Abstract
Objective
To determine whether treatment with clarithromycin for respiratory tract infections was associated with an increased risk of cardiovascular (CV) events, arrhythmias or all-cause mortality compared with other antibiotics.
Design
Retrospective cohort design comparing clarithromycin monotherapy for lower (LRTI) or upper respiratory tract infection (URTI) with other antibiotic monotherapies for the same indication.
Setting
Routine primary care data from the UK Clinical Practice Research Datalink and inpatient data from the Hospital Episode Statistics (HES).
Participants
Patients aged ≥35 years prescribed antibiotic monotherapy for LRTI or URTI 1998–2012 and eligible for data linkage to HES.
Main outcome measures
The main outcome measures were: adjusted risk of first-ever CV event, within 37 days of initiation, in commonly prescribed antibiotics compared with clarithromycin. Secondarily, adjusted 37-day risks of first-ever arrhythmia and all-cause mortality.
Results
Of 700 689 treatments for LRTI and eligible for the CV analysis, there were 2071 CV events (unadjusted event rate: 29.6 per 10 000 treatments). Of 691 998 eligible treatments for URTI, there were 688 CV events (9.9 per 10 000 treatments). In LRTI and URTI, there were no significant differences in CV risk between clarithromycin and all other antibiotics combined: OR=1.00 (95% CI 0.82 to 1.22) and 0.82 (0.54 to 1.25), respectively. Adjusted CV risk in LRTI versus clarithromycin ranged from OR=1.42 (cefalexin; 95% CI 1.08 to 1.86) to 0.92 (doxycycline; 0.64 to 1.32); in URTI, from 1.17 (co-amoxiclav; 0.68 to 2.01) to 0.67 (erythromycin; 0.40 to 1.11). Adjusted mortality risk versus clarithromycin in LRTI ranged from 0.42 to 1.32; in URTI, from 0.75 to 1.43. For arrhythmia, adjusted risks in LRTI ranged from 0.68 to 1.05; in URTI, from 0.70 to 1.22.
Conclusions
CV events were more likely after LRTI than after URTI. When analysed by specific indication, CV risk associated with clarithromycin was no different to other antibiotics.
Keywords: respiratory tract infection, antibiotics, clarithromycin, mortality, arrhythmia, Cardiovascular event
Strengths and limitations of this study.
This study examined cardiovascular and other serious outcomes following therapy with clarithromycin and, for the first time, all other antibiotics prescribed for the same indication.
The study considered lower and upper respiratory tract indications separately, enabling outcomes to be compared by the severity of antibiotics’ indication.
Our use of linked primary and secondary care data from the Clinical Practice Research Datalink (CPRD) provided a large and nationally representative sample spanning 14 years and capturing data from more than 1.6 million prescriptions.
Our analyses were based on issued prescriptions; we were unable to determine from the data whether these were dispensed, whether patients were compliant, or whether patients had been advised to delay starting the prescribed antibiotic.
Indications for prescriptions are not directly recorded in CPRD and therefore had to be deduced.
Introduction
There is an association between the inflammatory response to acute infection and cardiovascular (CV) event risk. Lower respiratory tract infection (LRTI) appears to be a trigger of acute myocardial infarction and stroke.1–3 It has been postulated that some antibiotic drug classes—notably the macrolides—are associated with electrophysiological side effects such as QT prolongation, and therefore potentially increase the risk of CV events.4 The British National Formulary advises that macrolides ‘should be used with caution in patients with a predisposition to QT interval prolongation’.5 However, macrolides are often used to treat more severe infections which should be taken into account when evaluating CV risk since antibiotic prescribing for LRTIs is different from that for other indications. Thus, illness severity and the indication for antibiotic treatment should be taken into account when analysing and interpreting any potential association between antibiotic exposure and outcome events.
This study was motivated by a recent paper that reported a significantly increased risk of cardiac death associated with clarithromycin versus penicillin-V (adjusted rate ratio (ARR) 1.76; 95% CI 1.08 to 2.85) but not with roxithromycin (ARR=1.04; 1.08 to 2.85), another macrolide antibiotic.6 However, the study did not account for indication.
In a recent study of antibiotic treatment failure, we identified almost 11 million first-line antibiotic monotherapies, of which 39% were for upper respiratory tract infections (URTIs) and 29% for LRTIs.7 Here, we used the same data set to characterise the risk of various severe outcome events, including CV events and arrhythmia, in people exposed to clarithromycin for respiratory infections versus other antibiotics. For the first time, the site of the infection was accounted for while adjusting for other risk factors.
Methods
Data source
The data sources were primary care data from the Clinical Practice Research Datalink (CPRD) (formerly the General Practice Research Database) and linked secondary care data from the Hospital Episode Statistics (HES) for England.8 Approval for this study was granted by CPRD's Independent Scientific Advisory Committee (protocol 15_012).
CPRD contains clinically rich, pseudonymised, longitudinal data relating to more than 14 million patients, collected from 660 primary care practices throughout the UK (to January 2013). A proportion of participating practices based in England, representing about 50% of all CPRD patients, also take part in a linkage scheme by which the records of eligible patients are anonymously linked to other independent data sets.9 These include, from 1997 to 2012, the HES repository, which collates data on all hospital admissions occurring within National Health Service hospitals in England.10 Patients in linked practices are representative of the CPRD population as a whole, which is in turn broadly representative of the UK population.9 11
CPRD applies data quality markers at patient and general practice (GP) levels. Patient records are considered to have an acceptable research quality if they are internally consistent with regard to age, sex, registration and event dates and if the patient has been permanently registered with the practice. Contributing practices are assigned an ‘up to standard’ date from which their data are judged to be of an acceptable quality with regard to completeness, plausibility and continuity.12
Data recorded in CPRD include demographics; symptoms and diagnoses; prescriptions; immunisations; results of investigations; referrals to specialists and secondary care; feedback from other care settings; and lifestyle information relating to health behaviour, such as body mass index (BMI) and smoking status. Diagnoses in CPRD are recorded using the Read code classification and have been validated in a number of studies, showing a high positive predictive value.13 Prescriptions are well documented in the database as they are generated within and automatically recorded by the general practitioner's practice software. The HES data include primary and contributory causes of hospital admission coded using the International Classification of Diseases (ICD)-10 classification.
Patient selection
To improve ascertainment of CV events, only those research quality patients eligible to have their records linked to the HES data set were selected, thereby providing details of diagnoses and procedures related to hospital admissions.
Patients younger than 35 years of age at therapy initiation were excluded from the analysis due to the rarity of CV events in this age group, although antibiotics were commonly prescribed to these patients. Patients were required to have been registered at an up-to-standard practice for at least 365 days at therapy initiation so that their prior history could be reliably characterised. Those with prior CV events at therapy initiation were excluded because of the difficulty, otherwise, in distinguishing between new events associated with antibiotic exposure and the re-recording by the general practitioner of an earlier event considered relevant to the patient’s care. Patients with previous arrhythmia were excluded from the arrhythmia analysis for the same reason.
Identification of antibiotic therapy
The antibiotic therapies comprising the study cohorts were selected from a data set of first-line antibiotic monotherapies if they began between 1998 and 2012 and had a single associated diagnosis, by clinical code, for LRTI (eg, pneumonia, bronchitis, whooping cough) or URTI (eg, pharyngitis, laryngitis, tonsillitis, sinusitis).
Episodes of monotherapy were identified as one or more consecutive prescriptions for a single antibiotic separated by no more than 30 days and uninterrupted by prescriptions for other antibiotic drug substances; 98% of all antibiotic prescriptions were monotherapy. Only first-line therapies were selected, where these were defined as first-line if there were no prescriptions for other antibiotics in the preceding 30 days. To prevent previous antibiotic exposure from impacting on outcomes, a minimum of 90 days between therapies was required. Therapies were further excluded if follow-up from antibiotic initiation to the end of registration (for reasons other than death) or to the end of the data excerpt was <37 days, this period having been selected based on a presumed treatment course of 7 days plus 30 days’ follow-up. More than 80% of the antibiotics in our data set were prescribed on a regimen of 7 days or fewer, and 7 days was also the antibiotic treatment duration examined in the Danish study.6
For each of the two indications, the seven antibiotic agents most frequently prescribed were examined and reported separately; antibiotics less frequently prescribed for each indication were grouped as ‘other’. The index date was defined as the date of the first prescription in the therapy episode. More than one episode could be identified for any individual patient.
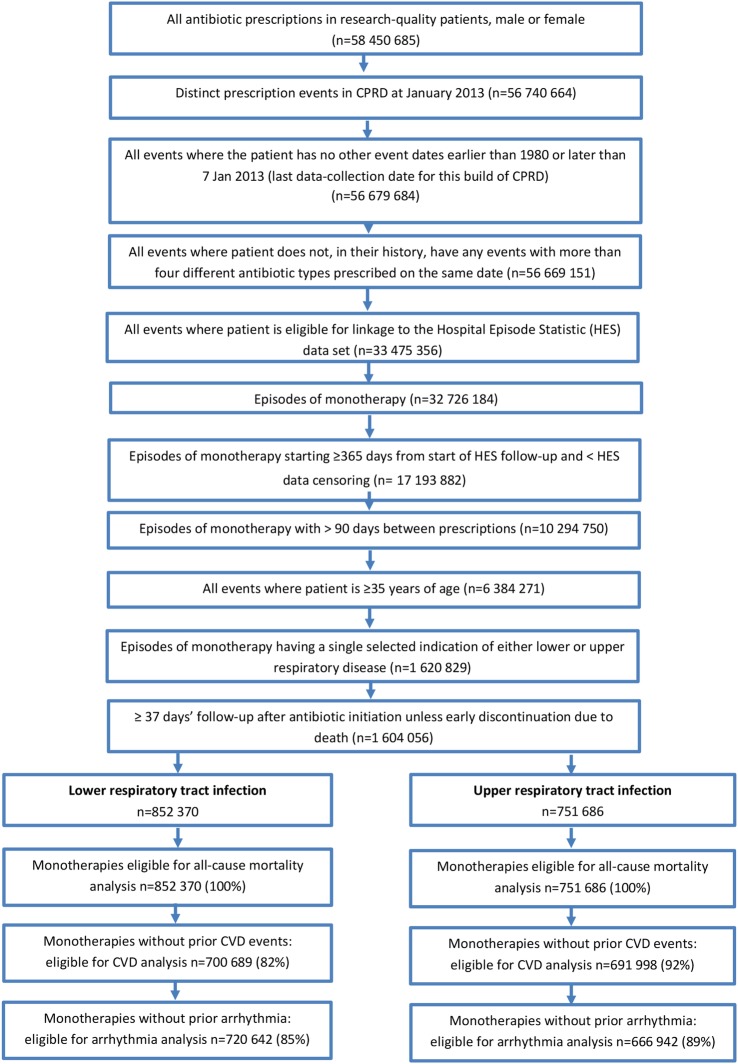
Figure 1 illustrates the overall process of selecting the study data.
Figure 1.
Data flow diagram for the selection of episodes of first-line antibiotic monotherapy reported in the CPRD. CPRD, Clinical Practice Research Datalink; CVD, cardiovascular disease.
Study end points
The primary end point was the occurrence of a first-ever CV event in the 37 days following antibiotic therapy initiation; this was determined for therapies for LRTI, URTI and overall, with the last of these enabling findings to be compared with previous studies.6 Secondary end points were 37-day all-cause mortality and 37-day first-ever arrhythmia. Each analysis compared clarithromycin with other antibiotics (the seven most commonly prescribed and the ‘other’ group) and with all antibiotics combined, for LRTI and URTI separately and together. Post hoc, following a recent publication comparing CV end points in clarithromycin and amoxicillin in the first 2 weeks of exposure for Helicobacter pylori eradication,14 we also analysed these end points in the 14 days following antibiotic initiation.
A CV event was defined as the first occurrence of fatal or non-fatal myocardial infarction, stroke, angina, or transient ischaemic attack, recorded by a Read or ICD-10 code in either the primary care or linked HES components of CPRD. An arrhythmia event was defined as a patient's first arrhythmia event recorded by a Read or ICD-10 code in these sources.
Statistical methods
The baseline characteristics of patients at antibiotic therapy initiation were determined for the most commonly prescribed antibiotics plus the ‘other’ group for each indication. Multivariable logistic regression was used to determine the independent associations between these antibiotics and 37-day CV events, 37-day all-cause mortality and 37-day arrhythmia events for LRTI and URTI.
LRTI and URTI indications were analysed separately, calculating separate ORs in order to investigate whether findings in previous studies might be due to differences in antibiotic prescription patterns between indications (LRTIs and URTIs were also analysed together). Clarithromycin was used as the reference category for the logistic regression. Candidate covariates were age, gender, smoking status, ethnicity, BMI, systolic blood pressure (SBP), total cholesterol (TC), diabetes, number of GP contacts in the prior year, Charlson comorbidity index, the number of antiplatelet, lipid-lowering and antihypertensive prescriptions in the year prior to index, year of antibiotic therapy initiation and the number of antibiotic therapies prescribed in the year prior to index.
Clarithromycin is an inhibitor of cytochrome CYP3A4 and so should not be combined with statins that are extensively metabolised by that enzyme. Statins not metabolised by CYP3A4 (rosuvastatin, pravastatin and fluvastatin) are therefore preferred for use in conjunction with clarithromycin. However, it has been reported that there may be an increased CV risk associated with these drug combinations.15 To test this hypothesis, a sensitivity analysis was planned that would include only those patients receiving statins not metabolised by CYP3A4; however, owing to low numbers of events, the analysis was not carried out. Concomitant statin use was therefore included as a categorical covariate in the model.
To allow for any potential non-linear effects of predictors on the outcome, continuous variables were considered for modelling using restricted cubic spline functions to allow for potential non-linear effects.
Multivariable logistic regression was used to determine the independent effects of antibiotic therapies for each of the two indications for outcomes in the 37 days and, post hoc, 14 days from initiation. All candidate covariates were included in the final model with no variable selection performed because it has been shown that excluding statistically insignificant variables does not improve predictive accuracy and makes accurate CIs hard to obtain. All statistical analyses were performed using R software (R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. http://www.r-project.org).
There were varying amounts of missing data for covariates such as BMI (66% missing), SBP (37%) and TC (69%; tables 1 and 2). The amount of missingness for certain covariates precluded the use of imputation techniques or the analysis of complete cases only. To address this, continuous covariates with missing values were categorised with an additional ‘missing/not recorded’ category. Similarly, categorical variables with missing values were recoded with an additional ‘missing/not recorded’ category.
Table 1.
Baseline characteristics: first-line antibiotic monotherapies prescribed for LRTI
| Clarithromycin | Amoxicillin | Cefalexin | Co-amoxiclav | Doxycycline | Erythromycin | Oxytetracycline | Other* | |
|---|---|---|---|---|---|---|---|---|
| Antibiotic monotherapies (all; mortality analysis), n | 42 920 | 582 817 | 28 850 | 37 161 | 20 568 | 74 800 | 13 817 | 51 437 |
| All patients, n | 35 911 | 381 218 | 24 456 | 32 313 | 18 139 | 58 146 | 12 040 | 42 931 |
| CVD-eligible therapies, n (%) | 35 202 (82.0%) | 482 054 (82.7%) | 22 513 (78.0%) | 29 444 (79.2%) | 16 621 (80.8%) | 63 391 (84.7%) | 11 527 (83.4%) | 39 937 (77.6%) |
| Arrhythmia-eligible therapies, n (%) | 34 934 (81.4%) | 496 126 (85.1%) | 23 699 (82.1%) | 30 741 (82.7%) | 16 689 (81.1%) | 64 373 (86.1%) | 11 991 (86.8%) | 42 089 (81.8%) |
| Women, n (%) | 25 462 (59.3%) | 320 708 (55.0%) | 18 257 (63.2%) | 19 106 (51.4%) | 11 649 (56.6%) | 45 912 (61.3%) | 7369 (53.3%) | 31 083 (60.4%) |
| Age at therapy initiation, years† | 51/63/74 | 49/61/73 | 52/64/76 | 53/65/76 | 52/64/74 | 47/59/71 | 51/62/72 | 53/65/77 |
| BMI, kg/m2, n (%): | ||||||||
| Underweight | 1010 (2.4%) | 10 935 (1.8%) | 638 (2.2%) | 939 (2.6%) | 555 (2.7%) | 1357 (1.8%) | 234 (1.7%) | 1249 (2.4%) |
| Normal range | 3974 (9.2%) | 51 694 (8.9%) | 2537 (8.8%) | 3507 (9.4%) | 2301 (11.2%) | 6107 (8.2%) | 1086 (7.9%) | 4727 (9.2%) |
| Overweight/preobese | 5412 (12.6%) | 75 168 (12.9%) | 3448 (11.9%) | 4521 (12.2%) | 3176 (15.4%) | 8726 (11.7%) | 1665 (12.1%) | 5966 (11.6%) |
| Obese (class I–II) | 4927 (11.5%) | 63 985 (11.0%) | 2987 (10.4%) | 3692 (9.9%) | 2822 (13.7%) | 8119 (10.9%) | 1318 (9.5%) | 4776 (9.3%) |
| Obese (class III) | 724 (1.7%) | 9199 (1.6%) | 468 (1.6%) | 553 (1.5%) | 423 (2.1%) | 1429 (1.9%) | 150 (1.1%) | 753 (1.5%) |
| Missing/not recorded | 26 873 (62.6%) | 371 836 (63.8%) | 18 772 (65.1%) | 23 949 (64.4%) | 11 291 (54.9%) | 49 062 (65.6%) | 9364 (67.7%) | 33 966 (66.0%) |
| Smoking, n (%): | ||||||||
| Never | 19 513 (45.5%) | 262 022 (45.0%) | 12 929 (44.8%) | 15 930 (42.9%) | 8553 (41.6%) | 34 309 (45.9%) | 6277 (45.4%) | 23 055 (44.8%) |
| Former | 12 893 (30.0%) | 162 763 (27.9%) | 8094 (28.1%) | 11 275 (30.3%) | 6707 (32.6%) | 19 630 (26.2%) | 3633 (26.3%) | 14 780 (28.7%) |
| Current | 10 190 (23.7%) | 152 764 (26.2%) | 7354 (25.5%) | 9544 (25.7%) | 5239 (25.5%) | 20 313 (27.2%) | 3782 (27.4%) | 12 741 (24.8%) |
| Missing/not recorded | 324 (0.8%) | 5268 (0.9%) | 473 (1.6%) | 412 (1.1%) | 69 (0.3%) | 548 (0.7%) | 125 (0.9%) | 861 (1.7%) |
| SBP, mm Hg, n (%): | ||||||||
| Normal | 4316 (10.1%) | 54623 (9.4%) | 2750 (9.5%) | 3544 (12.3%) | 2212 (10.8%) | 7110 (9.5%) | 937 (6.8%) | 4667 (9.1%) |
| Normal high | 12 177 (28.4%) | 157 921 (27.1%) | 7726 (26.8%) | 9817 (34.0%) | 6504 (31.6%) | 19 519 (26.1%) | 3204 (23.2%) | 12 536 (24.4%) |
| Hypertension | 12 471 (29.1%) | 170 779 (29.3%) | 8990 (31.2%) | 11 125 (38.6%) | 6010 (29.2%) | 21 197 (28.3%) | 4119 (29.8%) | 16 056 (31.2%) |
| Missing/not recorded | 13 956 (32.5%) | 199 494 (34.2%) | 9384 (32.5%) | 12 675 (43.9%) | 5842 (28.4%) | 26 974 (36.1%) | 5557 (40.2%) | 18 178 (35.3%) |
| GP contacts (year prior)† | 4/8/15 | 3/7/12 | 4/8/15 | 4/8/14 | 4/9/16 | 3/7/12 | 3/7/12 | 4/8/15 |
| Ethnicity, n (%) | ||||||||
| Non-white | 1400 (3.3%) | 19 092 (3.3%) | 693 (2.4%) | 1237 (3.3%) | 463 (2.3%) | 2344 (3.1%) | 219 (1.6%) | 1167 (2.3%) |
| Unknown | 4035 (9.4%) | 53 199 (9.1%) | 2682 (9.3%) | 3717 (10.0%) | 1746 (8.4%) | 7053 (9.4%) | 1284 (9.3%) | 5440 (10.6%) |
| White | 33 584 (78.2%) | 448 294 (76.9%) | 23 070 (80.0%) | 28 690 (77.2%) | 16 463 (80.1%) | 57 588 (77.0%) | 10 894 (78.8%) | 40 802 (79.3%) |
| Missing/not recorded | 3901 (9.1%) | 62 232 (10.7%) | 2405 (8.3%) | 3517 (9.5%) | 1896 (9.2%) | 7815 (10.5%) | 1420 (10.3%) | 4028 (7.8%) |
| Diabetes, n (%) | 4759 (11.1%) | 58 866 (10.1%) | 3262 (11.3%) | 4255 (11.4%) | 2369 (11.5%) | 6842 (9.1%) | 1069 (7.7%) | 5695 (11.1%) |
| TC, mmol/L, n (%) | ||||||||
| Desirable | 12 290 (28.6%) | 160 601 (27.6%) | 7725 (26.8%) | 10 232 (27.5%) | 7069 (34.4%) | 17 961 (24.0%) | 2881 (20.9%) | 12 460 (24.2%) |
| Borderline high | 2322 (5.4%) | 28 102 (4.8%) | 1419 (4.9%) | 1684 (4.5%) | 1108 (53.9%) | 3671 (4.9%) | 601 (4.3%) | 2306 (45.0%) |
| High | 908 (2.1%) | 10 722 (1.8%) | 533 (1.8%) | 619 (1.7%) | 408 (2.0%) | 1438 (1.9%) | 236 (1.7%) | 943 (1.8%) |
| Missing/not recorded | 27 400 (63.8%) | 383 392 (65.8%) | 19 173 (66.5%) | 24 626 (66.3%) | 11 983 (58.3%) | 51 730 (69.2%) | 10 099 (73.1%) | 35 728 (69.5%) |
| Lipid-lowering prescriptions (count year prior)†‡ | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/4 | 0/0/0 | 0/0/0 | 0/0/0 |
| Antihypertensive prescriptions (count year prior)†‡ | 0/0/7 | 0/0/7 | 0/0/7 | 0/0/7 | 0/0/7 | 0/0/6 | 0/0/6 | 0/0/7 |
| Antiplatelet prescriptions (count year prior)†‡ | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Antibiotic prescriptions (count year prior)†‡ | 1/2/3 | 1/1/2 | 1/2/3 | 1/2/3 | 1/2/3 | 1/1/2 | 1/1/3 | 1/2/3 |
*Forty-six antibiotics (10 most frequently used : penicillin-V, flucloxacillin, cefradine, azithromycin, tetracycline, ampicillin, cefadroxil, ofloxacin, levofloxacin, nitrofurantoin).
†Lower quartile/median/upper quartile.
‡Inclusive of index date.
BMI: <20 kg/m2—underweight, 20–24 kg/m2—normal, 25–29 kg/m2—overweight/preobese, 30–39 kg/m2—obese (class I—II), >40 kg/m2—obese (class III).
SBP: <120 mm Hg—normal, 120–139 mm Hg—normal high, >140 kg/m2—hypertension.
TC: <5.2 mmol/L—desirable, 5.2–6.1 mmol/L—borderline high, >6.1 mmol/L—high.
BMI, body mass index; CVD, cardiovascular disease; GP, general practitioner; LRTI, lower respiratory tract infection; SBP, systolic blood pressure; TC, total cholesterol.
Table 2.
Baseline characteristics: first-line antibiotic monotherapies prescribed for URTI
| Clarithromycin | Amoxicillin | Cefalexin | Co-amoxiclav | Doxycycline | Erythromycin | Penicillin-V | Other* | |
|---|---|---|---|---|---|---|---|---|
| Antibiotic monotherapies (all; mortality analysis), n | 20 303 | 380 258 | 23 581 | 25 823 | 72 226 | 66 607 | 121 238 | 41 650 |
| All patients, n | 17 818 | 280 614 | 20 251 | 22 862 | 56 392 | 53 510 | 103 580 | 36 916 |
| CVD-eligible therapies, n (%) | 18 657 (91.9%) | 345 636 (90.9%) | 21 122 (89.6%) | 23 874 (92.5%) | 67 649 (93.7%) | 61.854 (92.9%) | 115 936 (95.6%) | 37 270 (89.5%) |
| Arrhythmia-eligible therapies, n (%) | 17 600 (86.7%) | 335 160 (88.1%) | 20 618 (87.4%) | 22 852 (88.5%) | 63 806 (88.3%) | 59 401 (89.1%) | 111 211 (91.7%) | 36 294 (87.1%) |
| Women, n (%) | 13 958 (68.7%) | 244 927 (64.4%) | 16 693 (70.8%) | 16 379 (63.4%) | 50 164 (69.5%) | 46 352 (69.6%) | 76 028 (62.7%) | 28 531 (68.5%) |
| Age at therapy initiation, years† | 43/53/64 | 43/53/65 | 43/54/65 | 43/52/62 | 43/52/61 | 41/50/61 | 38/43/54 | 44/55/66 |
| BMI, kg/m2, n (%) | ||||||||
| Underweight | 293 (1.4%) | 4846 (1.3%) | 356 (1.5%) | 268 (1.1%) | 795 (1.2%) | 774 (1.2%) | 1028 (0.8%) | 584 (1.4%) |
| Normal range | 1851 (9.1%) | 32 753 (8.6%) | 2102 (8.9%) | 2022 (7.8%) | 6121 (7.8%) | 5190 (7.8%) | 8657 (7.1%) | 3637 (9.7%) |
| Overweight/preobese | 2448 (12.1%) | 45 419 (11.9%) | 2763 (11.7%) | 2801 (10.8%) | 7912 (10.7%) | 71 386 (10.7%) | 11 428 (9.4%) | 4807 (11.5%) |
| Obese (class I–II) | 2146 (10.6%) | 36 993 (9.7%) | 2163 (9.2%) | 2302 (8.9%) | 6342 (9.9%) | 6612 (9.9%) | 10 295 (8.5%) | 3869 (9.3%) |
| Obese (class III) | 349 (1.7%) | 5388 (1.4%) | 341 (1.4%) | 328 (1.3%) | 910 (1.7%) | 1133 (1.7%) | 1739 (1.4%) | 546 (1.3%) |
| Missing/not recorded | 13 216 (65.1%) | 254 859 (67.1%) | 15 856 (67.2%) | 18 102 (70.1) | 50 146 (68.7%) | 45 762 (68.7%) | 88 091 (72.7%) | 28 207 (67.7%) |
| Smoking, n (%): | ||||||||
| Never | 11 500 (56.7%) | 213 405 (56.1%) | 13 350 (56.6%) | 14 383 (55.7%) | 41 466 (57.4%) | 37 966 (57.0%) | 70 185 (57.9%) | 23 761 (57%) |
| Former | 4838 (23.8%) | 87 834 (23.1%) | 5332 (22.6%) | 5750 (22.3%) | 16 129 (22.3%) | 14 143 (21.2%) | 24 383 (20.1%) | 9230 (22.2%) |
| Current | 3889 (19.2%) | 77 209 (20.3%) | 4783 (20.3%) | 5548 (21.5%) | 14 365 (19.9%) | 14 209 (21.3%) | 25 886 (21.4%) | 8432 (20.2%) |
| Missing/not recorded | 76 (0.3%) | 1810 (0.5%) | 116 (0.5%) | 142 (0.5%) | 266 (0.4%) | 289 (0.4%) | 784 (0.6%) | 227 (0.5%) |
| SBP, mm Hg, n (%): | ||||||||
| Normal | 2400 (11.8%) | 41 534 (10.9%) | 2664 (11.3%) | 2762 (10.7%) | 7859 (10.9%) | 7481 (11.2%) | 14 592 (12.0%) | 4414 (10.6%) |
| Normal high | 5729 (28.2%) | 100 788 (26.5%) | 6198 (26.3%) | 6730 (26.1%) | 18 878 (26.1%) | 17 124 (25.7%) | 27 673 (22.8%) | 10 388 (24.9%) |
| Hypertension | 4418 (21.8%) | 89 787 (23.6%) | 5788 (24.5%) | 5506 (21.3%) | 15 461 (21.4%) | 14 297 (21.5%) | 18 593 (15.3%) | 10 494 (25.2%) |
| Missing/not recorded | 7756 (38.2%) | 148 149 (39.0%) | 8931 (37.9%) | 10 825 (41.9%) | 30 028 (41.6%) | 27 705 (41.6%) | 60 380 (49.8%) | 16 354 (39.3%) |
| GP contacts (year prior)† | 3/7/12 | 2/6/10 | 3/6/11 | 3/6/10 | 3/6/11 | 3/6/10 | 2/4/8 | 3/6/12 |
| Ethnicity, n (%) | ||||||||
| Non-white | 864 (4.2%) | 19 236 (5.1%) | 903 (3.8%) | 1083 (4.2%) | 2012 (2.8%) | 3262 (4.9%) | 6607 (5.0%) | 1499 (3.6%) |
| Unknown | 1831 (1.0%) | 33 981 (8.9%) | 2066 (8.8%) | 2435 (9.4%) | 7165 (9.9%) | 5967 (8.9%) | 11 548 (9.9%) | 3855 (9.3%) |
| White | 15 058 (74.2%) | 275 639 (72.5%) | 17 664 (74.9%) | 18 426 (71.4%) | 52 231 (72.3%) | 48 594 (73.0%) | 82 936 (68.4%) | 31 455 (75.5%) |
| Missing/not recorded | 2550 (12.6%) | 51 402 (13.5%) | 2948 (12.5%) | 3879 (15%) | 10 818 (15%) | 8784 (13.2%) | 20 287 (16.7%) | 4841 (11.6%) |
| Diabetes, n (%) | 1520 (7.5%) | 27 603 (7.3%) | 1677 (7.1%) | 1708 (6.6%) | 3985 (5.5%) | 4234 (6.4%) | 5681 (4.7%) | 3071 (7.4%) |
| TC, mmol/L, n (%): | ||||||||
| Desirable | 4669 (23.0%) | 84 779 (22.3%) | 5000 (21.2%) | 5215 (20.2%) | 13 842 (19.2%) | 12 507 (18.8%) | 17 136 (14.1%) | 8060 (19.4%) |
| Borderline high | 1069 (5.3%) | 18 072 (4.8%) | 1086 (4.6%) | 1144 (4.4%) | 3492 (4.8%) | 3106 (4.7%) | 3840 (3.2%) | 1914 (4.6%) |
| High | 451 (2.2%) | 6805 (1.8%) | 450 (1.9%) | 415 (1.6%) | 1355 (1.9%) | 1172 (1.8%) | 1459 (1.2%) | 736 (1.8%) |
| Missing/not recorded | 14 114 (69.5%) | 270 602 (71.2%) | 17 045 (72.3%) | 19 049 (73.8%) | 53 537 (74.1%) | 49 822 (74.8%) | 98 803 (81.5%) | 30 940 (74.3%) |
| Lipid-lowering prescriptions (count year prior)†‡ | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Antihypertensive prescriptions (count year prior)†‡ | 0/0/2 | 0/0/2 | 0/0/3 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/3 |
| Antiplatelet prescriptions (count year prior)†‡ | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Antibiotic prescriptions (count year prior)†‡ | 1/2/3 | 1/1/2 | 1/2/3 | 1/1/2 | 1/1/2 | 1/1/2 | 1/1/2 | 1/2/3 |
*Thirty-one antibiotics (10 most frequently used: ciprofloxacin, flucloxacillin, tetracycline, cefradine, cefadroxil, azithromycin, metronidazole, nitrofurantoin, ampicillin, co-fluampicil).
†Lower quartile/median/upper quartile.
‡Inclusive of index date.
BMI: <20 kg/m2—underweight, 20–24 kg/m2—normal, 25–29 kg/m2—overweight/preobese, 30–39 kg/m2—obese (class I–II), >40 kg/m2—obese (class III).
SBP: <120 mm Hg—normal, 120–139 mm Hg—normal high, >140 kg/m2—hypertension.
TC: <5.2 mmol/L—desirable, 5.2–6.1 mmol/L—borderline high, >6.1 mmol/L—high.
BMI, body mass index; CVD, cardiovascular disease; GP, general practitioner; URTI, upper respiratory tract infection; SBP, systolic blood pressure; TC, total cholesterol.
Patient involvement
No patients were involved in the development of the research question, in the design or conduct of this study, or in the development of outcome measures. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Patients and cases
At the end of 2012, CPRD contained records of 58 450 685 antibiotic prescriptions issued to patients with research quality records. From these, 1 604 056 first-line monotherapy episodes were identified, 852 370 (53%) of which were associated with LRTI and 751 686 (47%) with URTI (47%; figure 1). All of these therapies were eligible for inclusion in the all-cause mortality analysis. Selecting only those patients with no prior history of a CV event at treatment initiation, there were 1 392 687 eligible cases: 700 689 (50%) for LRTI and 691 998 (50%) for URTI; these therapies were therefore eligible for inclusion in the analysis of CV outcome. Patients had no prior history of arrhythmia at the initiation of 1 387 584 therapies, 720 642 (52%) for LRTI and 666 942 (48%) for URTI, thus eligible for inclusion in the analysis of arrhythmia.
Baseline characteristics
The overall demographics, biochemical test results, patterns of baseline comorbidity and numbers of prior therapies were generally similar between the antibiotics prescribed for LRTI and URTI, as detailed in tables 1 and 2. There were more women with URTIs (65.6% vs 56.3%), while patients with LRTIs were older (61.8 vs 53.3 years).
Antibiotic treatment
Within the LRTI group, the most commonly prescribed antibiotic was amoxicillin (68%), followed by erythromycin (9%), clarithromycin (5%), co-amoxiclav (4%), cefalexin (3%), doxycycline (2%) and oxytetracycline (2%). The most frequently prescribed antibiotic in the URTI group was amoxicillin (51%), followed by penicillin-V (16%), doxycycline (10%), erythromycin (9%), co-amoxiclav (3%), cefalexin (3%) and clarithromycin (3%). Thus, the most commonly prescribed antibiotics were the same for both respiratory infection categories, with the exception of oxytetracycline for LRTI and penicillin-V for URTI (almost exclusively for tonsillitis/pharyngitis). The eight most frequently prescribed antibiotics overall include the most commonly used antibiotics for both LRTIs and URTIs.
CV event risk
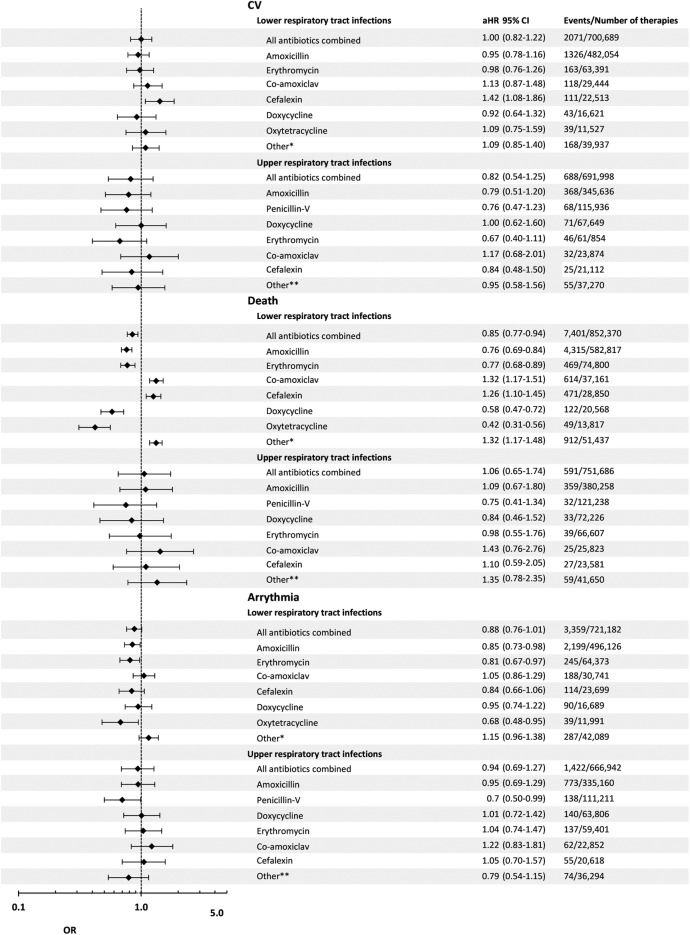
Within the LRTI group, the unadjusted first-ever CV event rates within 14 and 37 days of treatment initiation were 15.7 and 29.6 per 10 000 therapies, respectively (table 3). The adjusted 37-day risk of first-ever CV events was the same in clarithromycin therapies as in all other antibiotics combined (OR=1.00; 95% CI 0.82 to 1.22). When analysing each of the most frequently used antibiotics for LRTI separately versus clarithromycin, the 37-day adjusted risk of CV event was highest in cefalexin therapies (1.42; 1.08 to 1.86) and lowest in doxycycline therapies (0.92; 0.64 to 1.32; table 4; figure 2).
Table 3.
Crude event rates per 10 000 therapies for 14-day and 37-day all-cause mortality and CV and arrhythmia events, by first-line antibiotic monotherapy and by indication
| 14-day analysis |
37-day analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CV |
Death |
Arrhythmia |
CV |
Death |
Arrhythmia |
|||||||
| Events | Crude rate | Events | Crude rate | Events | Crude rate | Events | Crude rate | Events | Crude rate | Events | Crude rate | |
| Lower respiratory tract infection | ||||||||||||
| Overall | 1102 | 15.7 | 3690 | 43.2 | 1393 | 19.3 | 2071 | 29.6 | 7401 | 86.8 | 3359 | 49.3 |
| Amoxicillin | 686 | 14.2 | 2177 | 37.3 | 948 | 19.1 | 1326 | 27.5 | 4315 | 74.0 | 2199 | 44.3 |
| Erythromycin | 92 | 14.5 | 251 | 33.5 | 97 | 15.0 | 163 | 25.7 | 469 | 62.7 | 245 | 38.1 |
| Clarithromycin | 58 | 16.4 | 225 | 52.2 | 75 | 21.1 | 103 | 29.3 | 449 | 104.6 | 197 | 55.5 |
| Co-amoxiclav | 67 | 22.7 | 302 | 81.1 | 65 | 21.1 | 118 | 40.1 | 614 | 165.2 | 188 | 61.2 |
| Cefalexin | 71 | 31.5 | 251 | 86.9 | 48 | 20.2 | 111 | 49.3 | 471 | 163.3 | 114 | 48.1 |
| Doxycycline | 25 | 15.0 | 47 | 22.7 | 37 | 22.1 | 43 | 25.9 | 122 | 59.3 | 90 | 53.9 |
| Oxytetracycline | 16 | 13.9 | 17 | 12.3 | 16 | 13.3 | 39 | 33.8 | 49 | 35.5 | 39 | 32.5 |
| Other* | 87 | 21.8 | 420 | 81.6 | 107 | 25.4 | 168 | 42.1 | 912 | 177.3 | 287 | 55.1 |
| Upper respiratory tract infections | ||||||||||||
| Overall | 350 | 5.0 | 203 | 2.7 | 555 | 8.3 | 688 | 9.9 | 591 | 7.9 | 1422 | 21.3 |
| Amoxicillin | 187 | 5.4 | 122 | 3.2 | 316 | 9.4 | 368 | 10.6 | 359 | 9.4 | 773 | 23.1 |
| Penicillin-V | 40 | 10.7 | 15 | 1.2 | 45 | 4.0 | 68 | 5.9 | 32 | 2.6 | 138 | 12.4 |
| Doxycycline | 33 | 4.9 | 10 | 1.4 | 54 | 8.4 | 71 | 10.5 | 33 | 4.6 | 140 | 21.9 |
| Erythromycin | 25 | 4.0 | 15 | 2.2 | 51 | 8.6 | 46 | 7.4 | 39 | 5.9 | 137 | 23.1 |
| Co-amoxiclav | 14 | 5.9 | 6 | 2.3 | 23 | 10.0 | 32 | 13.4 | 25 | 9.7 | 62 | 27.1 |
| Cefalexin | 12 | 5.7 | 8 | 3.4 | 25 | 12.1 | 25 | 10.6 | 27 | 11.4 | 55 | 23.3 |
| Clarithromycin | 10 | 5.3 | 5 | 2.5 | 12 | 6.8 | 23 | 11.3 | 17 | 8.4 | 43 | 24.4 |
| Other† | 29 | 2.5 | 22 | 5.3 | 29 | 8.0 | 55 | 14.8 | 59 | 14.2 | 74 | 20.4 |
| Upper and lower respiratory tract infections combined | ||||||||||||
| Overall | 1452 | 10.4 | 3893 | 24.2 | 1948 | 14.0 | 2759 | 19.8 | 7992 | 49.8 | 4781 | 34.4 |
| Amoxicillin | 873 | 10.5 | 2299 | 23.8 | 87 | 16.3 | 1694 | 20.5 | 4674 | 48.5 | 2972 | 35.8 |
| Erythromycin | 117 | 9.3 | 266 | 18.8 | 148 | 11.9 | 209 | 16.7 | 508 | 35.9 | 382 | 30.9 |
| Penicillin-V | 43 | 3.6 | 22 | 1.8 | 53 | 4.6 | 73 | 6.1 | 52 | 4.2 | 148 | 13.0 |
| Doxycycline | 58 | 6.9 | 57 | 6.1 | 91 | 11.3 | 114 | 13.5 | 155 | 16.7 | 230 | 28.6 |
| Co-amoxiclav | 81 | 15.2 | 308 | 48.8 | 81 | 15.2 | 150 | 28.1 | 639 | 101.5 | 250 | 46.6 |
| Clarithromycin | 68 | 12.6 | 230 | 36.2 | 68 | 12.6 | 126 | 23.4 | 466 | 73.7 | 240 | 45.2 |
| Cefalexin | 83 | 19.0 | 259 | 49.4 | 83 | 19.0 | 136 | 25.4 | 498 | 95.0 | 169 | 38.1 |
| Oxytetracycline | 25 | 11.4 | 18 | 7.1 | 25 | 11.4 | 53 | 24.2 | 53 | 21.0 | 55 | 24.8 |
| Other‡ | 104 | 16.2 | 434 | 55.3 | 104 | 16.2 | 204 | 31.9 | 947 | 120.7 | 335 | 51.3 |
*Forty-six antibiotics (10 most frequently used: penicillin-V, flucloxacillin, cefradine, azithromycin, tetracycline, ampicillin, cefadroxil, ofloxacin, levofloxacin, nitrofurantoin).
†Thirty-one antibiotics (10 most frequently used: ciprofloxacin, flucloxacillin, tetracycline, cefradine, cefadroxil, azithromycin, metronidazole, nitrofurantoin, ampicillin, co-fluampicil).
‡Forty-nine antibiotics (10 most frequently used: trimethroprim, cefaclor, ciprofloxacin, flucloxacillin, cefradine, tetracycline, azithromycin, ampicillin, cefadroxil, nitrofurantoin, metronidazole).
CV, cardiovascular.
Table 4.
Adjusted ORs versus clarithromycin for 14-day and 37-day all-cause mortality and CV and arrhythmia events, by first-line antibiotic monotherapy and by indication
| 14-day OR (95% CI) |
37-day OR (95% CI) |
|||||
|---|---|---|---|---|---|---|
| CV events | All-cause mortality | Arrhythmia events | CV events | All-cause mortality | Arrhythmia events | |
| Lower respiratory tract infection | ||||||
| All antibiotics combined | 0.93 (0.71 to 1.21) | 0.83 (0.72 to 0.95) | 1.01 (0.80 to 1.27) | 1.00 (0.82 to 1.22) | 0.85 (0.77 to 0.94) | 0.88 (0.76 to 1.01) |
| Amoxicillin | 0.86 (0.66 to 1.13) | 0.75 (0.65 to 0.86) | 1.01 (0.80 to 1.28) | 0.95 (0.78 to 1.16) | 0.76 (0.69 to 0.84) | 0.85 (0.73 to 0.98) |
| Erythromycin | 0.98 (0.71 to 1.37) | 0.82 (0.69 to 0.99) | 0.87 (0.64 to 1.18) | 0.98 (0.76 to 1.26) | 0.77 (0.68 to 0.89) | 0.81 (0.67 to 0.97) |
| Co-amoxiclav | 1.17 (0.80 to 1.62) | 1.28 (1.07 to 1.53) | 0.98 (0.70 to 1.36) | 1.13 (0.87 to 1.48) | 1.32 (1.17 to 1.51) | 1.05 (0.86 to 1.29) |
| Cefalexin | 1.60 (1.26 to 2.27) | 1.28 (1.06 to 1.55) | 0.96 (0.66 to 1.38) | 1.42 (1.08 to 1.86) | 1.26 (1.10 to 1.45) | 0.84 (0.66 to 1.06) |
| Doxycycline | 0.96 (0.60 to 1.54) | 0.46 (0.33 to 0.64) | 0.99 (0.67 to 1.47) | 0.92 (0.64 to 1.32) | 0.58 (0.47 to 0.72) | 0.95 (0.74 to 1.22) |
| Oxytetracycline | 0.80 (0.46 to 1.39) | 0.29 (0.17 to 0.47) | 0.76 (0.44 to 1.31) | 1.09 (0.75 to 1.59) | 0.42 (0.31 to 0.56) | 0.68 (0.48 to 0.95) |
| Other* | 1.00 (0.71 to 1.39) | 1.16 (0.98 to 1.37) | 1.15 (0.85 to 1.55) | 1.09 (0.85 to 1.40) | 1.32 (1.17 to 1.48) | 1.15 (0.96 to 1.38) |
| Upper respiratory tract infection | ||||||
| All antibiotics combined | 0.99 (0.53 to 1.86) | 1.21 (0.50 to 2.98) | 1.39 (0.80 to 2.41) | 0.82 (0.54 to 1.25) | 1.06 (0.65 to 1.74) | 0.94 (0.69 to 1.27) |
| Amoxicillin | 0.94 (0.50 to 1.80) | 0.95 (0.35 to 2.63) | 1.44 (0.81 to 2.57) | 0.79 (0.51 to 1.20) | 1.09 (0.67 to 1.80) | 0.95 (0.69 to 1.29) |
| Penicillin-V | 1.03 (0.51 to 2.08) | 1.19 (0.38 to 3.71) | 0.85 (0.45 to 1.61) | 0.76 (0.47 to 1.23) | 0.75 (0.41 to 1.34) | 0.70 (0.50 to 0.99) |
| Doxycycline | 1.07 (0.53 to 2.18) | 0.67 (0.20 to 2.33) | 1.43 (0.76 to 2.67) | 1.00 (0.62 to 1.60) | 0.84 (0.46 to 1.52) | 1.01 (0.72 to 1.42) |
| Erythromycin | 0.85 (0.41 to 1.79) | 1.16 (0.37 to 3.65) | 1.42 (0.76 to 2.68) | 0.67 (0.40 to 1.11) | 0.98 (0.55 to 1.76) | 1.04 (0.74 to 1.47) |
| Co-amoxiclav | 1.19 (0.53 to 2.18) | 0.88 (0.22 to 3.57) | 1.68 (0.83 to 3.38) | 1.17 (0.68 to 2.01) | 1. 43 (0.76 to 2.67) | 1.22 (0.83 to 1.81) |
| Cefalexin | 0.96 (0.41 to 2.24) | 0.57 (0.27 to 2.57) | 1.73 (0.86 to 3.45) | 0.84 (0.48 to 1.50) | 1.10 (0.59 to 2.05) | 1.05 (0.70 to 1.57) |
| Other† | 1.21 (0.58 to 2.49) | 1.17 (0.37 to 3.70) | 1.13 (0.57 to 2.22) | 0.95 (0.58 to 1.56) | 1.35 (0.78 to 2.35) | 0.79 (0.54 to 1.15) |
| Lower and upper respiratory tract infections combined | ||||||
| All antibiotics combined | 0.90 (0.71 to 1.15) | 0.78 (0.68 to 0.89) | 1.01 (0.82 to 1.3) | 0.93 (0.78 to 1.11) | 0.80 (0.73 to 0.89) | 0.86 (0.76 to 0.98) |
| Amoxicillin | 0.86 (0.67 to 1.10) | 0.72 (0.63 to 0.83) | 1.05 (0.84 to 1.30) | 0.90 (0.75 to 1.08) | 0.74 (0.67 to 0.82) | 0.85 (0.75 to 0.98) |
| Erythromycin | 0.92 (0.68 to 1.24) | 0.78 (0.65 to 0.96) | 0.93 (0.72 to 1.22) | 0.88 (0.71 to 1.01) | 0.73 (0.65 to 0.84) | 0.84 (0.72 to 0.99) |
| Penicillin-V | 0.79 (0.53 to 1.16) | 0.25 (0.16 to 0.40) | 0.60 (0.42 to 0.75) | 0.71 (0.53 to 0.95) | 0.29 (0.21 to 0.39) | 0.57 (0.47 to 0.71) |
| Doxycycline | 0.86 (0.61 to 1.23) | 0.34 (0.26 to 0.46) | 0.95 (0.70 to 1.27) | 0.90 (0.70 to 1.17) | 0.45 (0.37 to 0.54) | 0.86 (0.72 to 1.03) |
| Co-amoxiclav | 1.13 (0.82 to 1.57) | 1.26 (1.06 to 1.50) | 1.07 (0.79 to 1.44) | 1.13 (0.89 to 1.44) | 1.32 (1.16 to 1.49) | 1.08 (0.90 to 1.29) |
| Cefalexin | 1.41 (1.02 to 1.95) | 1.18 (0.98 to 1.42) | 1.06 (0.78 to 1.45) | 1.25 (0.98 to 1.59) | 1.17 (1.02 to 1.33) | 0.87 (0.71 to 1.06) |
| Oxytetracycline | 0.88 (0.55 to 1.40) | 0.26 (0.17 to 0.42) | 0.76 (0.48 to 1.21) | 1.00 (0.72 to 1.38) | 0.37 (0.28 to 0.50) | 0.63 (0.47 to 0.84) |
| Other‡ | 1.01 (0.75 to 1.38) | 1.15 (0.97 to 1.35) | 1.08 (0.82 to 0.42) | 1.07 (0.86 to 1.34) | 1.28 (1.14 to 1.44) | 1.07 (0.90 to 1.26) |
*Forty-six antibiotics (10 most frequently used: penicillin-V, flucloxacillin, cefradine, azithromycin, tetracycline, ampicillin, cefadroxil, ofloxacin, levofloxacin, nitrofurantoin).
†Thirty-one antibiotics (10 most frequently used: ciprofloxacin, flucloxacillin, tetracycline, cefradine, cefadroxil, azithromycin, metronidazole, nitrofurantoin, ampicillin, co-fluampicil).
‡Forty-nine antibiotics (10 most frequently used: trimethroprim, cefaclor, ciprofloxacin, flucloxacillin, cefradine, tetracycline, azithromycin, ampicillin, cefadroxil, nitrofurantoin, metronidazole).
CV, cardiovascular.
Figure 2.
Forest plot of the adjusted ORs (with 95% CIs) for 37-day all-cause mortality and CV and arrhythmia events, by first-line antibiotic monotherapy and by indication. The reference category is treatment with clarithromycin. CV, cardiovascular.
Within the URTI group, the unadjusted first-ever CV event rates within 14 and 37 days of antibiotic initiation were 5.0 and 9.9 CV events per 10 000 therapies (table 3). The adjusted 37-day risk of a CV event was lower in all other antibiotics combined than in clarithromycin therapies (0.82; 0.54 to 1.25). When analysing each of the most frequently used antibiotics for URTI separately versus clarithromycin, the adjusted 37-day risk of a CV event was highest in co-amoxiclav therapies (1.17; 0.68 to 2.01) and lowest in erythromycin therapies (0.67; 0.40 to 1.11).
Within the LRTI and URTI combined group, the adjusted rates were 10.4 and 19.8 CV events per 10 000 therapies in the 14 and 37 days following initiation, respectively (table 3). The adjusted 37-day risk of a CV event was lower in all other antibiotics combined than in clarithromycin therapies (0.93; 0.78 to 1.11). When analysing each of the most frequently used antibiotics for URTI separately versus clarithromycin, the 37-day risk of CV risk was highest in cefalexin therapies (1.25; 0.98 to 1.59) and lowest in penicillin-V therapies (0.71; 0.53 to 0.95).
The 14-day risk of CV events in the LRTI group was comparable between all other antibiotics combined and clarithromycin (0.93; 0.71 to 1.21). In the URTI group, there was no difference (0.99; 0.53 to 1.86; table 4).
All-cause mortality risk
Within the LRTI group, unadjusted all-cause mortality rates of 43.2 and 86.8 deaths per 10 000 therapies were found in the 14 and 37 days following initiation, respectively (table 3). The adjusted 37-day risk of all-cause mortality was lower in all antibiotics combined than in clarithromycin therapies (0.85; 0.77 to 0.94). When analysing each of the most frequently used antibiotics for LRTI separately versus clarithromycin, the adjusted 37-day all-cause mortality risk was highest in co-amoxiclav therapies (1.32; 1.17 to 1.51) and lowest in oxytetracycline therapies (0.42; 0.31 to 0.56; table 4; figure 2).
In the URTI group, there were unadjusted rates of 2.7 and 7.9 deaths per 10 000 therapies, in the 14 and 37 days following initiation, respectively (table 3). The adjusted 37-day risk of all-cause mortality was higher in all other antibiotics combined than in clarithromycin therapies (1.06; 0.65 to 1.74). When analysing each of the most frequently used antibiotics for URTI separately versus clarithromycin, the adjusted 37-day all-cause mortality risk was highest in co-amoxiclav therapies (1.43; 0.76 to 2.67) and lowest in penicillin-V therapies (0.75; 0.41 to 1.34).
In the LRTI and URTI combined group, there were corresponding unadjusted rates of 24.2 and 49.8 deaths per 10 000 therapies, respectively. The adjusted 37-day risk of all-cause mortality was higher in all other antibiotics combined than in clarithromycin therapies (0.80; 0.73 to 0.89). When analysing each of the most frequently used antibiotics for LRTI and URTI combined versus clarithromycin, the 37-day all-cause mortality risk was highest in co-amoxiclav therapies (1.32; 1.16 to 1.49) and lowest in penicillin-V therapies (0.29; 0.21 to 0.39).
The 14-day OR for all-cause mortality in the LRTI group was similar to the 37-day result (0.83; 0.72 to 0.95, table 4); however, in the URTI analysis, the 14-day OR differed slightly from the 37-day result (1.21; 0.50 to 2.98). However, the CIs of the 14-day and 37-day ORs for all-cause mortality spanned 1.
Arrhythmia event risk
Within the LRTI group, there were unadjusted rates of 19.3 and 49.3 arrhythmia events per 10 000 therapies within 14 and 37 days of therapy initiation, respectively (table 3). The adjusted 37-day risk of first-ever arrhythmia was lower in all other antibiotics combined than in clarithromycin therapies (0.88; 0.76 to 1.01). When analysing each of the most frequently prescribed antibiotics for LRTI separately, the adjusted 37-day risk of arrhythmia was highest in co-amoxiclav therapies (1.05; 0.86 to 1.29) and lowest in oxytetracycline therapies (0.68; 0.48 to 0.95) compared with clarithromycin therapies (table 4; figure 2).
Within the URTI group, there were unadjusted rates of 8.3 and 21.3 events per 10 000 therapies within 14 and 37 days of therapy initiation, respectively (table 3). The adjusted 37-day risk of arrhythmia was lower in all other antibiotics combined than in clarithromycin therapies (0.94; 0.69 to 1.27). When analysing each of the most frequently prescribed antibiotics for URTI separately, the adjusted 37-day risk of arrhythmia was highest in co-amoxiclav therapies (1.22; 0.83 to 1.81) and lowest in penicillin-V therapies (0.70; 0.50 to 0.99) compared with clarithromycin.
Within the LRTI and URTI combined group, there were unadjusted rates of 14.0 and 34.4 arrhythmia events per 10 000 therapies, within the 14 and 37 days following therapy initiation (table 3). The adjusted 37-day risk of arrhythmia was lower in all other antibiotics combined than in clarithromycin therapies (0.86; 0.76 to 0.98). When analysing each of the most frequently prescribed antibiotics for URTI separately, the adjusted 37-day risk of arrhythmia was highest in co-amoxiclav therapies (1.08; 0.90 to 1.29) and lowest in penicillin-V therapies (0.57; 0.47 to 0.71) compared with clarithromycin.
The 14-day risk of arrhythmia in LRTI showed no difference between other antibiotics combined and clarithromycin therapies (1.01; 0.80 to 1.27). However, in the URTI group, the 14-day risk of arrhythmia was higher in all other antibiotics combined than in clarithromycin therapies (1.39; 0.80 to 2.41; table 4).
Interaction with statins not metabolised by cytochrome CYP3A4
In total, 13 176 therapies for LRTI and 7613 therapies for URTI were associated with prescriptions for statins not metabolised by cytochrome CYP3A4: rosuvastatin, pravastatin or fluvastatin. Among these, there were 80 deaths in the LRTI group and 9 deaths in the URTI group within 37 days of antibiotic initiation. In the statin-associated therapies for LRTI, there were also 23 first-ever CV events and 60 first-ever arrhythmia events within 37 days of antibiotic initiation; in those for URTI, there were 9 first-ever CV events and 24 first-ever arrhythmia events within 37 days of initiation.
Antibiotic therapies associated with rosuvastatin, pravastatin or fluvastatin included 801 clarithromycin therapies for LRTI and 255 clarithromycin therapies for URTI. Out of the 80 deaths in the statin-associated LRTI therapies, 6 were in therapies for clarithromycin. None of the nine deaths in the URTI therapies were in patients receiving clarithromycin therapies.
There were four CV events and one arrhythmia event in the statin-associated clarithromycin therapies for LRTI. There were no CV events or arrhythmia events in the statin-associated therapies for URTI.
A statistical model was not run due to the low number of events.
Discussion
This study was motivated by other studies concluding that there was increased CV risk associated with macrolide antibiotics, including clarithromycin. Here, we assessed the risk of a first-ever CV event, as well as that of all-cause mortality and first-ever cardiac arrhythmia associated with antibiotics prescribed for URTIs and LRTIs, using clarithromycin as the referent.
Our findings do not support the hypothesis that treatment of respiratory tract infections with clarithromycin increased the risk of CV events, cardiac arrhythmias or all-cause mortality. We found a large difference in antibiotic prescription patterns and event rates between patients treated for upper and lower respiratory infections. The majority of penicillin-V and doxycycline prescriptions were associated with URTI, whereas the majority of clarithromycin prescriptions were associated with LRTI, which dramatically increases CV event risks due to differential age and comorbidity. However, when the respiratory indication was not accounted for—as in the study by Svanström et al6—our findings also showed a significant difference between penicillin-V and clarithromycin, with a 37-day OR of 0.29 (95% CI 0.21 to 0.39) for penicillin-V versus clarithromycin for all-cause mortality. However, when upper and lower respiratory indications for penicillin-V and clarithromycin were investigated separately, a non-significant reduction in 37-day all-cause mortality was found in penicillin-V for URTI, with CIs spanning 1. Furthermore, pooled findings for doxycycline versus clarithromycin demonstrated a lower 37-day OR of 0.45 (95% CI 0.37 to 0.54) for all-cause mortality, but when split by indication the OR became 0.58 (0.47 to 0.72) for LRTI and 0.84 (0.46 to 1.52) for URTI.
For all-cause mortality, there were differences in odds in relation to the upper or lower location of the respiratory tract infection, but these patterns did not implicate clarithromycin specifically: some antibiotics were associated with a higher risk and others with a lower risk compared with clarithromycin. The risks of a first-ever CV event and arrhythmia with clarithromycin treatment were comparable with those observed with other antibiotics in patients with upper or lower respiratory infections. This was also the case where there was co-administration of statins not metabolised by cytochrome CYP3A4.
This study differed from previous studies in that it was more wide-ranging in the types of antibiotics evaluated. Other studies targeted specific antibiotics.16
Three outcomes were analysed here: all-cause mortality, first-ever CV event and first-ever arrhythmia. Macrolide antibiotics can inhibit the delayed potassium rectifier current in cardiac muscle cells and prolong the repolarisation phase of the cardiac cycle, manifesting as a lengthening of the QTc interval on an ECG17 18 and increasing the risk of tachyarrhythmia such as torsades des pointes.19 While extremely rare with oral administration of antibiotics at usual clinical doses and in people with healthy hearts, the risks of cardiac arrhythmia are potentially greater with administration of intravenous doses, co-administration with other inhibitors of CYP3A4, and in patients with heart disease, especially those with left ventricular dysfunction or inherited abnormalities of cardiac repolarisation (long QT syndrome). Despite this, our findings do not suggest an excess risk associated with clarithromycin therapy compared with other commonly prescribed antibiotics.
Following a recent publication evaluating various CV end points in clarithromycin and amoxicillin in the first 2 weeks of H. pylori eradication, we added the 14-day end point.13 Overall, we did not observe major differences between our primary end points at 14 days compared with 37 days.
Further, the relatively low risk of all-cause mortality associated with commonly used antibiotics in primary care suggests that any intrinsic risk of death and/or CV event and/or arrhythmic event associated with clarithromycin therapy is of minimal clinical importance at a population level where there is no prior history of CVD or arrhythmia. Any clinical impact from a possible proarrhythmic effect of clarithromycin appears to be offset by the treatment of the underlying infection, reducing the intensity and duration of inflammatory influences on the vessel wall and myocardium. In our previous study of antibiotic treatment failure in primary care, it was shown that the antibiotic failure rates in LRTIs were lowest for clarithromycin (19.2%) and amoxicillin (18.8%) in 2012. In order to prevent previous antibiotic interactions impacting on outcomes, we applied a minimum period of 90 days between antibiotic therapies. This meant that the majority of therapies considered treatment failures in the original study (switching to an alternative antibiotic within 30 days of treatment) were excluded from this analysis; therefore, antibiotics with a higher treatment failure rate were more likely to be excluded.
Since CPRD collates data from routine practice, there will inevitably be missing and erroneous data, coding imperfections and variation in medical practice. This is mitigated to some extent by selecting only research quality patient data from up-to-standard practices. A limitation of this study was the inability to stratify our analyses by severity of infection. However, since we selected only first-line monotherapies for analysis, it may be assumed that the associated infections were less severe due to the single course of antibiotics required.
There was no confirmation that the patients were compliant with the antibiotic prescription's dosage instructions and intended duration. Similarly, it is not known to what extent prescribers advised patients to delay starting the prescribed antibiotics.
We did not examine risk in patients ill enough to require therapy with multiple antibiotics in combination; nor did we examine patients whose antibiotic treatment was initiated in hospital. Macrolides are often used for indications other than respiratory tract infection, including skin and soft tissue infections, but patients treated for non-respiratory indications were not included in this analysis. These findings therefore apply to patients aged 35 years and older managed for respiratory tract infections in primary care. It would be of value to investigate patterns of antibiotic use and associated risk in people with pre-existing CV disease.
Conclusion
CV events were much more likely to be associated with LRTIs than with URTIs, irrespective of which antibiotic was prescribed. There was no evidence of increased risk of CV events in people without prior CV disease. Prescribers should take the individual patient's risk profile into consideration when prescribing an antibiotic; however, in this study of routine clinical data, no evidence was found that clarithromycin caused more cardiac events than other commonly used antibiotics.
Footnotes
Contributors: CJC and CAB developed the study protocol. Data extraction and analysis were carried out by BJ, EB and SJ-J, supervised by CJC. CCB advised on the interpretation of the study findings. CAB and MO provided statistical expertise. HdV, EB and SJ-J contributed to the writing of the manuscript. Owing to the conditions of the data license, co-authors from the funding body did not have access to the source CPRD data. However, they did have access to processed data. All other authors had full access to all of the data (including statistical reports and tables) in the study. All authors contributed to, read and approved the final manuscript, and all authors take responsibility for the integrity of the data and the accuracy of the data analysis. CJC is the guarantor, had final responsibility for the decision to submit for publication, and takes overall responsibility for all aspects of the study and this manuscript.
Funding: This study was supported by Abbott Healthcare Products (Mylan since February 2015), producers of branded clarithromycin in developed markets. This article has been reviewed for scientific content by the former Abbott (now Mylan) employees Mario Ouwens and Hans Friedrich Koch, and one employee HdV contributed to the writing.
Competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) CAB is a contractor of, EB, BJ and SJ-J are employed by, and CJC is a director of Pharmatelligence, a research consultancy receiving funding from Mylan for the submitted work; (2) HdV is employed by, and MO was employed at time of writing by Mylan. Mario Ouwens was an employee of Mylan at time of writing, however, is now an employee of Astra Zeneca.
Ethics approval: Studies using the CPRD are covered by ethics approval granted by the Trent Multicentre Research Ethics Committee (reference 05/MRE04/87). This study was granted CPRD Independent Scientific Advisory Committee approval on 26 March 2015, protocol number 15_012R2.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J 2008;29:96–103. 10.1093/eurheartj/ehm516 [DOI] [PubMed] [Google Scholar]
- 2.Smeeth L, Thomas SL, Hall AJ et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351:2611–18. 10.1056/NEJMoa041747 [DOI] [PubMed] [Google Scholar]
- 3.Corrales-Medina VF, Alvarez KN, Weissfeld LA et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015;313:264–74. 10.1001/jama.2014.18229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antzelevitch C, Sun ZQ, Zhang ZQ et al. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol 1996;28:1836–48. 10.1016/S0735-1097(96)00377-4 [DOI] [PubMed] [Google Scholar]
- 5.Joint Formulary Committee. British National Formulary (online). http://www.medicinescomplete.com (accessed 20 Aug 2015).
- 6.Svanström H, Pasternak B, Hviid A. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ 2014;349:g4930 10.1136/bmj.g4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie CJ, Berni E, Jenkins-Jones S et al. Antibiotic treatment failure in four common infections in UK primary care 1991–2012: longitudinal analysis. BMJ 2014;349:g5493 10.1136/bmj.g5493 [DOI] [PubMed] [Google Scholar]
- 8.Clinical Practice Research Datalink (CPRD). Clinical Practice Research Datalink. http://www.cprd.com/intro.asp (accessed 04/05/2016).
- 9.Gallagher AM, Puri S, van Staa TP. Linkage of the General Practice Research Database (GPRD) with other data sources. Pharmacoepidemiol Drug Saf 2011;20:S230 10.1002/pds.2206 [DOI] [Google Scholar]
- 10.Health & Social Care Information Centre. Hospital Episode Statistics. http://www.hscic.gov.uk/hes (accessed 04/05/2016).
- 11.Hollowell J. The General Practice Research Database: quality of morbidity data. Popul Trends 1997;87:36–40. [PubMed] [Google Scholar]
- 12.Herrett E, Gallagher AM, Bhaskaran K et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrett E, Thomas SL, Schoonen WM et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. 10.1111/j.1365-2125.2009.03537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong AYS, Root A, Douglas IJ et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 2016;352:h6926 10.1136/bmj.h6926 [DOI] [PubMed] [Google Scholar]
- 15.Li DQ, Kim R, McArthur E et al. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ 2015;187:174–80. 10.1503/cmaj.140950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray WA, Murray KT, Hall K et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90. 10.1056/NEJMoa1003833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volberg WA, Koci BJ, Su W et al. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther 2002;302:320–7. 10.1124/jpet.302.1.320 [DOI] [PubMed] [Google Scholar]
- 18.Abo-Salem E, Fowler JC, Attari M et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther 2014;32:19–25. 10.1111/1755-5922.12054 [DOI] [PubMed] [Google Scholar]
- 19.Shaffer D, Singer S, Korvick J et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002;35:197–200. 10.1086/340861 [DOI] [PubMed] [Google Scholar]