| Clinically significant diarrhea (n = 61) | 37 (51) | 22 (65) | 2 (40) |
| No clinically significant diarrhea (n = 19) | 16 (22) | 3 (9) | 0 (0) |
| Unable to determine if patient had clinically significant diarrhea (n = 31) | 19 (26) | 9 (27) | 3 (60) |
| Fever | 10 (14) | 6 (18) | 2 (40) |
| Leukocytosisa | 13 (18) | 17 (50) | 0 (0) |
| Abdominal pain | 22 (31) | 20 (59) | 2 (40) |
| Abdominal tendernessa | 12 (17) | 14 (41) | 2 (40) |
| Toxigenic culture positive | 4 (6) | 4 (12) | 1 (20) |
| Positive EIAa | 0 (0) | 3 (9) | 1 (20) |
| CDI within 30 days after index EIA | 1 (1) | 0 (0) | 0 (0) |
| Died within 90 days of EIA | 5 (7) | 5 (15) | 3 (60) |
| Median survival (days)b | 90 | 90 | 76 |
Chi-square tests indicate a P value of <0.05.
Log rank tests indicate a P value of <0.01.
TABLE 4.
Positive Clostridium difficile EIA resultsa
| Variable | Pretest probability (no.)b |
|
|---|---|---|
| Medium (n = 3) | High (n = 1) | |
| Positive toxigenic culture | 1 | 1 |
| Pretest empiric CDI treatment | 1 | 1 |
| Posttest CDI treatment | 1 | 1 |
| Clinical symptoms | ||
| Fever | 0 | 0 |
| Leukocytosis | 1 | 0 |
| Clinically significant diarrhea | 3 | 0 |
| Abdominal pain | 2 | 0 |
| Abdominal tenderness | 1 | 1 |
| CT scan in previous 7 days | 0 | 1c |
| Other reasons for diarrhea | ||
| Chemotherapy | 1 | 0 |
| Died within 90 days | 1 | 1 |
There were a total of 4 positive EIA results.
There were 0 low pretest probability patients with positive EIA results.
CT scan findings indicated diffuse bowel wall edema and hyperemia in colon.
Of the five patients with a high pretest probability of CDI, two (40%) of these patients had CSD. The presence of CSD could not be determined for the other three patients due to a lack of information from the patient or clinical team about the patient's stool type or stool frequency. Further examination of these five high pretest probability patients indicate that they all had high medical acuity and/or underlying immunosuppression, none were on laxatives, and three were on empiric treatment for CDI prior to testing. Two patients were critically ill in the medical intensive care unit, and four had an active hematopoietic malignancy.
One patient with a high pretest probability of CDI had a positive toxin EIA and a negative TC result. This patient had a history of a hematopoietic malignancy with recent chemotherapy and treatment with broad-spectrum antibiotics for health care-associated pneumonia a month prior to the positive EIA. At the time of the positive toxin EIA, the patient was toxic appearing, neutropenic, had diarrhea documented by the clinical provider, and received empiric CDI treatment with metronidazole. A computed tomography (CT) scan of the abdomen demonstrated diffuse bowel wall edema of the colon. The patient was ultimately diagnosed with a perforated bowel due to diverticulitis and associated Escherichia coli bacteremia.
Toxigenic culture.
C. difficile was recovered in culture from the stool of 14 patients, of which 9 were toxigenic. Four isolates were positive for tcdA, tcdB, and cdtA and cdtB. The remaining five had only tcdA and tcdB detected. The following five different strain types were identified: PCR ribotype 027 (n = 3), 014/020 (n = 3), one each of ribotypes 015/046 and 106/174, and a strain type previously characterized at Washington University (WU) but without a match in the European Centre for Disease Prevention and Control (ECDC)-Cardiff reference strains named WU8 (4). Characteristics of these nine patients are given in Table 5 and are stratified by pretest probability of CDI. Two culture-positive patients had positive toxin EIA results and were treated for CDI. None of the seven patients with a positive TC but negative EIA developed CDI within 30 days after the index EIA or died within 90 days after the index toxin EIA date. Only one TC-positive/EIA-negative patient had been started on empiric treatment with oral metronidazole prior to culture. One patient who had a positive index EIA and positive index TC (ribotype 027) died within 90 days of the toxin EIA.
TABLE 5.
Positive Clostridium difficile toxigenic culture resultsa
| Variable | Pretest probability (no.) |
||
|---|---|---|---|
| Low (n = 4) | Medium (n = 4) | High (n = 1) | |
| Ribotype | 027 (2) | 014/020 (2) | 106/174 |
| 014/020 | 027 | ||
| WU8 | 015/046 | ||
| Positive EIA | 0 | 2 | 0 |
| Pretest empiric CDI treatment | 1 | 0 | 0 |
| Posttest empiric CDI treatment | 1 | 0 | 0 |
| Clinical symptoms | |||
| Fever | 1 | 0 | 1 |
| Leukocytosis | 1 | 0 | 0 |
| Clinically significant diarrhea | 4 | 3 | 1 |
| Abdominal pain | 3 | 2 | 1 |
| Abdominal tenderness | 2 | 1 | 1 |
| CT scan within previous 7 days | 2b | 0 | 0 |
| Other reasons for diarrhea | |||
| Laxative | 0 | 1 | 0 |
| Chemotherapy | 0 | 2 | 1 |
| Developed CDI within 30 daysc | 0 | 0 | 0 |
| Died within 90 days | 0 | 1 | 0 |
There were a total of 9 patients with positive TC results. Five additional patients had nontoxigenic C. difficile detected in their stool.
CT scan findings indicated malrotation and focal pancreatitis.
Does not include results of index toxin EIA.
There was no significant difference in survival at 90 days post-EIA between patients who were culture positive and EIA negative (n = 7) and patients who were culture negative and EIA negative (n = 100) (0% EIA−/TC+ died versus 11% EIA−/TC−; log rank, P = 0.37).
Repeat testing.
Eight patients had a repeat test ordered within 96 h of the index test. Five of these patients were assigned a low pretest probability of CDI, two had a medium probability, and one had a high probability. Two of these patients had an index test which was EIA negative and TC positive (both were positive for tcdA and tcdB; ribotypes 106/174 and 014/020). The patient with ribotype 106/174 had been assigned a high pretest probability of CDI, had not received empiric CDI treatment, and had a negative repeat EIA but a positive TC. The patient with ribotype 014/020 had been assigned a medium pretest probability of CDI, had not received empiric CDI treatment, and had a negative repeat EIA and a negative TC. None of the eight patients with repeat tests within 96 h were treated for CDI or were diagnosed with CDI within 30 days, including the patients with cultures that were positive for toxigenic C. difficile. One of the eight patients died within 90 days for reasons unrelated to CDI (oncology patient with low pretest probability of CDI).
Only 1 out of the 111 patients had a positive C. difficile EIA within the 30 days after the index EIA and negative TC. This patient had a history of acute myeloid leukemia and, at the time of the negative index EIA and TC tests, was classified as having a low probability of CDI. After the index test, the patient underwent hematopoietic stem cell transplantation (HSCT) and subsequently developed neutropenic fever and diarrhea. Seven days after the index test, the patient had a positive EIA and TC (positive for tcdA, tcdB, and cdtA and cdtB; PCR ribotype 126).
DISCUSSION
This study adds to the growing body of evidence that laboratory testing alone is insufficient to confirm a diagnosis of CDI. Existing data suggest that many patients tested for C. difficile do not have a clinical syndrome compatible with CDI and/or have alternative causes of diarrhea. The results of this study emphasize the tenet that a patient's clinical presentation should be taken into account prior to ordering, and when interpreting, laboratory testing for C. difficile.
Of the patients for whom we were able to determine diarrhea severity, only 76% had CSD. A substantial portion of patients had other medical conditions or were on laxatives that may have caused diarrhea at the time of the C. difficile test. Similarly, in a study designed to validate a PCR assay for C. difficile, Peterson et al. found that 39% of submitted stool samples came from patients who had fewer than three diarrheal stools per day (9). Su et al. performed a clinical review during an evaluation of a NAAT-based assay and found that 21% of patients no longer had diarrhea (≥3 loose stools within 24 h) at the time of sample collection (10). In our study, 16% were receiving a laxative within 24 h prior to C. difficile testing, including a patient receiving polyethylene glycol in preparation for a colonoscopy. The use of laxatives prior to C. difficile testing was previously documented in studies by Buckel et al. and Dubberke et al., which noted that up to 19% to 44% of patients tested for C. difficile had documented laxative use in the 48 h prior to stool collection (5, 11).
We were unable to assess for the presence of CSD in 28% of patients due to the absence of data from the patient or clinical team on the patient's stool consistency or frequency. Reasons for the lack of data included patient factors, such as the inability to communicate due to critical illness or dementia, as well as health care worker factors, such as a lack of documentation of clinical symptoms and stool frequency. The inability of patients to communicate their symptoms is a limitation that clinicians face on a daily basis, as many patients and caregivers are unable to consistently communicate due to their medical comorbidities (for example, debilitating stroke or critical illness). Improved methods for the documentation of stool consistency and frequency in the medical health record may help clinicians accurately determine which patients should be tested for C. difficile.
In this sample, 65% of patients had a low pretest probability for CDI. Notably, none of these patients had a positive C. difficile EIA, and none of the four low-probability patients colonized with toxigenic C. difficile developed CDI-attributable complications. Alternate causes of diarrhea were found in many of these patients, and often their clinical presentations were not concerning for CDI after thoughtful review. This suggests that C. difficile testing may not be indicated in patients with a low pretest probability for CDI.
There were some notable cases in this cohort that warrant further discussion. One patient with a high pretest probability for CDI had a positive EIA but a negative TC result. This patient had been on empiric CDI treatment with metronidazole prior to testing and was ultimately diagnosed with a perforated bowel due to diverticulitis and associated Escherichia coli bacteremia. A potential explanation for the negative culture is that the metronidazole inhibited growth in culture. Alternatively, the EIA was falsely positive, and all symptoms were due to diverticulitis and bacteremia. Another patient had a positive EIA and TC 7 days after a negative index EIA and TC. This patient had a history of acute myeloid leukemia, and at the time of the index test, she was classified as having a low probability of CDI. Within that 7-day time period, the patient underwent HSCT and subsequently developed neutropenic fever and diarrhea. The median incubation period from C. difficile acquisition to CDI is <7 days (12). Given the continued hospital exposure and immunocompromised state, it is likely that this patient acquired C. difficile and developed CDI after the negative index test.
None of the seven patients with a negative index EIA but positive TC developed CDI within 30 days after the index EIA or died within 90 days after the index test. Of the eight patients who had a repeat test ordered within 96 h of a negative index test, two patients had an index test which was EIA negative and TC positive. Neither had received empiric CDI treatment, both had negative repeat EIAs, one had a positive repeat TC, and neither was diagnosed with CDI or died within 30 days of the index EIA. Although patients may have clinical syndromes concerning for CDI, they may be colonized with C. difficile and not have actual CDI. These findings are consistent with prior literature; Polage et al. demonstrated that C. difficile-attributable complications are rare among patients with a negative C. difficile toxin EIA (13). In another study, Polage et al. demonstrated that presentation and CDI-related complications were no different in patients that had negative toxin EIA and PCR tests than they were in patients with negative toxin EIA and positive PCR tests (14).
There are several limitations to this study. First, it was relatively small and it was conducted over a short time frame at a single institution; a larger study with a longer assessment period would allow for more robust statistical analyses of CDI-related outcomes and mortality. Second, the pretest CDI probabilities were assigned by a single physician. As we have emphasized, CDI is a diagnosis that relies heavily on clinical judgment, and our results may have been biased by the assessments of one physician's judgment. Outcomes based on CDI probability suggest that this is not the case; median survival was shortest in the high pretest probability group (90, 90, and 76 days in the low, medium, and high probability groups, respectively). In the current investigation, we did not perform a molecular test for C. difficile; however, previous studies have shown that when PCR is used to detect C. difficile in hospitalized patients with diarrhea, the sensitivity approaches that of TC (7, 15).
This study provides additional emphasis on the importance of patients' clinical symptoms for the interpretation of C. difficile diagnostic assays. Assignment of a pretest probability for CDI at the time of testing is a novel approach for assessing the impact of the overall clinical picture on the interpretation of C. difficile assay results. As we assessed patients in real-time and did not exclude patients who could not communicate their stool characteristics or frequency, this study is generalizable to the circumstances that clinicians encounter on a daily basis. Data regarding the patient's symptoms and clinical exam were collected prospectively; we were not limited by retrospective medical record data. Given the prospective data collection, we were able to collect a more complete record of a patient's stool characteristics, as often diarrhea is incompletely captured in a medical chart. Further prospective studies of this nature would be of value.
Clinicians and health care facilities continue to search for a C. difficile test that is simultaneously rapid, sensitive, and specific for CDI. Certainly, more research is needed on diagnostic methods for CDI, especially given the important consideration of asymptomatic colonization. However, perhaps the difficulties encountered in CDI diagnosis are less a failure of diagnostic technology than an overreliance on diagnostic tests and an underreliance on clinical assessments. Moving forward, methods and guidelines to diagnose CDI that couple laboratory-based C. difficile detection with clinical assessments need to be developed.
MATERIALS AND METHODS
Setting.
This prospective cohort study was performed at Barnes-Jewish Hospital, a 1,250-bed tertiary care hospital in St. Louis, MO, from August to October 2014. The study was part of a quality-improvement initiative to improve C. difficile testing practices. The Washington University (WU) Human Research Protection Office approved publication of the results of this quality improvement project. Inpatients who were ≥18 years old were eligible if they had a C. difficile EIA ordered.
Clinical evaluation.
Consecutive inpatients with C. difficile testing ordered were approached to participate. A physician (J.H.K.) interviewed patients and evaluated their symptoms, stool characteristics and frequency, and performed an abdominal exam. Stool characteristics were assessed with the Bristol stool scale, and clinically significant diarrhea (CSD) was defined as ≥3 Bristol type 6 or 7 stools per day or abdominal pain plus Bristol type 6 or 7 stools (5, 16, 17). If the patient and/or clinical team was unable to provide information regarding the stool type or frequency, CSD was documented as “unable to be determined.” Additional data obtained included patient demographics, vitals, antibiotics, laxatives (docusate, senna, polyethylene glycol, lactulose, enemas), tube feeds, CDI history, imaging, and white blood cell counts (WBC). Patients were then assigned a high, medium, or low pretest probability of CDI. This rating was assigned prior to the availability of the patients' C. difficile testing results. The pretest probability for CDI was determined based on a combination of the patient's stool history, clinical signs, symptoms, abdominal exam, laboratory test results, and radiology findings. If there were other more probable causes of diarrhea, such as the presence of a laxative or underlying gastrointestinal pathology (mucositis related to chemotherapy), this was also taken into consideration. If CSD was unable to be determined, all other data gathered was used to assist in the determination of pretest probability. For example, if CSD was unable to be determined but the patient had other exam, laboratory, and radiographic findings highly consistent with CDI, the patient would be assigned a high pretest probability for CDI.
Laboratory testing for C. difficile.
Patients' stool samples were submitted to the clinical laboratory for testing with the TechLab Tox A/B II EIA (Blacksburg, VA); testing was rejected on formed stools (3). If available, remnant stool was frozen at −80°C. Culture for C. difficile was performed as previously described (18). Briefly, an aliquot of stool was heated at 80°C for 10 min followed by inoculation into cycloserine-cefoxitin mannitol broth with taurocholate and lysozyme (Anaerobe Systems, Morgan Hill, CA). Colonies resembling C. difficile were identified using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) using the Vitek MS platform (bioMérieux, Durham, NC) (19). Isolates were evaluated for the presence of tcdA, tcdB, and binary toxin genes (cdtA and cdtB) by multiplex PCR (4, 20, 21) and were characterized by PCR ribotyping (21).
Statistical analysis.
Patients' assigned pretest probability of CDI based on clinical signs and symptoms was compared with the toxin EIA results and TC results. Chi-square analyses and the log rank test were used for analyses (SPSS version 21; IBM Statistics, Armonk, NY).
ACKNOWLEDGMENTS
This work was supported by grants from the Foundation of Barnes-Jewish Hospital (grant 7915-77) and the Prevention Epicenters Program of the Centers for Disease Control and Prevention (grant U54CK000162). J.H.K. was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, subaward KL2TR000450, from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH).
J.H.K., K.A.R., and T.H. report no conflicts of interest. C.A.D.B. reports grants from bioMérieux, Accelerate Diagnostics, Theravance, and Cepheid outside this submitted work. E.R.D. reports grants and personal fees from Sanofi Pasteur, Merck, and Rebiotix, and personal fees from Valneva, GlaxoSmithKline, and Summit outside this submitted work.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
REFERENCES
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnham C-AD, Dubberke ER, Kociolek LK, Polage CR, Riley TV. 2016. Clostridium difficile-diagnostic and clinical challenges. Clin Chem 62:310–314. doi: 10.1373/clinchem.2015.243717. [DOI] [PubMed] [Google Scholar]
- 3.Dubberke ER, Burnham C-AD. 2015. Diagnosis of Clostridium difficile infection: treat the patient, not the test. JAMA Intern Med 175:1801–1802. doi: 10.1001/jamainternmed.2015.4607. [DOI] [PubMed] [Google Scholar]
- 4.Alasmari F, Seiler SM, Hink T, Burnham C-AD, Dubberke ER. 2014. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 59:216–222. doi: 10.1093/cid/ciu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubberke ER, Han Z, Bobo L, Hink T, Lawrence B, Copper S, Hoppe-Bauer J, Burnham C-AD, Dunne WM Jr. 2011. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol 49:2887–2893. doi: 10.1128/JCM.00891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett JG, Gerding DN. 2008. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 46(Suppl):S12–S18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 7.Burnham C-AD, Carroll KC. 2013. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 26:604–630. doi: 10.1128/CMR.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan DJ, Leekha S, Croft L, Burnham C-AD, Johnson JK, Pineles L, Harris AD, Dubberke ER. 2015. The importance of colonization with Clostridium difficile on infection and transmission. Curr Infect Dis Rep 17:499. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LR, Manson RU, Paule SM, Hacek DM, Robicsek A, Thomson RB Jr, Kaul KL. 2007. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis 45:1152–1160. doi: 10.1086/522185. [DOI] [PubMed] [Google Scholar]
- 10.Su WY, Mercer J, Van Hal SJ, Maley M. 2013. Clostridium difficile testing: have we got it right? J Clin Microbiol 51:377–378. doi: 10.1128/JCM.02189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckel WR, Avdic E, Carroll KC, Gunaseelan V, Hadhazy E, Cosgrove SE. 2015. Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 36:217–221. doi: 10.1017/ice.2014.19. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 13.Polage CR, Chin DL, Leslie JL, Tang J, Cohen SH, Solnick JV. 2012. Outcomes in patients tested for Clostridium difficile toxins. Diagn Microbiol Infect Dis 74:369–373. doi: 10.1016/j.diagmicrobio.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen H, Huang B, Tang Y, Lee L, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. 2015. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 175:1792–1801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande A, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Pant C, Hernandez AV. 2011. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis 53:e81–e90. doi: 10.1093/cid/cir505. [DOI] [PubMed] [Google Scholar]
- 16.Peterson LR, Robicsek A. 2009. Does my patient have Clostridium difficile infection? Ann Intern Med 151:176–179. doi: 10.7326/0003-4819-151-3-200908040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SJ, Heaton KW. 1997. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 18.Hink T, Burnham C-AD, Dubberke ER. 2013. A systematic evaluation of methods to optimize culture-based recovery of Clostridium difficile from stool specimens. Anaerobe 19:39–43. doi: 10.1016/j.anaerobe.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner O, Mochon A, Branda J, Burnham C-AD, Bythrow M, Ferraro M, Ginocchio C, Jennemann R, Manji R, Procop GW, Richter S, Rychert J, Sercia L, Westblade L, Lewinski M. 2014. Multi-centre evaluation of mass spectrometric identification of anaerobic bacteria using the VITEK MS system. Clin Microbiol Infect 20:335–339. doi: 10.1111/1469-0691.12317. [DOI] [PubMed] [Google Scholar]
- 20.Antikainen J, Pasanen T, Mero S, Tarkka E, Kirveskari J, Kotila S, Mentula S, Kononen E, Virolainen-Julkunen AR, Vaara M, Tissari P. 2009. Detection of virulence genes of Clostridium difficile by multiplex PCR. APMIS 117:607–613. doi: 10.1111/j.1600-0463.2009.02509.x. [DOI] [PubMed] [Google Scholar]
- 21.Westblade LF, Chamberland RR, MacCannell D, Collins R, Dubberke ER, Dunne WM Jr, Burnham C-AD. 2013. Development and evaluation of a novel, semiautomated Clostridium difficile typing platform. J Clin Microbiol 51:621–624. doi: 10.1128/JCM.02627-12. [DOI] [PMC free article] [PubMed] [Google Scholar]