ABSTRACT
Signaling through Toll-like receptors (TLRs), the main receptors in innate immunity, is essential for the defense of mucosal surfaces. It was previously shown that systemic TLR5 stimulation by bacterial flagellin induces an immediate, transient interleukin-22 (IL-22)-dependent antimicrobial response to bacterial or viral infections of the mucosa. This process was dependent on the activation of type 3 innate lymphoid cells (ILCs). The objective of the present study was to analyze the effects of flagellin treatment in a murine model of oral infection with Yersinia pseudotuberculosis (an invasive, Gram-negative, enteropathogenic bacterium that targets the small intestine). We found that systemic administration of flagellin significantly increased the survival rate after intestinal infection (but not systemic infection) by Y. pseudotuberculosis. This protection was associated with a low bacterial count in the gut and the spleen. In contrast, no protection was afforded by administration of the TLR4 agonist lipopolysaccharide, suggesting the presence of a flagellin-specific effect. Lastly, we found that TLR5- and MyD88-mediated signaling was required for the protective effects of flagellin, whereas neither lymphoid cells nor IL-22 was involved.
KEYWORDS: interleukin-22, TLR5, Yersinia pseudotuberculosis, flagellin, intestine, mouse infection, Toll-like receptors
INTRODUCTION
The cross talk between mucosal immune cells and intestinal epithelial cells is essential for controlling the bacterial flora in the gut and protecting against pathogenic microbes (1, 2). Many studies have demonstrated that interleukin-22 (IL-22) has a role in the host's immune defense and in tissue-protective and regenerative functions in the gastrointestinal tract (3, 4). IL-22 is a cytokine from the IL-10 family. It is produced by T helper 17 (Th17) lymphocytes, γδ T cells, or NKT cells. Most of the IL-22 in the gut is produced by type 3 innate lymphoid cells (ILC3) (5–8). IL-22 targets epithelial cells and mesenchymal stromal cells but not immune cells, since the IL-22R1 subunit of the IL-22 receptor is not expressed by hematopoietic cells (9). IL-22 induces the signal transducer and activator of transcription-3 (STAT-3) pathway, which in turn leads to the induction of antimicrobial molecules (like C-type lectin RegIIIγ and calgranulin S100A9) (10), mucin production (11), and intestinal wound healing (12, 13). Innate IL-22 production by ILC3 requires transactivation by IL-23 (plus IL-1β, in some cases) secreted by dendritic cells (6), and this process has been linked to colonization resistance and protection against Citrobacter rodentium via the production of RegIIIγ (7, 14–17). In contrast, it was recently shown that IL-22 can promote Salmonella colonization, indicating that the cytokine's role depends on the pathogen in question (18).
Earlier research has shown that immunostimulatory treatments can promote the production of IL-22 in the gut and thus boost antibacterial defenses. Indeed, systemic administration of flagellin induces IL-22 production by ILC3 in the spleen and in the intestinal mucosa (19, 20). Flagellin (the main protein component of the bacterial flagella) binds to Toll-like receptor 5 (TLR5), an innate receptor expressed at the surface of epithelial and dendritic cells (21), and can also be sensed by NAIP5, a cytosolic protein associated with the NLRC4 inflammasome (22, 23). It has been demonstrated that the IL-22 production induced by flagellin was linked to the production of IL-23 by intestinal lamina propria CD103+ CD11b+ dendritic cells and TLR5 signaling (20). Interestingly, flagellin administration induces IL-22-dependent protection against vancomycin-resistant Enterococcus faecalis infections and against rotavirus infections (10, 24). Systemic flagellin administration also protects against Clostridium difficile colitis or Salmonella enterica serovar Typhimurium infection, although it has not yet been established whether this protection requires IL-22 (25, 26). Much as in the intestine, flagellin-mediated protection against Streptococcus pneumoniae respiratory infections is associated with IL-22 production by lung ILC3 (27).
In the present study, we hypothesized that flagellin treatment could protect against intestinal infection by Yersinia pseudotuberculosis, an invasive, Gram-negative enteropathogen responsible for enteritis and mesenteric lymphadenitis. Y. pseudotuberculosis targets the ileum, actively translocates across the intestinal barrier through M cells, infects the Peyer's patches and mesenteric lymph nodes, and disseminates to the spleen and the liver (28). We found that preexposing mice to flagellin protected against intestinal infection (but not systemic infection) by Y. pseudotuberculosis, and that this protection was independent of IL-22.
RESULTS
Administration of flagellin protects against intestinal infection (but not systemic infection) by Y. pseudotuberculosis.
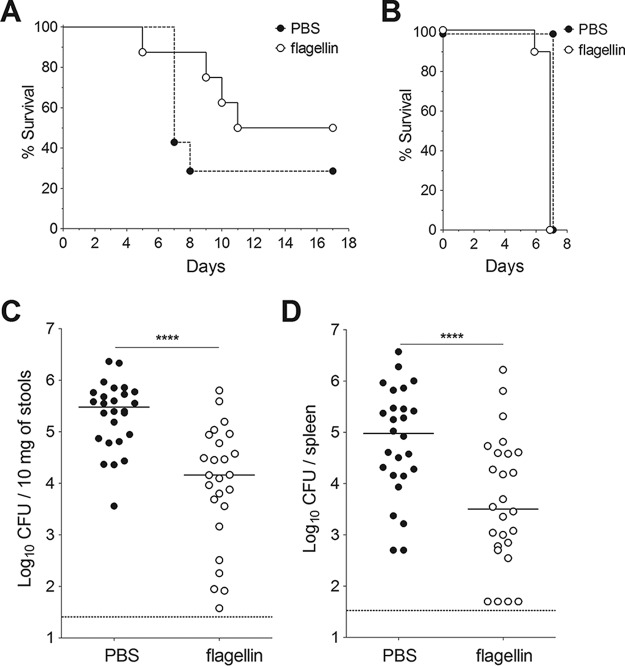
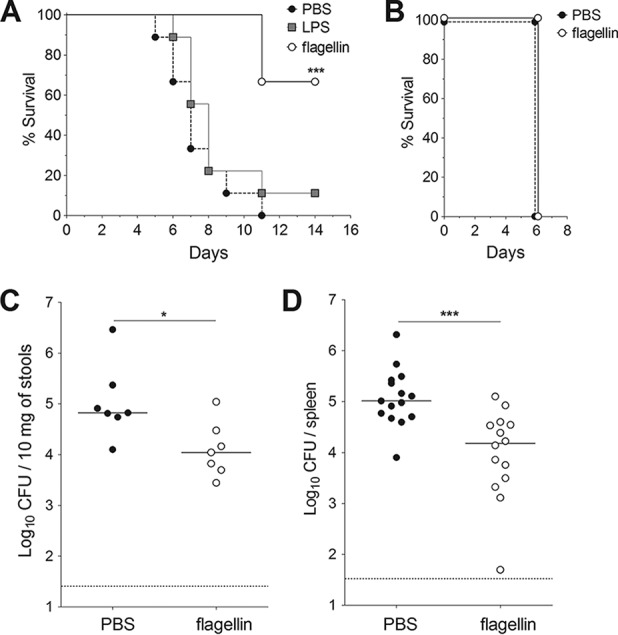
We first looked at whether the TLR5 agonist flagellin could induce resistance against oral infection with Y. pseudotuberculosis. To this end, BALB/c mice were injected intraperitoneally with TLR agonists and intragastrically infected 30 min later with a lethal dose of Y. pseudotuberculosis. Although all animals that had received PBS alone died within 11 days, only 33% of the animals that received flagellin died (Fig. 1A). Consistent with these findings, the Y. pseudotuberculosis count in stools of flagellin-treated animals was six times lower than that in samples from untreated animals (Fig. 1C), and the count in the spleen of animals was seven times lower (Fig. 1D). In contrast, the TLR4 agonist lipopolysaccharide (LPS) did not induce any protection, since LPS-treated animals rapidly died after oral Y. pseudotuberculosis infection (only 1 out of the 9 treated animals survived) (Fig. 1A). Production of IL-6 and CXCL2 in the blood and the ileum revealed that both flagellin and LPS significantly induced systemic and local inflammatory responses in mice (Fig. 2). However, LPS triggered a stronger response in blood, whereas flagellin induced a higher response in the ileum. Altogether, these results indicate that the protection is specific for flagellin and is not due to overall stimulation of innate defense mechanisms. When animals were infected intravenously with Y. pseudotuberculosis (in order to bypass intestinal tissue invasion), flagellin did not elicit a protective effect (Fig. 1B). These results show that systemic administration of flagellin protects against mucosal (but not systemic) Y. pseudotuberculosis infection.
FIG 1.
Flagellin protects against intestinal infection (but not systemic infection) by Y. pseudotuberculosis. Female BALB/c mice were treated intraperitoneally with flagellin (5 μg) or LPS (5 μg) in phosphate-buffered saline (PBS) or with PBS alone. The animals were challenged 30 min later with an intragastric inoculation of 5 × 108 CFU of Y. pseudotuberculosis (A, C, and D) or an intravenous injection of 103 CFU of Y. pseudotuberculosis (B). (A) The survival of mice (n = 9) after an oral challenge was monitored for 14 days. One of three representative survival experiments is shown here. Statistical significance was determined using a log rank test compared to the untreated mice (***, P < 0.001). (B) The survival of mice (n = 10) after an intravenous challenge was monitored for 7 days. Bacterial counts in the stools (C) and the spleen (D) were determined 72 h after the oral challenge. CFU counts for individual mice (n = 7 to 14) are shown. The solid line corresponds to the median value, and the dashed line represents the detection threshold. Data from flagellin-treated and untreated mice were compared in a Mann-Whitney test (*, P < 0.05; ***, P < 0.001).
FIG 2.
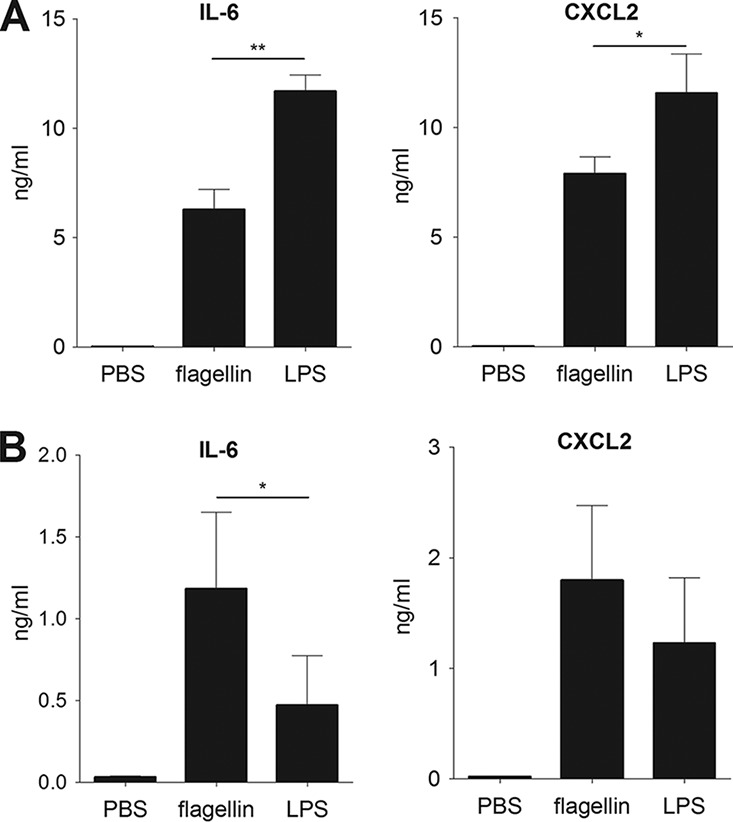
Flagellin and LPS activate systemic and local inflammation. BALB/c mice (n = 5) were treated intraperitoneally with flagellin (5 μg) or LPS (5 μg) in PBS or with PBS alone. Blood and ileum were sampled 2 h posttreatment. IL-6 and CXCL2 levels were measured by ELISA in serum (A) and ileum homogenates (B). The data are shown as the means ± SD. Data from flagellin-treated and LPS-treated mice were compared in a Mann-Whitney test (*, P < 0.05; **, P < 0.01).
It has previously been shown that BALB/c and C57BL/6 mice differ in their susceptibility to Yersinia infection (29). BALB/c mice have been classified as susceptible, whereas C57BL/6 mice are resistant because of their ability to mount strong IL-12, gamma interferon (IFN-γ), and Th1 responses. We therefore analyzed the ability of flagellin treatment to promote protection against oral infection by Y. pseudotuberculosis in C57BL/6 animals (Fig. 3). Consistent with the trend of improved survival, we observed a significant increase of bacterial clearance upon flagellin treatment. Indeed, the bacterial counts in the stools and spleen of flagellin-treated mice were 20- and 65-fold lower, respectively, than in untreated animals (Fig. 3A, C, and D). Analysis of the time course of Y. pseudotuberculosis colonization of the stools and spleen of C57BL/6 mice showed that flagellin-associated protection was observed from 72 h following infection (see Fig. S1 in the supplemental material). As seen in BALB/c mice, flagellin provided protection in C57BL/6 mice when Y. pseudotuberculosis was administered by the oral but not the systemic route (Fig. 3B). In conclusion, flagellin-mediated protection against Y. pseudotuberculosis is independent of the animal's intrinsic resistance to oral infection.
FIG 3.
Flagellin protects against Y. pseudotuberculosis intestinal infection in Yersinia-resistant C57BL/6 mice. Female C57BL/6 mice were treated intraperitoneally with flagellin (5 μg) in PBS or with PBS alone 30 min before an intragastric challenge with 5 × 108 CFU of Y. pseudotuberculosis (A, C, and D) or an intravenous challenge with 103 CFU of Y. pseudotuberculosis (B). Survival was monitored daily for 17 days (n = 7 to 8) (A) or 7 days (n = 10) (B). Survival data from flagellin-treated and untreated mice were compared in a log rank test. Bacterial counts were determined in the stools (C) and the spleen (D). CFU counts for individual mice (n = 25 to 26) at 72 h postinfection are shown. The solid bar corresponds to the median value, and the dashed line represents the detection threshold. Data from flagellin-treated and untreated mice were compared in a Mann-Whitney test (****, P < 0.0001).
TLR5 signaling is specifically required for flagellin-mediated protection against Y. pseudotuberculosis.
Two innate receptors are able to recognize flagellin: TLR5 detects extracellular flagellin, whereas the cytoplasmic NAIP5/NLRC4 complex detects intracellular flagellin. To define the receptors' respective contributions to the flagellin-induced protection, we compared the activity of recombinant flagellins impaired in their ability to stimulate TLR5 (rFliC89–96*) (30) or the NAIP5/NLRC4 complex (rFliC492stop) to that of unmutated recombinant flagellin (rFliC) (Fig. S2). As expected, rFliC89–96* was unable to trigger TLR5-specific responses (e.g., the systemic production of CXCL2 and CCL20 after intravenous injection), in contrast to rFliC or rFliC492stop (Fig. S2A and B). rFliC492stop was unable to induce the NLRC4-dependent processing of procaspase-1 seen with rFliC (Fig. S2C and D). Treatment with rFliC protected mice against Y. pseudotuberculosis infection, since the bacterial counts in stools and spleen were 20 and 28 times lower, respectively, than in untreated, infected animals (Fig. 4A and B). Similar effects were obtained after treatment with rFliC492stop, the flagellin unable to bind to NLRC4: the bacterial counts in the stools and spleen of treated mice were 29 and 10 times lower, respectively, than those for untreated animals (Fig. 4A and B). In contrast, protection was abrogated when mice were treated with rFliC89–96* prior to infection (Fig. 4A and B).
FIG 4.
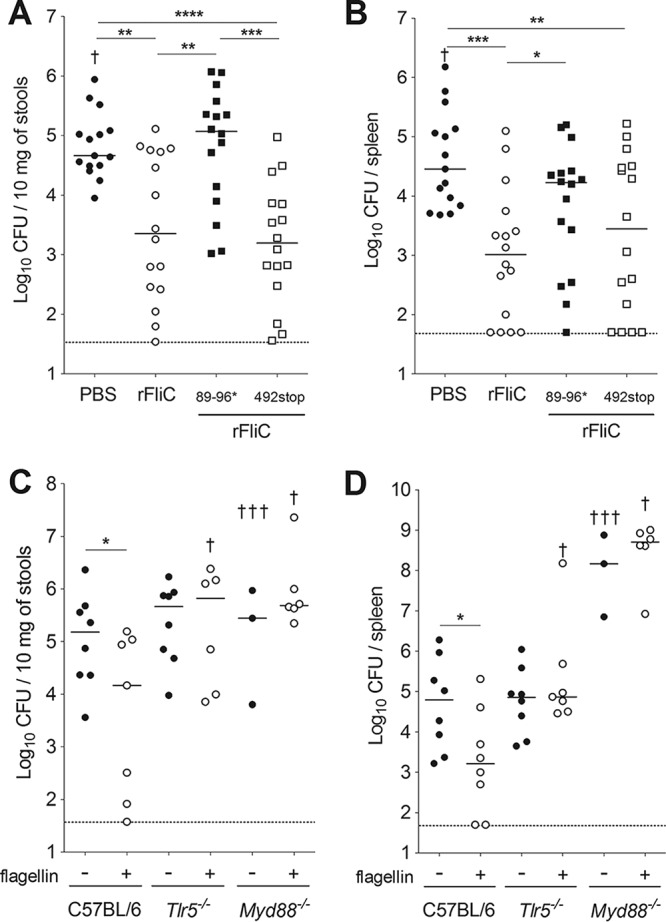
Flagellin-mediated antibacterial defenses require TLR5 signaling. (A and B) C57BL/6 mice were treated intraperitoneally with 5 μg of the histidine-tagged flagellin rFliC, histidine-tagged mutant flagellins (rFliC89/96* or rFliC492stop), or PBS alone 30 min before an intragastric challenge with 5 × 108 CFU of Y. pseudotuberculosis. (C and D) Tlr5−/− or Myd88−/− mice were treated with 5 μg of native flagellin in PBS or with PBS alone 30 min before an intragastric challenge with 5 × 108 CFU of Y. pseudotuberculosis. Bacterial counts were determined in the stools (A and C) and the spleen (B and D). CFU counts for individual mice (n = 7 to 16) at 72 h postinfection are shown. The solid bar corresponds to the median value, and the dashed line represents the detection threshold. The dagger symbol indicates animals that died. Bacterial counts were not determined in these animals. Intergroup differences were analyzed in a Mann-Whitney test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
We also found that flagellin treatment was not associated with low bacterial counts in Tlr5−/− mice compared with those in C57BL/6 animals; this finding demonstrates that TLR5 signaling is required for the protection (Fig. 4C and D). Flagellin-mediated protection was also abolished in mice lacking myeloid differentiation factor 88 (MyD88), which is required for TLR5 signaling (Fig. 4C and D). It is noteworthy that high bacterial counts were found in the spleen of Myd88−/− mice infected with Y. pseudotuberculosis, confirming that MyD88 is critical for protection against Yersinia infection (31). Taken as a whole, these results showed that flagellin exerts its protective effect through the activation of TLR5.
Flagellin induces a transient inflammatory response independently of Y. pseudotuberculosis infection.
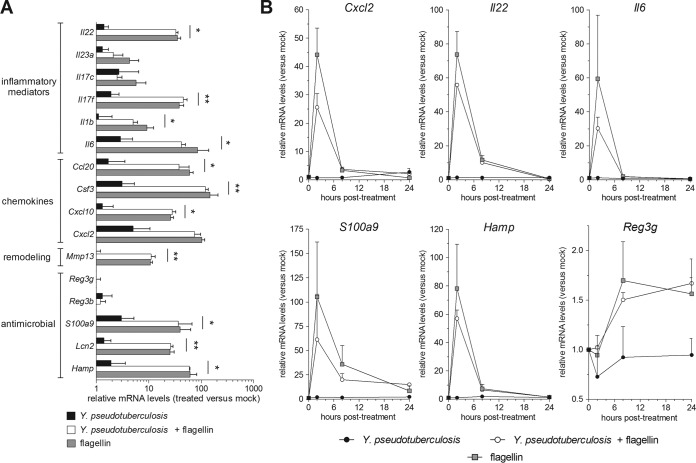
To characterize the flagellin-mediated response, we analyzed gene expression in the ileum of C57BL/6 mice treated with flagellin and infected (or not) with Y. pseudotuberculosis. Two hours after flagellin treatment, we observed strong expression of genes coding for inflammatory cytokines (e.g., IL-6, IL-1β, and IL-17F), chemokines (e.g., CCL20 and CXCL10), remodeling protein (MMP13), and antimicrobial molecules (S100A9, lipocalin-2, and hepcidin) (Fig. 5A). As expected, flagellin administration triggered a 100-fold relative increase in Il22 mRNA levels within 2 h. Interestingly, flagellin-dependent gene expression occurred in both infected and noninfected animals, indicating that Y. pseudotuberculosis infection does not modify flagellin's biological activity per se. We next analyzed the time course of the flagellin-mediated transcriptional response (Fig. 5B). In both infected and uninfected mice, flagellin rapidly upregulated the transcription of Cxcl2, Il22, Il6, Hamp, and S100a9 genes. This was followed by a strong decrease in mRNA levels 8 h posttreatment and a return to baseline after 24 h (Fig. 5B). However, the gene coding for RegIIIγ displayed a delayed expression profile; expression started 8 h posttreatment and was maintained until 24 h, thus confirming a previous report (19). In summary, the flagellin-induced gene expression in the ileum was not changed by Y. pseudotuberculosis infection.
FIG 5.
Y. pseudotuberculosis infection does not influence the flagellin-specific gene expression signature. (A and B) Female C57BL/6 mice (n = 4) were treated intraperitoneally with flagellin (5 μg) in PBS or with PBS alone (mock). Thirty minutes later, the animals were challenged intragastrically with 5 × 108 CFU of Y. pseudotuberculosis. One group of mice was treated with flagellin but not Y. pseudotuberculosis. Ilea were sampled postinfection and mRNA was quantified by quantitative reverse transcription-PCR. mRNA levels are expressed relative to those of uninfected and PBS-treated animals (the mock group; arbitrarily set to a value of 1) and are shown as the means ± SD. (A) Analysis of gene expression at 2 h posttreatment. Intergroup differences compared to flagellin-treated animals were analyzed in a Limma test with Benjamini-Hochberg FDR correction (*, P < 0.05; **, P < 0.01). Significant differences in mRNA expression levels were not found between flagellin and Y. pseudotuberculosis plus flagellin groups. (B) A time course analysis of gene expression.
We found that unlike flagellin, LPS was not able to induce protection against oral infection with Y. pseudotuberculosis (Fig. 1A). We compared the transcriptional response to LPS and flagellin for a set of 10 genes known to be activated by TLR stimulation (19). Whereas both flagellin and LPS were associated with similar expression levels in the spleen, flagellin was 10- to 100-fold more potent than LPS in the ileum (Fig. S3). These data corroborate our previous observations (Fig. 2) (19) and suggest that flagellin-mediated protection correlated with a specific, intestinal innate response.
IL-22 is not required for flagellin-mediated protection.
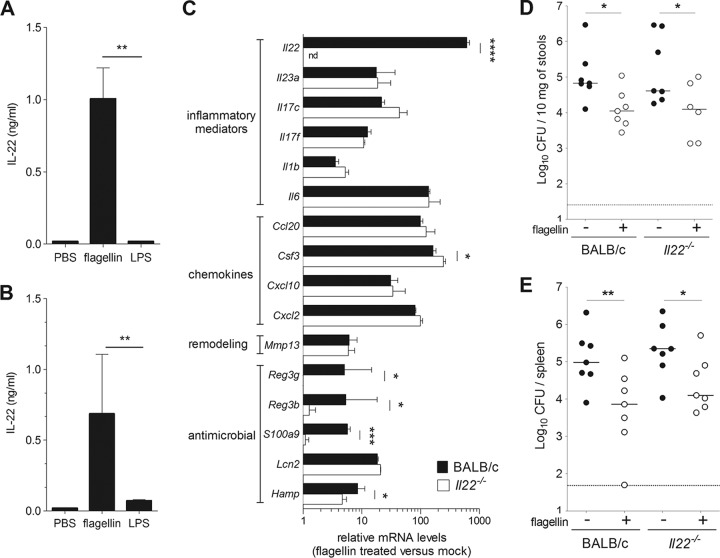
Given that TLR5 stimulation strongly enhanced intestinal production of IL-22, a cytokine that is critical for mucosal defense (Fig. 5) (10, 19, 20, 24, 25), we further investigated the role of IL-22 in flagellin-mediated protection against Y. pseudotuberculosis. First, we found that flagellin administration induced a strong production of IL-22 in blood and ileum (Fig. 6A and B). Interestingly, treatment with LPS was unable to stimulate IL-22 production in the systemic (blood) and local (ileum) compartments (Fig. 6A and B). A transcriptional analysis of ileum samples 2 h after flagellin injection indicated that the overall signatures in Il22−/− mice (BALB/c background) and in BALB/c littermates were similar, except, as expected, for the absence of expression of Il22 and the reduced expression of three well-defined IL-22 target genes (Reg3b, Reg3g, and S100a9) in the knockout animal (Fig. 6C) (20). Finally, flagellin-treated BALB/c and Il22−/− mice were inoculated with Y. pseudotuberculosis, and bacterial counts in the spleen and stools were measured. As in littermate BALB/c (IL-22 proficient) mice, bacterial counts were significantly lower in flagellin-treated Il22−/− mice than in untreated Il22−/− mice (Fig. 6D and E). Similar protection was observed in Il22−/− mice with the C57BL/6 background (data not shown). Taken as a whole, these results indicate that IL-22 is not required for flagellin-mediated protection and suggest that the antimicrobial molecules RegIIIβ, RegIIIγ, and S100A9 are not involved in flagellin's mode of action.
FIG 6.
Flagellin-mediated protection does not require IL-22. (A and B) Female BALB/c mice (n = 5) were treated intraperitoneally with flagellin (5 μg) or LPS (5 μg) in PBS or with PBS alone. Blood and ileum were sampled 2 h posttreatment. IL-22 levels were measured by ELISA in serum (A) and ileum homogenates (B). The data are presented as means ± SD. Data from flagellin-treated and LPS-treated mice were compared in a Mann-Whitney test (**, P < 0.01). (C) Il22−/− mice and wild-type BALB/c littermates (n = 3) were treated intraperitoneally with PBS alone or flagellin (5 μg) in PBS. The ileum was sampled at 2 h for quantification of mRNA levels using quantitative reverse transcription-PCR. mRNA levels are expressed relative to the PBS group (mock). nd, not detected. The data are presented as means ± SD. Intergroup differences were analyzed in a Limma test with Benjamini-Hochberg FDR correction (*, P < 0.05; ***, P < 0.001; ****, P < 0.0001). (D and E) BALB/c and Il22−/− mice were treated intraperitoneally with flagellin (5 μg) in PBS or with PBS alone 30 min before an intragastric challenge with 5 × 108 CFU of Y. pseudotuberculosis. Bacterial counts were determined in the stools (D) and the spleen (E). CFU counts for individual mice (n = 7) at 72 h postinfection are shown. The solid line corresponds to the median value, and the dashed line represents the detection threshold. Statistical significance was assessed in a Mann-Whitney test (*, P < 0.05; **, P < 0.01).
Lastly, we also studied flagellin-mediated protection in Rag2−/− Il2rg−/− mice (Fig. 7). These mice lack T and B lymphocytes and cells that depend on the common γ-chain (natural killer cells, natural killer T cells, and all subsets of ILCs). Innate production of IL-22 is known to be impaired in Rag2−/− Il2rg−/− mice due to the specific absence of ILC3 (7, 19). First, we observed that the bacterial counts after Y. pseudotuberculosis infection were 15-fold higher in the spleen and 27-fold lower in the stools of Rag2−/− Il2rg−/− mice than in wild-type C57BL/6 mice (Fig. 3C and D; Fig. 7). This indicates that Rag2−/− Il2rg−/− mice can control the mucosal colonization but not the dissemination of Y. pseudotuberculosis. Despite this difference of susceptibility, flagellin was still able to increase the bacterial clearance in Rag2−/− Il2rg−/− mice. Indeed, the bacterial counts in stools and spleen were 5.6- and 9-fold lower, respectively, in Rag2−/− Il2rg−/− animals treated systemically with flagellin than in mock-treated, infected animals (Fig. 7). These data indicate that lymphoid cells (including ILC3) are not required for flagellin-mediated protection.
FIG 7.
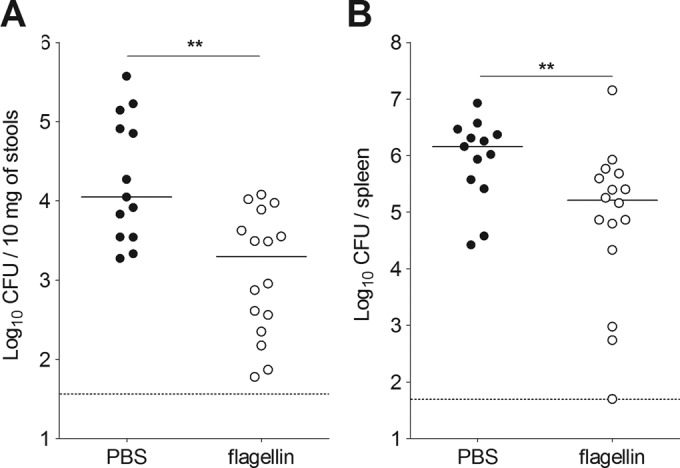
Flagellin protection does not require B, T, NK, and innate lymphoid cells. Rag2−/− Il2rg−/− mice were treated intraperitoneally with flagellin (5 μg) in PBS or with PBS alone 30 min before an intragastric challenge with 5 × 108 CFU of Y. pseudotuberculosis. Bacterial counts were determined in the stools (A) and the spleen (B). CFU counts for individual mice (n = 13 to 16) at 72 h postinfection are shown. The solid bar corresponds to the median value, and the dashed line represents the detection threshold. Intergroup differences were analyzed in a Mann-Whitney test (**, P < 0.01).
DISCUSSION
In the present study, we showed that flagellin administration protects against intestinal (but not systemic) infection with Y. pseudotuberculosis. These data strongly suggest that TLR5 signaling has a mucosal protective effect. We and others have already shown that TLR5 signaling induces the rapid production of IL-22 by stimulating ILC3 in a dendritic cell-dependent manner (19, 20). Since IL-22 reportedly has a protective role against intestinal and intra-abdominal pathogens (14, 24, 32), we hypothesized that this cytokine was also involved in flagellin-mediated mucosal protection of mice against Y. pseudotuberculosis. Surprisingly, we found that IL-22 is not required for this protection. Moreover, our present results suggest that neither ILC3 nor the IL-22-dependent antimicrobial agents S100A9, RegIIIβ, and RegIIIγ are required for protection.
These observations contrast with animal studies of infections with the enteric bacterium C. rodentium, in which IL-22 and RegIIIγ proteins are essential for intestinal protection (14). The differential involvement of IL-22 in models of experimental infections with Citrobacter and Yersinia might be related to differences in the pathogenicity of these two Gram-negative microorganisms. C. rodentium is an extracellular attaching/effacing pathogen that is related to enteropathogenic Escherichia coli and damages the colonic epithelium (14). In contrast, Y. pseudotuberculosis does not colonize the epithelial surface; in fact, it invades intestinal tissues and displays a pronounced tropism for ileal Peyer's patches, mesenteric lymph nodes, and the spleen (28). This invasive feature might make Y. pseudotuberculosis resistant to an IL-22-mediated, epithelial, antibacterial response. The infection site (colon versus ileum) might also contribute to specific expression of IL-22-dependent protection. For example, IL-22 might profoundly influence resistance to colonization of colon, where the microbial density is significantly higher than in the ileum.
Our present results also showed that the protection against Y. pseudotuberculosis infection requires TLR5 and thus agree with a previous study in which impaired TLR5 signaling was associated with chronic yersiniosis in humans (33). This protection was also specific for flagellin, since a TLR4 agonist did not induce protection. This disparity can be attributed to the differences in cellular expression levels of TLR4 and TLR5; TLR4 is mainly expressed by hematopoietic cells, whereas TLR5 is mainly expressed in the epithelium (34). Moreover, the responsiveness of hematopoietic cells and epithelial cells to TLR4 and TLR5 agonists is associated with gut-specific features. For instance, the gut epithelium's tolerance to LPS is a hallmark feature related to the absence (or lack of function) of signaling molecules like IRAK-1 or coreceptors like CD14 (35). Furthermore, it has been shown that although the intestinal lamina propria contains dendritic cells expressing TLR5 and TLR4, the response to TLR5 stimulation is significantly stronger than the response to TLR4 stimulation (36). We confirmed this scenario because flagellin, but not LPS, induced a strong innate response in the ileum (see Fig. S3 in the supplemental material).
Our results strongly suggest that nonlymphoid cells have a major role in flagellin-mediated protection against Y. pseudotuberculosis. Recently published data showed that TLR5 signaling deficiency in gut epithelium is involved in spontaneous, low-grade, intestinal inflammation in a process modulated by the microbiota (37). Accordingly, the systemic administration of flagellin is known to activate gut epithelial cells (38). Flagellin's protective activity is not restricted to the gut, since intranasal administration is effective against respiratory infections and depends on the TLR5-mediated activation of epithelial cells and neutrophil recruitment (39, 40). Taken as a whole, these findings suggest that epithelial cells have a role in the flagellin-mediated protection against Y. pseudotuberculosis.
Neutrophils constitute the first line of inducible defense against infection. In the present study, we confirmed that flagellin administration induces the rapid, transient transcriptional activation of genes encoding chemokines involved in chemoattraction of neutrophils in the intestine, as described previously (19). Instillation of flagellin into the respiratory tract in both naive and infected animals is associated with the recruitment of neutrophils into lungs (30, 39–41). It remains to be seen whether, in the intestine, neutrophils contribute to flagellin-mediated protection against Y. pseudotuberculosis. However, in vivo and in vitro studies have shown that Y. pseudotuberculosis is partially resistant to killing by human neutrophils (42–44); this suggests that flagellin-mediated protection involves additional or unrelated mechanisms. Along with neutrophils, other myeloid cells may also contribute to this protection, because macrophages, monocytes, and dendritic cells are all targets of flagellin-mediated signaling. Interestingly, stimulation of TLR signaling is known to shift the anti-inflammatory effects of Y. pseudotuberculosis on macrophages toward inflammatory pyroptosis, a process that contributes to bacterial clearance (45).
In agreement with previous studies, we found that flagellin stimulates the transcription of genes encoding antimicrobial molecules (19, 20, 46, 47). Although RegIIIβ was found to be associated with intestinal resistance to Y. pseudotuberculosis (31), the present study suggests that this C-type lectin and other IL-22-induced antimicrobial molecules (such as S100A9 and RegIIIγ) are not essential for flagellin-mediated protection. We identified other antimicrobial compounds (unrelated to IL-22 activation) that might have a role in the clearance of Y. pseudotuberculosis; these include lipocalin-2 (which binds to bacterial siderophores) and hepcidin (which influences the levels of iron available for bacterial growth). Other antimicrobial candidates include the cathelicidin-related antimicrobial peptide or the β-defensins, which also have been linked to TLR5 signaling (47, 48). Understanding the anti-Yersinia mechanisms that are primed by TLR5 signaling and contribute to mucosal protection remains an important topic for future research.
MATERIALS AND METHODS
Bacterial preparation.
Yersinia pseudotuberculosis (serogroup O:1, strain IP32777) (49) was grown as described previously (50). Briefly, Luria-Bertani Lennox (LB) (AthenaES, Baltimore, MD) plates were streaked from a −80°C culture stock. Bacterial inocula were prepared from overnight cultures in LB broth at 28°C. This culture was monitored spectrometrically (optical density at 600 nm [OD600]) and diluted to the appropriate concentration. The number of bacteria (in CFU) was confirmed by plating serial dilutions on LB agar plates.
The murine model of infection.
Six- to 20-week-old BALB/cJ or C57BL/6J mice (Janvier Laboratories, Saint-Berthevin, France), Tlr5−/−, Myd88−/−, Il22−/−, and Rag2−/− Il2rg−/− mice (all backcrossed with C57BL/6J), and Il22−/− mice (backcrossed with BALB/cJ) were maintained in individually ventilated cages and handled in a vertical laminar-flow cabinet (class II A2; ESCO, Hatboro, PA). All experiments complied with current national and institutional regulations and ethical guidelines (B59-350009; Institut Pasteur de Lille, Lille, France). Mice were infected with Y. pseudotuberculosis via the intravenous or intragastric route, as described previously (50). Briefly, animals were orally challenged with 5 × 108 bacteria in 200 μl of water or intravenously infected with 103 bacteria in 100 μl of phosphate-buffered saline (PBS). These bacterial inocula correspond to lethal doses established according to the 50% lethal dose (LD50) values defined previously as 107.3 by the oral route and <102 by the intravenous route (51, 52). PBS alone was administered in control experiments. The mice were observed daily for signs of illness. For the determination of bacterial counts in stools and spleen, mice were sacrificed at selected time points by the intraperitoneal injection of 5.47 mg of sodium pentobarbital. Stools and spleens were weighed and collected in PBS. Samples were then homogenized with an UltraTurrax homogenizer (IKA-Werke, Staufen, Germany), and viable counts were determined by plating serial dilutions onto LB agar plates (for spleens) or LB agar plates containing vancomycin (50 μg/ml) and irgasan (1 μg/ml) (for stools) in order to specifically enable Yersinia growth. CFU were counted after 48 h of culture at 28°C.
Administration of flagellin and LPS.
Native flagellin FliC from Salmonella enterica serovar Typhimurium and the recombinant flagellins rFliC and rFliC89–96* (both harboring an amino-terminal histidine tag) were produced as described previously (30, 53). rFliC is equivalent to the native protein, whereas rFliC89–96* contains TLR5-nonsignaling residues at positions 89 to 96. The recombinant flagellin FliC492stop was generated by site-directed mutagenesis of a plasmid harboring rFliC cloned into the expression vector pET22b+. A premature stop codon was introduced in the plasmid coding for FliC492stop in order to truncate the last two C-terminal residues involved in NAIP5/NLRC4 complex recognition (54). Recombinant flagellins were produced in E. coli BL21(DE3), purified using fast protein liquid chromatography (GE Healthcare, Pittsburgh, PA), and depleted of LPS using Triton X-114 extraction and polymyxin B columns (Thermo Fisher Scientific, Waltham, MA). In a Limulus assay (Associates of Cape Cod Inc., East Falmouth, MA), the residual LPS concentration was determined to be less than 20 pg per μg of flagellin. To ensure that the flagellins were mostly monomers, the corresponding samples were heated for 10 min at 65°C before use. Flagellins (5 μg in 200 μl PBS), ultrapure LPS from E. coli (5 μg in 200 μl PBS; serotype 0111:B4; InvivoGen, Toulouse, France), or PBS alone was administered intraperitoneally 30 min prior to the bacterial challenge.
Gene expression.
Total RNA was extracted with the NucleoSpin RNA II kit (Macherey-Nagel, Durel, Germany) and reverse transcribed with the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). The cDNA was amplified using SYBR green-based real-time PCR on a 7300 real-time PCR system (Applied Biosystems). Primers used in the study are listed in Table S1 in the supplemental material. For high-throughput analysis, TaqMan low-density arrays (Applied Biosystems) were used (references of the assays/probes are listed in Table S2). The analysis was carried out using real-time StatMiner software (Integromics) or ThermoCloud RQ software (Thermo Fisher Scientific). Relative mRNA levels (2−ΔΔCT) were determined by comparing (i) the PCR cycle thresholds (CT) for the gene of interest and the housekeeping genes (Actb and/or B2m) (ΔCT) and (ii) the ΔCT values for flagellin-treated and untreated groups (ΔΔCT). The CT threshold was set to 33 cycles.
Caspase 1 activation assay.
The procaspase-1 processing assay was performed as previously described (55). Briefly, bone marrow-derived macrophages isolated from wild-type C57BL/6 and Nlrc4−/− mice were incubated with streptolysin O (SLO) (Sigma-Aldrich, St. Louis, MO) in the presence or absence of recombinant flagellins for 2 h. Cell lysates were prepared in Laemmli buffer and transferred to nitrocellulose membranes. Processing of procaspase-1 was followed by immunoblotting with caspase-1-specific antibody (Adipogen, San Diego, CA).
Cytokine assays.
CXCL2, CCL20, IL-6, and IL-22 levels were measured by enzyme-linked immunosorbent assay (ELISA) in serum or ileum tissue homogenates prepared with T-PER reagent (Pierce; Rockford, IL) supplemented with protease inhibitor cocktail (Roche Diagnostics, Switzerland) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis.
Results were expressed as the means ± standard deviations (SD) or the median, as indicated. Intergroup differences were analyzed using the Mann-Whitney test and the log rank test (GraphPad Prism 5.0a). The Limma test with Benjamini-Hochberg false discovery rate (FDR) correction was used for high-throughput PCR with TaqMan low-density arrays (Applied Biosystems) as described previously (19). The threshold for statistical significance was set to a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by University Lille, INSERM, CNRS, Institut Pasteur de Lille. R.P. was funded by a Ph.D. fellowship from Ministère de la Recherche et de l'Enseignement Supérieur.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00806-16.
REFERENCES
- 1.Caballero S, Pamer EG. 2015. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol 33:227–256. doi: 10.1146/annurev-immunol-032713-120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abreu MT. 2010. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 3.Dudakov JA, Hanash AM, van den Brink MR. 2015. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eidenschenk C, Rutz S, Liesenfeld O, Ouyang W. 2014. Role of IL-22 in microbial host defense. Curr Topics Microbiol Immunol 380:213–236. [DOI] [PubMed] [Google Scholar]
- 5.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. 2009. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnenberg GF, Artis D. 2015. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med 21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity 21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. 2010. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis 201:534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Investig 118:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. 2011. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. 2015. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity 42:731–743. doi: 10.1016/j.immuni.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science (New York, NY) 336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M. 2014. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40:262–273. doi: 10.1016/j.immuni.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, Sebbane F, Benecke A, Renauld JC, Hardt WD, Ryffel B, Sirard JC. 2010. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J Immunol 185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. 2012. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 22.Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, Dermody TS, Ouyang W, Williams IR, Vijay-Kumar M, Gewirtz AT. 2014. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science (New York, NY) 346:861–865. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarchum I, Liu M, Lipuma L, Pamer EG. 2011. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun 79:1498–1503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. 2008. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol 180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 27.Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, Chabalgoity JA, Renauld JC, Eberl G, Benecke AG, Trottein F, Faveeuw C, Sirard JC. 2014. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis 210:493–503. doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 28.Smego RA, Frean J, Koornhof HJ. 1999. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis 18:1–15. doi: 10.1007/s100960050219. [DOI] [PubMed] [Google Scholar]
- 29.Autenrieth IB, Beer M, Bohn E, Kaufmann SH, Heesemann J. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun 62:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porte R, Fougeron D, Munoz-Wolf N, Tabareau J, Georgel AF, Wallet F, Paget C, Trottein F, Chabalgoity JA, Carnoy C, Sirard JC. 2015. A Toll-like receptor 5 agonist improves the efficacy of antibiotics in the treatment of primary and influenza-associated pneumococcal mouse infections. Antimicrob Agents Chemother 59:6064–6072. doi: 10.1128/AAC.01210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dessein R, Gironella M, Vignal C, Peyrin-Biroulet L, Sokol H, Secher T, Lacas-Gervais S, Gratadoux JJ, Lafont F, Dagorn JC, Ryffel B, Akira S, Langella P, Nunez G, Sirard JC, Iovanna J, Simonet M, Chamaillard M. 2009. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut 58:771–776. doi: 10.1136/gut.2008.168443. [DOI] [PubMed] [Google Scholar]
- 32.Zheng M, Horne W, McAleer JP, Pociask D, Eddens T, Good M, Gao B, Kolls JK. 2016. Therapeutic role of interleukin 22 in experimental intra-abdominal Klebsiella pneumoniae infection in mice. Infect Immun 84:782–789. doi: 10.1128/IAI.01268-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netea MG, van der Leij F, Drenth JP, Joosten LA, te Morsche R, Verweij P, de Jong D, Kullberg BJ, van der Meer JW. 2010. Chronic yersiniosis due to defects in the TLR5 and NOD2 recognition pathways. Neth J Med 68:310–315. [PubMed] [Google Scholar]
- 34.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 35.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. 2006. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. 2008. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol 9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 37.Chassaing B, Ley RE, Gewirtz AT. 2014. Intestinal epithelial cell Toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147:1363–1377. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirard JC, Didierlaurent A, Cayet D, Sierro F, Rumbo M. 2009. Toll-like receptor 5- and lymphotoxin beta receptor-dependent epithelial Ccl20 expression involves the same NF-kappaB binding site but distinct NF-kappaB pathways and dynamics. Biochim Biophys Acta 1789:386–394. doi: 10.1016/j.bbagrm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Janot L, Sirard JC, Secher T, Noulin N, Fick L, Akira S, Uematsu S, Didierlaurent A, Hussell T, Ryffel B, Erard F. 2009. Radioresistant cells expressing TLR5 control the respiratory epithelium's innate immune responses to flagellin. Eur J Immunol 39:1587–1596. doi: 10.1002/eji.200838907. [DOI] [PubMed] [Google Scholar]
- 40.Munoz N, Van Maele L, Marques JM, Rial A, Sirard JC, Chabalgoity JA. 2010. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun 78:4226–4233. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honko AN, Mizel SB. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun 72:6676–6679. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laws TR, Davey MS, Green C, Cooper IA, Titball RW, Lukaszewski RA. 2011. Yersinia pseudotuberculosis is resistant to killing by human neutrophils. Microbes Infect 13:607–611. doi: 10.1016/j.micinf.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Thorslund SE, Ermert D, Fahlgren A, Erttmann SF, Nilsson K, Hosseinzadeh A, Urban CF, Fallman M. 2013. Role of YopK in Yersinia pseudotuberculosis resistance against polymorphonuclear leukocyte defense. Infect Immun 81:11–22. doi: 10.1128/IAI.00650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolan HG, Durand EA, Mecsas J. 2013. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe 14:306–317. doi: 10.1016/j.chom.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergsbaken T, Cookson BT. 2007. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog 3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao N, Kumar A, Jyot J, Yu FS. 2010. Flagellin-induced corneal antimicrobial peptide production and wound repair involve a novel NF-kappaB-independent and EGFR-dependent pathway. PLoS One 5:e9351. doi: 10.1371/journal.pone.0009351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. 2010. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol 185:1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogushi K, Wada A, Niidome T, Mori N, Oishi K, Nagatake T, Takahashi A, Asakura H, Makino S, Hojo H, Nakahara Y, Ohsaki M, Hatakeyama T, Aoyagi H, Kurazono H, Moss J, Hirayama T. 2001. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J Biol Chem 276:30521–30526. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- 49.Simonet M, Falkow S. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect Immun 60:4414–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnoy C, Mullet C, Muller-Alouf H, Leteurtre E, Simonet M. 2000. Superantigen YPMa exacerbates the virulence of Yersinia pseudotuberculosis in mice. Infect Immun 68:2553–2559. doi: 10.1128/IAI.68.5.2553-2559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collyn F, Lety MA, Nair S, Escuyer V, Ben Younes A, Simonet M, Marceau M. 2002. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect Immun 70:6196–6205. doi: 10.1128/IAI.70.11.6196-6205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniel C, Sebbane F, Poiret S, Goudercourt D, Dewulf J, Mullet C, Simonet M, Pot B. 2009. Protection against Yersinia pseudotuberculosis infection conferred by a Lactococcus lactis mucosal delivery vector secreting LcrV. Vaccine 27:1141–1144. doi: 10.1016/j.vaccine.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 53.Nempont C, Cayet D, Rumbo M, Bompard C, Villeret V, Sirard JC. 2008. Deletion of flagellin's hypervariable region abrogates antibody-mediated neutralization and systemic activation of TLR5-dependent immunity. J Immunol 181:2036–2043. doi: 10.4049/jimmunol.181.3.2036. [DOI] [PubMed] [Google Scholar]
- 54.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. 2010. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matusiak M, Van Opdenbosch N, Vande Walle L, Sirard JC, Kanneganti TD, Lamkanfi M. 2015. Flagellin-induced NLRC4 phosphorylation primes the inflammasome for activation by NAIP5. Proc Natl Acad Sci U S A 112:1541–1546. doi: 10.1073/pnas.1417945112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.