Abstract
The mechanism of zinc absorption has not been delineated, but kinetic studies show that both passive and carrier-mediated processes are involved. We have identified a low molecular mass zinc-binding protein in the soluble fraction of rat intestinal mucosa that could function as an intracellular zinc carrier. The protein was not detected in liver or pancreas, suggesting a role specific to the intestine. The protein binds zinc during transmucosal zinc transport and shows signs of saturation at higher luminal zinc concentrations, characteristics consistent with a role in carrier-mediated zinc absorption. Microsequence analysis of the protein purified by gel-filtration HPLC and SDS/PAGE showed complete identity within the first 41 N-terminal amino acids with the deduced protein sequence of cysteine-rich intestinal protein [Birkenmeier, E. H. & Gordon, J. I. (1986) Proc. Natl. Acad. Sci. USA 83, 2516-2520]. These investigators showed that the gene for this protein is developmentally regulated in neonates during the suckling period, conserved in many vertebrate species, and predominantly expressed in the small intestine. Cysteine-rich intestinal protein contains a recently identified conserved sequence of histidine and cysteine residues, the LIM motif, which our results suggest confers metal-binding properties that are important for zinc transport and/or functions of this micronutrient.
Full text
PDF
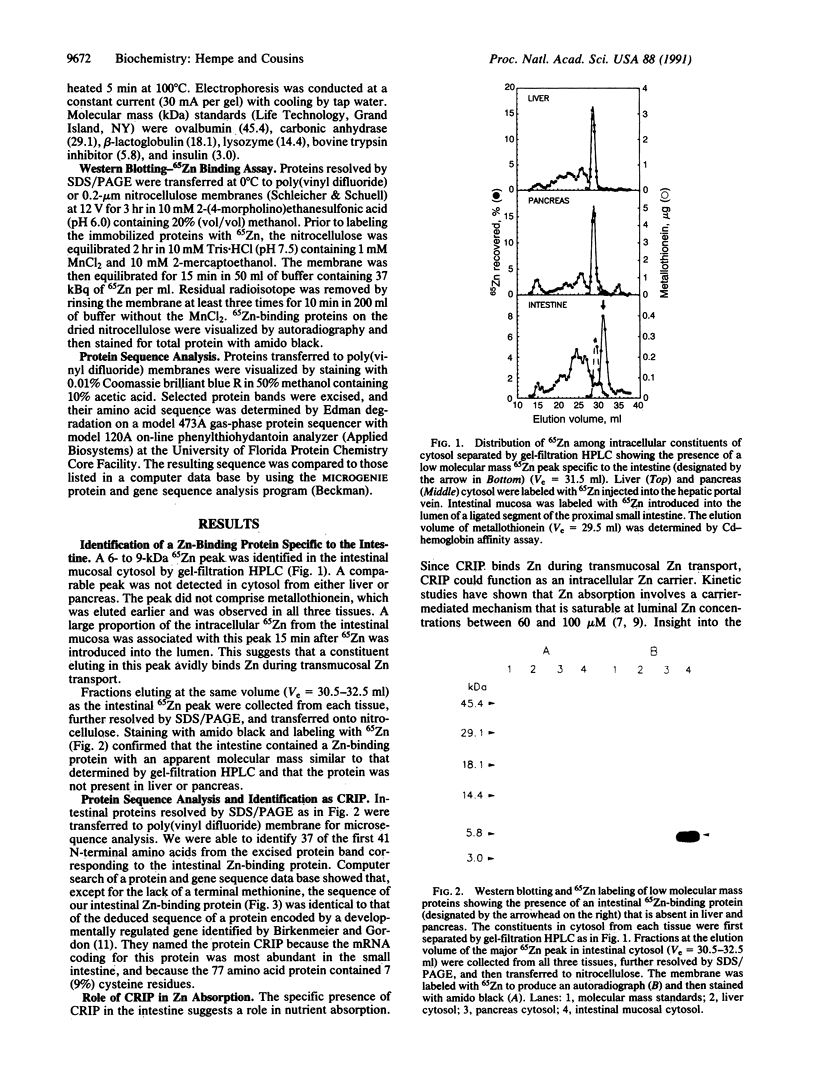

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Gordon J. I. Developmental regulation of a gene that encodes a cysteine-rich intestinal protein and maps near the murine immunoglobulin heavy chain locus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2516–2520. doi: 10.1073/pnas.83.8.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Foroni L., Kennedy M., Rabbitts T. H. The rhombotin gene belongs to a class of transcriptional regulators with a potential novel protein dimerisation motif. Oncogene. 1990 Jul;5(7):1103–1105. [PubMed] [Google Scholar]
- Bronner F. Intestinal calcium absorption: mechanisms and applications. J Nutr. 1987 Aug;117(8):1347–1352. doi: 10.1093/jn/117.8.1347. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Davies N. T. Studies on the absorption of zinc by rat intestine. Br J Nutr. 1980 Jan;43(1):189–203. doi: 10.1079/bjn19800078. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Toal B. F. Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol. 1982 Oct;66(1):134–142. doi: 10.1016/0041-008x(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Freyd G., Kim S. K., Horvitz H. R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990 Apr 26;344(6269):876–879. doi: 10.1038/344876a0. [DOI] [PubMed] [Google Scholar]
- Ghishan F. K., Sobo G. Intestinal maturation: in vivo zinc transport. Pediatr Res. 1983 Feb;17(2):148–151. doi: 10.1203/00006450-198302000-00013. [DOI] [PubMed] [Google Scholar]
- Hempe J. M., Carlson J. M., Cousins R. J. Intestinal metallothionein gene expression and zinc absorption in rats are zinc-responsive but refractory to dexamethasone and interleukin 1 alpha. J Nutr. 1991 Sep;121(9):1389–1396. doi: 10.1093/jn/121.9.1389. [DOI] [PubMed] [Google Scholar]
- Hempe J. M., Cousins R. J. Effect of EDTA and zinc-methionine complex on zinc absorption by rat intestine. J Nutr. 1989 Aug;119(8):1179–1187. doi: 10.1093/jn/119.8.1179. [DOI] [PubMed] [Google Scholar]
- Hoadley J. E., Leinart A. S., Cousins R. J. Kinetic analysis of zinc uptake and serosal transfer by vascularly perfused rat intestine. Am J Physiol. 1987 Jun;252(6 Pt 1):G825–G831. doi: 10.1152/ajpgi.1987.252.6.G825. [DOI] [PubMed] [Google Scholar]
- Hoadley J. E., Leinart A. S., Cousins R. J. Relationship of 65Zn absorption kinetics to intestinal metallothionein in rats: effects of zinc depletion and fasting. J Nutr. 1988 Apr;118(4):497–502. doi: 10.1093/jn/118.4.497. [DOI] [PubMed] [Google Scholar]
- Hurley L. S., Duncan J. R., Sloan M. V., Eckhert C. D. Zinc-binding ligands in milk and intestine: a role in neonatal nutrition? Proc Natl Acad Sci U S A. 1977 Aug;74(8):3547–3549. doi: 10.1073/pnas.74.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. J., Holt D., Webb M., Carter N. D. Physiological zinc-binding proteins of medium molecular weight in the rat gut. Br J Nutr. 1986 Mar;55(2):369–377. doi: 10.1079/bjn19860043. [DOI] [PubMed] [Google Scholar]
- Karlsson O., Thor S., Norberg T., Ohlsson H., Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990 Apr 26;344(6269):879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- Kowarski S., Blair-Stanek C. S., Schachter D. Active transport of zinc and identification of zinc-binding protein in rat jejunal mucosa. Am J Physiol. 1974 Feb;226(2):401–407. doi: 10.1152/ajplegacy.1974.226.2.401. [DOI] [PubMed] [Google Scholar]
- Norton D. S., Heaton F. W. Distribution of copper and zinc among protein fractions in the cytoplasm of rat tissues. J Inorg Biochem. 1980 Aug;13(1):1–9. doi: 10.1016/s0162-0134(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Oestreicher P., Cousins R. J. Zinc uptake by basolateral membrane vesicles from rat small intestine. J Nutr. 1989 Apr;119(4):639–646. doi: 10.1093/jn/119.4.639. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Isolation of an intestinal metallothionein induced by parenteral zinc. Biochem Biophys Res Commun. 1977 Mar 21;75(2):286–294. doi: 10.1016/0006-291x(77)91041-5. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Seal C. J., Heaton F. W. Zinc transfer among proteins in rat duodenum mucosa. Ann Nutr Metab. 1987;31(1):55–60. doi: 10.1159/000177248. [DOI] [PubMed] [Google Scholar]
- Steel L., Cousins R. J. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol. 1985 Jan;248(1 Pt 1):G46–G53. doi: 10.1152/ajpgi.1985.248.1.G46. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Heuchel R., Schaffner W., Kägi J. H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991 Feb 25;279(2):310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]