ABSTRACT
Recent studies have shown that immune responses against the cell-traversal protein for Plasmodium ookinetes and sporozoites (CelTOS) can inhibit parasite infection. While these studies provide important evidence toward the development of vaccines targeting this protein, it remains unknown whether these responses could engage the Plasmodium falciparum CelTOS in vivo. Using a newly developed rodent malaria chimeric parasite expressing the P. falciparum CelTOS (PfCelTOS), we evaluated the protective effect of in vivo immune responses elicited by vaccination and assessed the neutralizing capacity of monoclonal antibodies specific against PfCelTOS. Mice immunized with recombinant P. falciparum CelTOS in combination with the glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) or glucopyranosyl lipid adjuvant-liposome-QS21 (GLA-LSQ) adjuvant system significantly inhibited sporozoite hepatocyte infection. Notably, monoclonal antibodies against PfCelTOS strongly inhibited oocyst development of P. falciparum and Plasmodium berghei expressing PfCelTOS in Anopheles gambiae mosquitoes. Taken together, our results demonstrate that anti-CelTOS responses elicited by vaccination or passive immunization can inhibit sporozoite and ookinete infection and impair vector transmission.
KEYWORDS: CelTOS, parasite, malaria, transgenic, vaccine
INTRODUCTION
The most recent report by the World Health Organization indicates that in 2013 there were approximately 198 million malaria cases worldwide and an estimated 584,000 deaths (1). While global efforts to reduce the impact of the disease have shown progress over the last decade, malaria still represents a major burden to humanity, especially to populations living in African countries. Strategic vector-control interventions, accurate diagnostic tests, and effective antimalarial drugs are fundamental requirements in the fight against malaria. However, it is acknowledged that the development of a fully efficient vaccine is critical to achieve malaria eradication.
To date, RTS,S is the most advanced malaria vaccine candidate. This formulation is based on a portion of the most abundant protein on the surface of the malaria sporozoite, the circumsporozoite protein (CSP). Notably, RTS,S has shown potential to decrease the incidence of severe malaria in children and to prevent infection in 30 to 50% of its recipients (2–4). While these findings are encouraging, additional improvements may be required to accomplish a more broadly protective formulation.
A likely way to increase the effectiveness of subunit malaria vaccines is the development of formulations incorporating multiple parasite antigens (5). Besides CSP, a number of recent studies have explored the protective effect of immune responses targeting different sporozoite antigens (6–9). One of these antigens is the cell-traversal protein for ookinetes and sporozoites (CelTOS), which has a critical role in the establishment of malaria infections in both mosquito and vertebrate hosts (10). Importantly, antibody and T-cell responses against CelTOS have shown to mediate cross-species protection against a heterologous Plasmodium species challenge and to confer sterile protection to immunized mice (11).
In this study, we utilized the Pfenex platform to express Plasmodium falciparum CelTOS (PfCelTOS) protein that can be grown and purified under good manufacturing practices. Further, we report on the use of a newly generated chimeric Plasmodium berghei parasite expressing the PfCelTOS and demonstrate that immune responses against PfCelTOS can inhibit sporozoite hepatocyte infection. In addition, we also show that monoclonal antibodies against this protein inhibit sporozoite infectivity in vivo and significantly impair parasite development in Anopheles gambiae mosquitoes. Our results demonstrate that immune responses against CelTOS not only have the potential to inhibit Plasmodium infection but also decrease malaria transmission.
RESULTS
Expression and purification of recombinant CelTOS protein.
Plasmodium falciparum CelTOS (PfCelTOS) protein has previously been produced cytoplasmically in Escherichia coli (11); however, this material was generated on a small scale using an affinity tag, adding 16 amino acids to the N terminus of mature PfCelTOS. To enable vaccine development and later clinical manufacturing, a Pseudomonas fluorescens production strain encoding mature CelTOS with no added amino acids was developed. A gene encoding mature CelTOS, optimized for expression in P. fluorescens, by DNA2.0 using DNA2.0's proprietary algorithm in combination with the codon usage table for Pfenex's P. fluorescens strain MB214, was fused in frame with a variety of secretion leaders (12) to generate expression plasmids, which were screened in combination with an array of host strains to identify an optimal production strain. Samples grown at a 0.5-ml scale were evaluated for titer and intact mass. Strains with low levels of clipping (<0.3%) that showed titers of up to 0.2 g/liter at the 0.5-ml scale were selected to advance to 2-liter bioreactors to produce material for preclinical testing. Unoptimized titers ranged from ∼0.7 to over 1 g/liter. A three-step purification scheme was developed to purify recombinant PfCelTOS (PfrCelTOS). The final purified material had low endotoxin levels (<12 endotoxin units [EU]/mg) and corresponded to fully intact mature CelTOS, with no detectable clipped species.
Generation of PbANKA-PfCelTOS(r)PbCelTOSCelTOS chimeric parasites.
We developed a rodent challenge model, which involved creating a chimeric P. berghei parasite line where the P. berghei celtos coding sequence (CDS) (Pbceltos) gene was replaced with the P. falciparum celtos CDS (Pfceltos), amplified from genomic DNA of the P. falciparum NF54 strain. In addition to expressing PfCelTOS, these chimeric parasites constitutively express the fusion green fluorescent protein (GFP)-luciferase reporter protein (see Fig. S1 and S3 in the supplemental material). Correct replacement of the Pbceltos CDS by the Pfceltos CDS in the chimeric line was confirmed by diagnostic Southern analysis of chromosomes separated by pulsed-field gel electrophoresis and diagnostic PCR on genomic DNA (Fig. S1). Immunofluorescence microscopy of chimeric and wild-type (WT) sporozoites using sera from mice immunized with PfCelTOS and PbCelTOS (13) confirmed the expression of PfCelTOS in sporozoites of chimeric parasite line 2258cl2 (Fig. S1). Chimeric parasites showed normal asexual blood stage multiplication in mice (data not shown), and oocyst and sporozoite production in Anopheles stephensi mosquitoes was comparable to that of WT P. berghei parasites (see Table S3 in the supplemental material).
Immunization with PfrCelTOS impairs sporozoite infection in vivo.
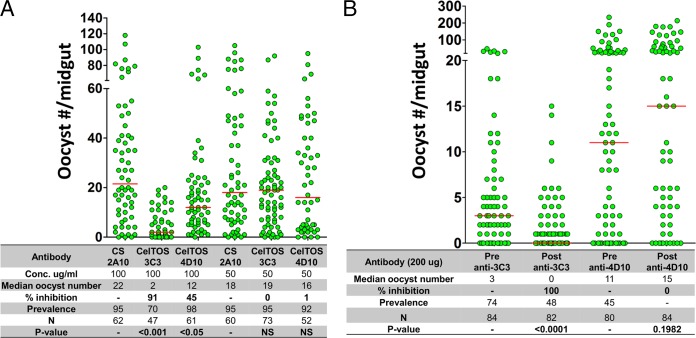
In experiments to evaluate the protective effect of immune responses elicited by PfrCelTOS, we found that mice immunized with 20 μg of PfrCelTOS in combination with the glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) or glucopyranosyl lipid adjuvant-liposome-QS21 (GLA-LSQ) adjuvant system had a significantly reduced parasite liver burden after challenge against chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. Mice immunized with 20 μg PfrCelTOS and GLA-SE had a 60% reduction of liver parasite burden compared to that in mice immunized with GLA-SE only (Fig. 1). In addition, animals immunized with 20 μg PfrCelTOS and GLA-LSQ had a 77% reduction of liver parasite load compared to that in naive controls after challenge with PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites (Fig. 1).
FIG 1.
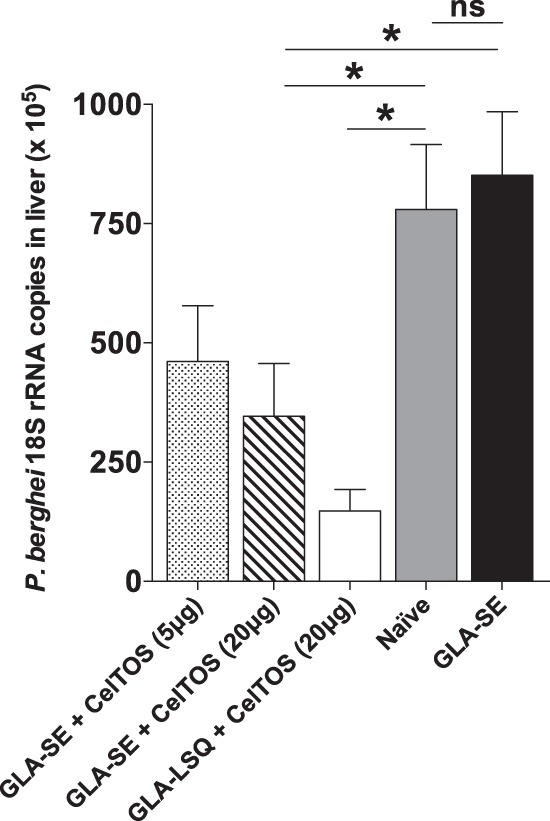
Immunization with PfrCelTOS inhibits sporozoite hepatocyte infection. Immunizations with 20 μg PfrCelTOS in combination with GLA-SE or GLA-LSQ conferred significant protection against challenge with chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. Mice immunized with 5 μg PfrCelTOS and GLA-SE had only a modest reduction of liver parasite burden (46% inhibition compared to the GLA-SE control group). The graph represents the results of one experiment. Values are means ± standard errors of the means (SEM) (n = 5 mice per group; *, P < 0.05; ns, not significant).
Sera raised against PfrCelTOS recognize P. falciparum sporozoites.
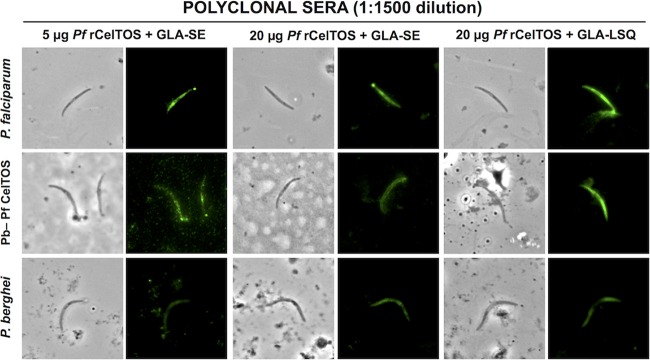
We performed immunofluorescence assays (IFAs) to determine the reactivity of polyclonal sera generated in mice immunized with PfrCelTOS. We showed that the vaccine-induced antibodies are capable of recognizing P. falciparum 3D7 and chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. Sera from mice immunized with 5 μg or 20 μg of PfrCelTOS in combination with GLA-SE yielded a fluorescent signal at up to a 1:1,500 dilution when tested against both parasite lines. IFA titers of polyclonal sera generated in mice immunized with 20 μg of PfrCelTOS and GLA-LSQ reached 1:4,500 against the PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites and 1:13,500 against P. falciparum sporozoites. We also found that the anti-CelTOS polyclonal sera were able to recognize WT P. berghei sporozoites, with titers comparable to those reached with PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites (Fig. 2). Preimmune sera or sera from mice injected with adjuvants only did not recognize sporozoites by IFA (see Fig. S4 in the supplemental material).
FIG 2.
Polyclonal sera and monoclonal antibodies bind CelTOS on sporozoites. P. falciparum (3D7), chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites, and P. berghei (ANKA) sporozoites were stained with polyclonal sera.
Passive transfer of MAb 4D10 partially inhibits sporozoite hepatocyte infection.
In view of the results obtained after immunizations with the PfrCelTOS, we developed monoclonal antibodies (MAbs) 3C3 and 4D10, which are specific for the P. falciparum CelTOS sequences CESQSMNKIGDDLAE and RGNNGHNSSSSLYNC, respectively. Both MAb 3C3 and MAb 4D10 reacted weakly against P. falciparum and PbANKA-PfCelTOS(r)PbCelTOSCelTOS sporozoites (data not shown). No cross-reactivity was observed against WT P. berghei. To determine whether these antibodies were capable of neutralizing sporozoite hepatocyte infection in vivo, we passively transferred 300 μg of each antibody into naive mice immediately prior to challenge with chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. Passive transfer of MAb 4D10 modestly reduced sporozoite infectivity up to 48% compared to that in naive controls. However, the parasite burden in mice receiving MAb 3C3 was not significantly different from that of naive controls that did not receive antibodies but were injected with parasites (see Fig. S5A in the supplemental material). Mice receiving control antibodies that do not bind to sporozoites had a parasite burden comparable to that observed in naive mice (see Fig. S5B in the supplemental material).
Anti-CelTOS monoclonal antibodies bind to P. falciparum ookinetes and inhibit oocyst formation.
Given the critical role of CelTOS for ookinete development, we sought to determine if monoclonal antibodies targeting this protein bind to P. falciparum ookinetes and inhibit oocyst formation in A. gambiae mosquitoes. Immunofluorescence assays showed that MAb 3C3, but not MAb 4D10, clearly binds to ookinetes isolated from mosquito midguts (Fig. 3). This is in contrast to the weak fluorescence observed with sporozoites. In a standard membrane feeding assay (SMFA), P. falciparum gametocyte cultures were mixed with different concentrations of MAb 3C3, MAb 4D10, or control antibody 2A10 and then fed to A. gambiae mosquitoes. Eight days after feeding, we determined the median oocyst number, calculated as described in Materials and Methods. We found that in mosquitoes fed with gametocyte cultures incubated with 0.1 mg/ml of irrelevant antibody (2A10), the median oocyst number was 22. In contrast, in mosquitoes fed with gametocyte cultures incubated with 0.1 mg/ml of MAb 3C3, the median oocyst number was reduced to 2; i.e., there was a 91% inhibition. The same concentration of MAb 4D10 inhibited only 45% of developing oocysts. No inhibitory effect was observed when the monoclonal antibodies were mixed with gametocytes at 0.05 mg/ml (Fig. 4A).
FIG 3.
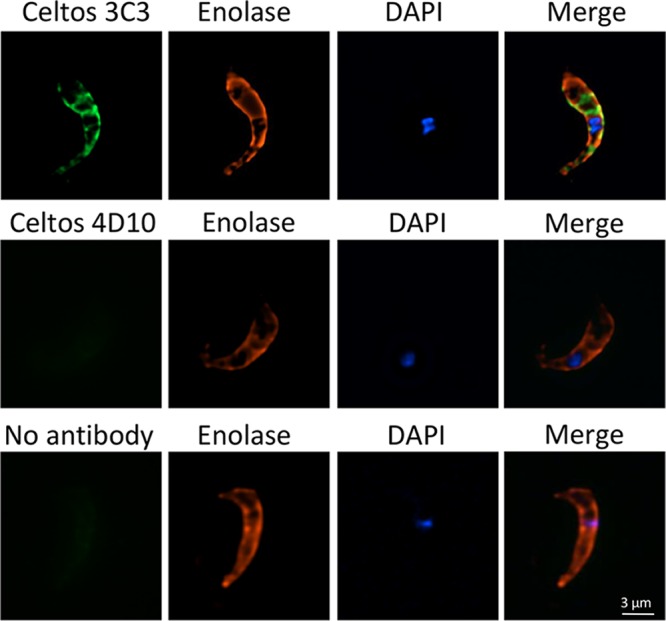
Monoclonal antibody 3C3 binds to P. falciparum ookinetes. Ookinetes were isolated from mosquito midguts 24 h after feeding on an infectious blood meal. Ookinetes were incubated with either MAb 3C3 or MAb 4D10. Anti-P. falciparum enolase antibody (34) was used as a positive control.
FIG 4.
Inhibition of P. falciparum oocyst formation by monoclonal antibodies. The effect MAbs 3C3 and 4D10 on P. falciparum (A) and PbANKA-PfCelTOS(r)PbCelTOSCelTOS (B) oocyst formation was determined by SMFAs and PAFAs, respectively. (A) Increasing concentrations of the antibodies were added to P. falciparum gametocyte cultures and fed to A. gambiae female mosquitoes. Control groups of mosquitoes were fed with cultures to which the anti-P. falciparum CSP monoclonal antibody 2A10 was added (35). (B) A. gambiae female mosquitoes (preantibody) were fed on mice infected with PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. After feeding, mice were injected intravenously with 200 μg of either MAb 3C3 or MAb 4D10. A second group of mosquitoes (postantibody) were fed on the antibody-treated mice. Percent inhibition is defined as [(control median oocyst number − experimental median oocyst number)/control median oocyst number] × 100. Prevalence, percentage of infected mosquitoes. N, number of mosquitoes analyzed. Horizontal bars represent the median number of oocysts from two independent experiments for results shown in panels A and B. Statistical significance was determined using one-way ANOVA with the Bonferroni multiple-comparison test (A) or the Mann-Whitney U test (B).
In addition, passive-administration feeding assays (PAFAs) were performed to determine the effect of monoclonal antibodies on oocyst formation by PbANKA-PfCelTOS(r)PbCelTOSCelTOS chimeric parasites. Oocyst numbers were determined in A. gambiae mosquitoes fed on mice infected with chimeric parasites, before and after passive administration of either MAb 3C3 or MAb 4D10 (Fig. 4B). The median oocyst number obtained in mice before transfer of MAb 3C3 was 3, and this was reduced to 0 after antibody transfer. In contrast, transfer of MAb 4D10 did not reduce oocyst formation. These results are in agreement with our previous results showing that MAb 3C3 binds to P. falciparum ookinetes and inhibits oocyst formation (Fig. 3 and 4A).
DISCUSSION
In this study, we report the protective effect of functional immune responses and antibodies against the P. falciparum CelTOS protein. To evaluate in vivo responses against this protein, a new chimeric P. berghei parasite line expressing the P. falciparum CelTOS was generated. Chimeric rodent parasites expressing orthologous proteins from human malaria species are practical tools to evaluate immune responses elicited by vaccination or natural infection in vivo (14–19). The newly generated PbANKA-PfCelTOS(r)PbCelTOSCelTOS chimeric parasite develops normally throughout blood and mosquito stages, and its infectivity is comparable to that of WT P. berghei sporozoites.
Importantly, we used an expression system that has shown to robustly produce recombinant full-length malaria antigen in a conformationally accurate and biologically active state (20). Further, this protein was produced without the use of expression tags. Although tags are useful research tools, they may potentially impact activity and are not appropriate for inclusion in a final vaccine product.
Immune responses were induced by immunization with P. falciparum rCelTOS in combination with the adjuvant systems GLA-SE or GLA-LSQ. This recombinant protein was produced in P. fluorescens, a standardized platform that has been previously used for generating recombinant P. falciparum CSP (20). Mice immunized with 5 μg or 20 μg of PfrCelTOS in combination with GLA-SE or GLA-LSQ mounted immune responses that inhibited infection of chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS sporozoites. The induced antibodies bind to P. falciparum sporozoites, PfCelTOS in chimeric sporozoites, and WT P. berghei as determined by IFA. The cross-reactivity between PfCelTOS and PbCelTOS in WT P. berghei parasites is consistent with previous studies indicating cross-reactivity between CelTOS expressed in P. berghei and P. falciparum (11), which is likely due to the significant similarities between the amino acid sequences of the P. falciparum and P. berghei CelTOS proteins.
To address the protective and transmission-blocking capacity of antibodies against CelTOS, the MAbs 3C3 and 4D10 were developed. In passive-transfer experiments, we demonstrate that MAb 4D10 can partially inhibit hepatocyte infection by chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites, while MAb 3C3 appears to have no discernible effect on neutralizing sporozoite infection. In contrast, in SMFA experiments, we found that MAb 3C3 is capable of strongly inhibiting P. falciparum oocyst development in A. gambiae mosquitoes. MAb 4D10 was also capable of significantly inhibiting oocyst development, but its effect was not as strong as that observed with MAb 3C3. In support of these data, MAb 3C3 also inhibited oocyst formation of PfCelTOS(r)PbCelTOSCelTOS chimeric parasites. The fact that MAb 3C3 had a stronger oocyst-inhibitory effect than MAb 4D10 may suggest that MAb 3C3's epitope is more available for antibody targeting during the early developmental stages of the parasite within mosquitoes. However, in contrast to the results obtained with monoclonal antibodies, we did not detect an inhibitory effect of anti-CelTOS polyclonal sera on P. falciparum oocyst formation (not shown). It is also worth mentioning that another group mapped a CelTOS peptide (amino acids [aa] 21 to 40) that overlaps with our MAb 4D10 peptide (aa 26 to 40) (21) and found that this peptide bound to liver-derived cell lines (HeLa and HepG2). Therefore, it may have been predicted that MAb 4D10 would be protective at the liver stage and that this region may not have been critical for the oocyst stage. Further studies will be necessary to determine if CelTOS undergoes conformational changes throughout the parasite's life cycle and if certain motifs of this protein become available for antibody targeting at different developmental stages.
We have shown that in vivo immune responses and monoclonal antibodies against the P. falciparum CelTOS can inhibit sporozoite hepatocyte infection and impair the parasite's development in mosquito vectors. Our results add to the evidence supporting the protective effect of immune responses against this protein and provide further proof of its potential as a target for vaccine development (7, 11, 22, 23). Additionally, the current study shows for the first time that antibodies against CelTOS not only inhibit sporozoite infection but also inhibit parasite development in mosquitoes. While the anti-CelTOS antibody responses may not be sufficient to confer sterile immunity, our findings should encourage further research aimed at generating a multiantigenic vaccine that could include CelTOS to potentially target the malaria parasite at different developmental stages.
MATERIALS AND METHODS
Recombinant CelTOS protein.
Recombinant P. falciparum CelTOS (PfrCelTOS) was produced in a Pseudomonas fluorescens platform using Pfenex Expression Technology (Pfenex, Inc., San Diego, CA) (24) Briefly, the P. falciparum 3D7 (isolate BAD97684) mature CelTOS coding sequence, corresponding to UniProt Q53UB8 amino acids 25 to 182, was optimized for expression in P. fluorescens (DNA2.0, Menlo Park, CA) and cloned into 20 expression plasmids, each with a different P. fluorescens periplasmic secretion leader/ribosome binding site combination. The CelTOS-encoding sequence was cloned in frame with the secretion leader-coding sequence in each plasmid and transformed into 50 P. fluorescens host strains. The resulting expression strains were then screened for protein yield and quality by SDS-capillary gel electrophoresis (CGE) and intact mass analysis, respectively.
A subset of the tested strains was advanced to the 2-liter bioreactor scale for the production of material for preclinical testing. Cells were lysed by microfluidization using 2 passes at 15,000 lb/in2 (Microfluidics M-110Y high-pressure pneumatic homogenizer) and loaded into a TMAE HiCap (EMD) anion-exchange capture column, followed by polishing steps using hydroxyapatite (HA) (type I, 40 μm; Bio-Rad) mixed-mode chromatography and MonoQ (GE) strong anion-exchange chromatography. Endotoxin analyses were performed using the Limulus amebocyte lysate (LAL) Endosafe portable test system (PTS) (Charles River Laboratories) following manufacturer-supplied operating procedures. Protein samples were processed prior to intact mass analysis by liquid chromatography coupled to mass spectrometry (LC-MS) to detect any clipped species.
Antibody development.
The monoclonal antibodies (MAbs) 3C3 and 4D10 were generated by Precision Antibody, a division of A&G Pharmaceutical Inc. (Columbia, MD), using proprietary technology and mouse immunization protocols. MAb 3C3 was generated through immunizations with the CelTOS-derived synthetic peptide CESQSMNKIGDDLAE, whereas peptide RGNNGHNSSSSLYNC was used to generate MAb 4D10. Supernatants from the generated hybridomas were screened by ELISA using PfrCelTOS (Pfenex Inc.) and by Western blotting using a cell lysate from P. fluorescens CelTOS expression strains.
Generation of DNA constructs and genotyping of the chimeric parasites.
To generate the chimeric parasites where the P. berghei celtos coding sequence (CDS) (Pbceltos) (PBANKA_1432300) has been replaced by the P. falciparum celtos CDS (Pfceltos) (PF3D7_1216600), we used a 2-step gene insertion/marker out (GIMO) transfection protocol (25, 26). In the first step, we deleted the Pbceltos CDS and replaced it with the positive-negative selectable marker, to create a P. berghei celtos deletion GIMO line (PbANKA-CelTOS GIMO). In order to do this, we generated the pL1960 construct, which is based on the standard GIMO DNA construct pL0034 (25). This construct contains the positive-negative (hdhfr::yfcu) selection marker (SM) cassette, and was used to insert both the Pbceltos 5′ and 3′ gene-targeting regions (TR), encompassing the full-length promoter and transcription terminator sequences, respectively. The linear pL1960 DNA construct was introduced into PbGFP-Luccon parasites (676m1cl1) using standard methods of transfection (27). Transfected parasites were selected in mice by applying positive selection by providing pyrimethamine in the drinking water (27). Transfected parasites were cloned by limiting dilution (28), resulting in the PbANKA-CelTOS GIMO line (line 2217). Correct deletion of the Pbceltos CDS was confirmed by diagnostic PCR analysis on genomic DNA (gDNA) and Southern analysis of pulsed-field gel-separated chromosomes as described previously (29). Primers used for PCR genotyping are listed in Tables S1 and S2 in the supplemental material.
In the second step, we replaced the positive-negative SM in the PbANKA-CelTOS GIMO genome with the Pfceltos CDS by GIMO transfection to create the P. berghei chimeric CelTOS replacement line. This was achieved by modifying the construct used in the first step (pL1960); specifically, the hdfhr::yfcu SM cassette was removed and replaced with Pfceltos CDS sequence, generating plasmid pL1971. The Pfceltos CDS was amplified from genomic DNA of the NF54 strain of P. falciparum. The pL1971 construct was sequenced to ensure that there were no mutations in the Pfceltos CDS. The construct was linearized using ApaI and NotI restriction enzymes outside the 5′ and 3′ TRs before transfection. The construct was used to transfect parasites of the PbANKA-CELTOS GIMO line (line 2217cl1) using standard methods of GIMO transfection (25). Transfected parasites were selected in mice by applying negative selection by providing 5-fluorocytosine (5-FC) in the drinking water of mice (30). Negative selection results in selection of chimeric parasites where the hdhfr::yfcu SM in the celtos locus of the PbANKA-CelTOS GIMO line is replaced by the CDS of Pfceltos. Selected chimeric parasites were cloned by limiting dilution (28). Correct integration of the constructs into the genomes of chimeric parasites was analyzed by diagnostic PCR analysis on gDNA and Southern analysis of pulsed-field gel-separated chromosomes as described previously (29). Primers used for PCR genotyping are listed in Tables S1 and S2. This method creates chimeric “gene replacement” P. berghei parasites that lack the Pbceltos CDS but express PfCelTOS [PbANKA-PFCelTOS(r)PbCelTOS; line 2258cl2] under the control of the Pbceltos regulatory sequences.
Phenotyping of chimeric parasites.
Multiplication of blood stages in mice was determined during the cloning period as described previously (29, 31). Feeding of A. stephensi mosquitoes, determination of oocyst production, and sporozoite collection were performed as described previously (31). Expression of PfCelTOS in chimeric sporozoites was analyzed by immunofluorescence assay (IFA) using sera from mice immunized with PfCelTOS or PbCelTOS (diluted 1:100) (13). Purified sporozoites were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min on ice and then washed three times with PBS and blocked with 20 μl 10% fetal bovine serum (FCS) plus 1% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The excess blocking medium was removed, followed by the addition of 20 to 25 μl primary monoclonal antibody in 10% FCS plus 1% BSA in PBS (blocking medium) for 1 to 2 h at room temperature or overnight at 4°C. After incubation, the primary antibody was removed and the slides washed three times with PBS, followed by staining with the secondary antibody (Alexa Fluor 488 goat anti-mouse IgG from Life Technologies [catalog number A-11001]) diluted 1:800 in blocking medium for 1 h at room temperature. After washing three times with PBS, nuclei were stained with 2% Hoechst 33342 (Cell Signaling Technology, catalog number 4082S) in PBS for 10 min at room temperature, washed twice with PBS, and left to air dry; this was followed by addition of fluorescence mounting medium (Dako, code S3023). Coverslips were mounted onto the slides, and the slides were sealed with nail polish and left to dry overnight in the dark. The parasites in both blue and green channels were analyzed using a DMI-300B Leica fluorescence microscope and images processed using ImageJ software. The infectivity of chimeric sporozoites was determined by assessing the prepatent period in mice infected with chimeric sporozoites, which was comparable to that observed in mice infected with WT P. berghei sporozoites (see Fig. S1 and S2 in the supplemental material). These results demonstrate that chimeric P. berghei sporozoites expressing PfCelTOS instead of PbCelTOS are fully infectious and able to complete liver stage development in mice.
Immunizations and sporozoite challenge.
C57BL/6 mice were immunized with 5 μg or 20 μg of PfrCelTOS in combination with 5 μg of the adjuvant systems glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) and GLA-liposome-QS21 formulation (GLA-LSQ) (IDRI). Intramuscular immunizations were administered in the hind legs on study days 0, 14, and 35. Three weeks after the last immunization, mice were challenged with 5 × 103 sporozoites of the chimeric parasite line PbANKA-PfCelTOS(r)PbCelTOS (2258 cl2) delivered by intravenous (i.v.) tail vein injection.
IFAs.
Immunofluorescence assays (IFAs) were performed using air-dried sporozoites or ookinetes obtained from mosquito midguts dissected 24 h after feeding on an infectious blood meal. Briefly, a sporozoite suspension (4 × 105 to 6 × 105 sporozoites/ml) or ookinete suspension was air dried at room temperature on poly-l-lysine-coated slides (Tekdon Inc., Myakka City, FL). Parasites were fixed for 30 min using a 2% (wt/vol) paraformaldehyde (Sigma, St. Louis, MO)–1× phosphate-buffered saline (PBS) solution and washed 2 times. Sporozoites were then permeabilized for 30 min using a 0.1% (vol/vol) Triton (Roche, Mannheim, Germany)–1× PBS solution and washed. Antibody or serum samples diluted in 1% (wt/vol) bovine serum albumin (BSA) (Sigma, St. Louis, MO)–1× PBS (1% BSA–PBS) were incubated for 30 min at room temperature. Slides were then washed with 1% BSA–PBS and incubated for 30 min at room temperature with a secondary-antibody solution [Alexa Fluor 488 F(ab′)2 fragment of goat anti-mouse IgG(H+L); 2 mg/ml; (Invitrogen)]. Fluorescent sporozoites and ookinetes were visualized under an upright fluorescence microscope (Nikon Eclipse 90i).
Sporozoite challenge upon passive transfer of monoclonal antibodies.
Three hundred micrograms of the monoclonal antibody MAb 3C3 or MAb 4D10 was passively transferred into naive mice by i.v. tail vein injection 5 to 10 min prior to challenge with 2 × 103 chimeric PbANKA-PfCelTOS(r)PbCelTOSCelTOS sporozoites, delivered via the same route. Forty hours later, mice were euthanized and livers harvested to quantify the parasite load by reverse transcription-quantitative PCR (RT-qPCR) (32).
P. falciparum SMFA.
For the standard membrane feeding assay (SMFA), P. falciparum gametocyte cultures were diluted to 0.03% gametocytemia. Monoclonal antibodies were added to these cultures at a final concentration of 50 μg/ml or 100 μg/ml, and then the cultures were fed to A. gambiae female mosquitoes. Mosquitoes were kept at 27°C in a humidified chamber. Mosquito midguts were dissected 8 days after feeding, and the oocyst number per midgut was determined after staining in a 0.2% (wt/vol) mercury dibromofluorescein salt–1× PBS solution. Percent inhibition was defined as [(control median oocyst number − experimental median oocyst number)/control median oocyst number] × 100.
P. berghei PAFA.
Passive-administration feeding assays (PAFAs) were performed as previously described (33). Briefly, a group of A. gambiae mosquitoes (preantibody) were fed for 15 min on a mouse infected with PbANKA-PfCelTOS(r)PbCelTOSCelTOS parasites. The mouse was then injected i.v. with 200 μg of either MAb 3C3 or MAb 4D10. After 15 min of recovery to allow for antibody dissemination in the bloodstream, the mouse was used to feed a second group of A. gambiae mosquitoes (postantibody). Mosquito midguts were dissected 10 days after feeding, and the oocyst number per midgut was determined after staining in a 0.2% (wt/vol) mercury dibromofluorescein salt–1× PBS solution. Percent inhibition was defined as [(control median oocyst number − experimental median oocyst number)/control median oocyst number] × 100.
Mice.
Five- to 8-week-old female C57BL/6 mice were purchased from NCI (Frederick, MD). All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University.
Data analysis.
Statistical analysis comparing means was performed by a two-tailed unpaired Student t test with Welch's correction, unless otherwise stated in a figure legend or table. As stated in the legends of the figures, the statistical significance was determined using one-way analysis of variance (ANOVA) with Bonferroni multiple-comparison tests or Mann-Whitney or Mantel-Cox analysis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Annie Mo, Program Officer for the Parasitology and International Programs Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, for helpful insights and discussions. D.A.E., J.V.-R., Y.F.-G., and F.Z. thank the Bloomberg Family Foundation for continued support. This work was partially funded through NIAID/DMID contract number AI-N01-054210 to Leidos Inc.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00498-16.
REFERENCES
- 1.Anonymous. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.RTS,S Clinical Trials Partnership. 2014. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, Ouedraogo S, Sandrine Y, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Odero C, Oneko M, Otieno K, et al. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 4.Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, Owusu R, Shomari M, Leach A, Jongert E, Salim N, Fernandes JF, Dosoo D, Chikawe M, Issifou S, Osei-Kwakye K, Lievens M, Paricek M, Moller T, Apanga S, Mwangoka G, Dubois MC, Madi T, Kwara E, Minja R, Hounkpatin AB, Boahen O, Kayan K, Adjei G, Chandramohan D, Carter T, Vansadia P, Sillman M, Savarese B, Loucq C, Lapierre D, Greenwood B, Cohen J, Kremsner P, Owusu-Agyei S, Tanner M, Lell B. 2011. Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis 11:741–749. doi: 10.1016/S1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- 5.Good MF, Doolan DL. 2010. Malaria vaccine design: immunological considerations. Immunity 33:555–566. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Kester KE, Gray Heppner D Jr, Moris P, Ofori-Anyinam O, Krzych U, Tornieporth N, McKinney D, Delchambre M, Ockenhouse CF, Voss G, Holland C, Beckey JP, Ballou WR, Cohen J. 2014. Sequential phase 1 and phase 2 randomized, controlled trials of the safety, immunogenicity and efficacy of combined pre-erythrocytic vaccine antigens RTS,S and TRAP formulated with AS02 adjuvant system in healthy, malaria naive adults. Vaccine 32:6683–6691. doi: 10.1016/j.vaccine.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann-Leitner ES, Hosie H, Trichilo J, Deriso E, Ranallo RT, Alefantis T, Savranskaya T, Grewal P, Ockenhouse CF, Venkatesan MM, Delvecchio VG, Angov E. 2013. Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria. Front Immunol 4:176. doi: 10.3389/fimmu.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, Walsh DS, Yoon IK, Prosperi C, Juompan LY, Lanar DE, Krzych U, Hall BT, Ware LA, Stewart VA, Williams J, Dowler M, Nielsen RK, Hillier CJ, Giersing BK, Dubovsky F, Malkin E, Tucker K, Dubois MC, Cohen JD, Ballou WR, Heppner DG Jr. 2010. Recombinant liver stage antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Ferraro B, Talbott KT, Balakrishnan A, Cisper N, Morrow MP, Hutnick NA, Myles DJ, Shedlock DJ, Obeng-Adjei N, Yan J, Kayatani AK, Richie N, Cabrera W, Shiver R, Khan AS, Brown AS, Yang M, Wille-Reece U, Birkett AJ, Sardesai NY, Weiner DB. 2013. Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infect Immun 81:3709–3720. doi: 10.1128/IAI.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. 2006. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol 59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann-Leitner ES, Mease RM, De La Vega P, Savranskaya T, Polhemus M, Ockenhouse C, Angov E. 2010. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS One 5:e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retallack DM, Schneider JC, Mitchell J, Chew L, Liu H. 2007. Transport of heterologous proteins to the periplasmic space of Pseudomonas fluorescens using a variety of native signal sequences. Biotechnol Lett 29:1483–1491. doi: 10.1007/s10529-007-9415-5. [DOI] [PubMed] [Google Scholar]
- 13.Longley RJ, Salman AM, Cottingham MG, Ewer K, Janse CJ, Khan SM, Spencer AJ, Hill AV. 2015. Comparative assessment of vaccine vectors encoding ten malaria antigens identifies two protective liver-stage candidates. Sci Rep 5:11820. doi: 10.1038/srep11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. 2002. A new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol 169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 15.Bauza K, Malinauskas T, Pfander C, Anar B, Jones EY, Billker O, Hill AV, Reyes-Sandoval A. 2014. Efficacy of a Plasmodium vivax malaria vaccine using ChAd63 and modified vaccinia Ankara expressing thrombospondin-related anonymous protein as assessed with transgenic Plasmodium berghei parasites. Infect Immun 82:1277–1286. doi: 10.1128/IAI.01187-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Zhang D, Pan W. 2009. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS One 4:e6894. doi: 10.1371/journal.pone.0006894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Koning-Ward TF, O'Donnell RA, Drew DR, Thomson R, Speed TP, Crabb BS. 2003. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J Exp Med 198:869–875. doi: 10.1084/jem.20030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mlambo G, Maciel J, Kumar N. 2008. Murine model for assessment of Plasmodium falciparum transmission-blocking vaccine using transgenic Plasmodium berghei parasites expressing the target antigen Pfs25. Infect Immun 76:2018–2024. doi: 10.1128/IAI.01409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramjanee S, Robertson JS, Franke-Fayard B, Sinha R, Waters AP, Janse CJ, Wu Y, Blagborough AM, Saul A, Sinden RE. 2007. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25:886–894. doi: 10.1016/j.vaccine.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Noe AR, Espinosa D, Li X, Coelho-Dos-Reis JG, Funakoshi R, Giardina S, Jin H, Retallack DM, Haverstock R, Allen JR, Vedvick TS, Fox CB, Reed SG, Ayala R, Roberts B, Winram SB, Sacci J, Tsuji M, Zavala F, Gutierrez GM. 2014. A full-length Plasmodium falciparum recombinant circumsporozoite protein expressed by Pseudomonas fluorescens platform as a malaria vaccine candidate. PLoS One 9:e107764. doi: 10.1371/journal.pone.0107764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtidor H, Arevalo-Pinzon G, Bermudez A, Calderon D, Vanegas M, Patino LC, Patarroyo MA, Patarroyo ME. 2012. Binding activity, structure, and immunogenicity of synthetic peptides derived from Plasmodium falciparum CelTOS and TRSP proteins. Amino Acids 43:365–378. doi: 10.1007/s00726-011-1087-8. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse CF, Angov E. 2011. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine 29:5940–5949. doi: 10.1016/j.vaccine.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann-Leitner ES, Chaudhury S, Steers NJ, Sabato M, Delvecchio V, Wallqvist AS, Ockenhouse CF, Angov E. 2013. Computational and experimental validation of B and T-cell epitopes of the in vivo immune response to a novel malarial antigen. PLoS One 8:e71610. doi: 10.1371/journal.pone.0071610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Retallack DM, Jin H, Chew L. 2012. Reliable protein production in a Pseudomonas fluorescens expression system. Protein Expr Purif 81:157–165. doi: 10.1016/j.pep.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, Franke-Fayard BM, Janse CJ, Khan SM. 2011. A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One 6:e29289. doi: 10.1371/journal.pone.0029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salman AM, Mogollon CM, Lin JW, van Pul FJ, Janse CJ, Khan SM. 2015. Generation of transgenic rodent malaria parasites expressing human malaria parasite proteins. Methods Mol Biol 1325:257–286. doi: 10.1007/978-1-4939-2815-6_21. [DOI] [PubMed] [Google Scholar]
- 27.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. 2006. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Menard R, Janse C. 1997. Gene targeting in malaria parasites. Methods 13:148–157. doi: 10.1006/meth.1997.0507. [DOI] [PubMed] [Google Scholar]
- 29.Janse CJ, Ramesar J, Waters AP. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 30.Orr RY, Philip N, Waters AP. 2012. Improved negative selection protocol for Plasmodium berghei in the rodent malarial model. Malar J 11:103. doi: 10.1186/1475-2875-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annoura T, Ploemen IH, van Schaijk BC, Sajid M, Vos MW, van Gemert GJ, Chevalley-Maurel S, Franke-Fayard BM, Hermsen CC, Gego A, Franetich JF, Mazier D, Hoffman SL, Janse CJ, Sauerwein RW, Khan SM. 2012. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 30:2662–2670. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Bruña-Romero O, Hafalla JCR, González-Aseguinolaza G, Sano GI, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31:1499–1502. doi: 10.1016/S0020-7519(01)00265-X. [DOI] [PubMed] [Google Scholar]
- 33.Vega-Rodriguez J, Perez-Barreto D, Ruiz-Reyes A, Jacobs-Lorena M. 2015. Targeting molecular interactions essential for Plasmodium sexual reproduction. Cell Microbiol 17:1594–1604. doi: 10.1111/cmi.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh AK, Coppens I, Gardsvoll H, Ploug M, Jacobs-Lorena M. 2011. Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc Natl Acad Sci U S A 108:17153–17158. doi: 10.1073/pnas.1103657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, Nussenzweig V. 1985. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228:1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.