Epilepsy is common in Rett syndrome, but seizure burden over time is unclear. By following 778 affected individuals for 3939 person-years, Tarquinio et al. show that the lifetime risk of epilepsy is almost 90%, but that most patients experience remission. The results will contribute to the planning of therapeutic trials.
Keywords: epilepsy, epidemiology, prognosis, co-morbidity, genetics
Abstract
Epilepsy is common in Rett syndrome, an X-linked dominant disorder caused by mutations in the MECP2 gene, and in Rett-related disorders, such as MECP2 duplication. However, neither the longitudinal course of epilepsy nor the patterns of seizure onset and remission have been described in Rett syndrome and related conditions. The present study summarizes the findings of the Rett syndrome Natural History study. Participants with clinical Rett syndrome and those with MECP2 mutations without the clinical syndrome were recruited through the Rett Natural History study from 2006 to 2015. Clinical details were collected, and cumulative lifetime prevalence of epilepsy was determined using the Kaplan-Meier estimator. Risk factors for epilepsy were assessed using Cox proportional hazards models. Of 1205 participants enrolled in the study, 922 had classic Rett syndrome, and 778 of these were followed longitudinally for 3939 person-years. The diagnosis of atypical Rett syndrome with a severe clinical phenotype was associated with higher prevalence of epilepsy than those with classic Rett syndrome. While point prevalence of active seizures ranged from 30% to 44%, the estimated cumulative lifetime prevalence of epilepsy using Kaplan-Meier approached 90%. Specific MECP2 mutations were not significantly associated with either seizure prevalence or seizure severity. In contrast, many clinical features were associated with seizure prevalence; frequency of hospitalizations, inability to walk, bradykinesia, scoliosis, gastrostomy feeding, age of seizure onset, and late age of diagnosis were independently associated with higher odds of an individual having epilepsy. Aggressive behaviour was associated with lower odds. Three distinct patterns of seizure prevalence emerged in classic Rett syndrome, including those who did not have seizures throughout the study, those who had frequent relapse and remission, and those who had relentless seizures. Although 248 of those with classic Rett syndrome and a history of seizures were in terminal remission at last contact, only 74 (12% of those with a history of epilepsy) were seizure free and off anti-seizure medication. When studied longitudinally, point prevalence of active seizures is relatively low in Rett syndrome, although lifetime risk of epilepsy is higher than previously reported. While daily seizures are uncommon in Rett syndrome, prolonged remission is less common than in other causes of childhood onset epilepsy. Complete remission off anti-seizure medications is possible, but future efforts should be directed at determining what factors predict when withdrawal of medications in those who are seizure free is propitious.
Introduction
Rett syndrome, the most common cause of profound intellectual disability in females, is characterized by apparently normal early development followed by psychomotor regression and the emergence of midline hand stereotypies. Many, but not all, individuals experience seizures. The estimates of epilepsy prevalence in Rett syndrome range from 48% in the cross-sectional examination of the Natural History study (Glaze et al., 2010) to up to 94% in other studies (Steffenburg et al., 2001; Jian et al., 2007). Variability among previous studies results from a range of sample sizes, different criteria for diagnosis of Rett syndrome, and inconsistency in diagnosis of seizures. Therefore, interpretation of results within and across studies is difficult. Some studies have reported prevalence values at various ages, but no longitudinal study has reported the cumulative incidence of epilepsy, patterns of seizure onset and remission in various age groups, or the lifetime prevalence of epilepsy in Rett syndrome.
Examination of subjects in the cross-sectional report of the Natural History study revealed that 12% of spells termed ‘seizures’ by parents did not meet the clinical diagnosis of seizures. Because non-epileptic paroxysmal events are common in Rett syndrome, even epileptologists disagree on the classification of spells as seizures (Cardoza et al., 2011). However, clinical diagnosis by an experienced paediatric neurologist remains the gold standard for diagnosis of epilepsy (ILAE, 1993; Fisher et al., 2005). Indeed, in the absence of evaluation by an expert in conjunction with video-EEG, parents misclassify non-epileptic spells as seizures in as many as 82% of cases (Glaze et al., 1998).
Despite a number of cross-sectional studies, incidence, prevalence, and risk factors for epilepsy, as well as prognosis and response to treatment of seizures in Rett syndrome remain unclear (Glaze et al., 2010; Bao et al., 2013; Nissenkorn et al., 2015). Based on cross-sectional analyses we previously estimated point prevalence of epilepsy in classic Rett syndrome at 30–40% (Glaze et al., 2010). The current study examined the cumulative risk of epilepsy, the prevalence of epilepsy and the course of epilepsy in a large prospective, longitudinal cohort of subjects with clinically diagnosed Rett syndrome, methyl-CpG binding protein 2 gene (MECP2) mutation without Rett syndrome, and those with duplications of MECP2. We were particularly interested in studying the effect of type of mutation and clinical characteristics on the age of onset and course of epilepsy in Rett syndrome. Due to the complex nature of anti-seizure drug prescribing practices, medication efficacy and polypharmacy will be addressed in a separate manuscript.
Materials and methods
Participants
Participants were recruited from 2006 to 2015 through the multicentre Rett Natural History study at one of eight US sites (four study sites and four travel clinics) and evaluated every 6 to 12 months. The Rett Natural History Consortium is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health. Diagnoses of Rett syndrome or other related phenotypes and epilepsy were performed by a study neurologist or geneticist (D.G.G., W.E.K., J.L.N., A.K.P., and S.A.S.) with extensive clinical experience in Rett syndrome. Consensus criteria (Hagberg et al., 2002; Neul et al., 2010) were used to categorize participants as having classic Rett syndrome, atypical Rett syndrome, or a mutation in MECP2 but not fulfilling clinical criteria for Rett syndrome. All participants had MECP2 testing; participants with clinical Rett syndrome were included even if they lacked a mutation. Further details have been published previously, including age of symptom onset and diagnosis, and age and cause of death (Glaze et al., 2010; Tarquinio et al., 2012, 2015a,b). History of seizure onset, frequency, and treatment of seizures, as well as associated features and comorbidities was recorded at the first encounter and updated at each subsequent visit. Demographic data including race and ethnicity, type of residence, and parental age; socioeconomic data (median income and population density) were collected at the first visit or estimated using US census data based on postal address. A study physician completed a full neurological examination and an anthropometrist recorded somatic measurements.
Two quantitative severity scales, the Motor Behavioral Assessment and Clinical Severity Score described previously (Tarquinio et al., 2012), were administered at each visit to characterize severity using both dynamic and static items measuring the individual’s abilities, dysfunction, and comorbidities. Quality of life was assessed for participants using the 50-item Child Health Questionnaire and for caregivers using the 36-item Short Form Health Survey.
Data definitions and categorization
Participants were analysed in separate groups based on clinical and molecular differences. First, participants with clinical Rett syndrome were divided into classic and atypical based on diagnostic criteria. Those with MECP2 mutations without the Rett syndrome phenotype were analysed as a separate group. Those with atypical Rett syndrome were divided into mild and severe groups based on clinical phenotypical severity, as reported previously (Cuddapah et al., 2014). The Clinical Severity Score in atypical Rett syndrome has a bimodal distribution, suggesting that two distinct atypical groups exist; when the epilepsy variable is excluded (to avoid dependent associations), the nadir between the two distributions is at 19. These two groups were divided based on this nadir as follows: an ‘atypical severe’ category, characterized by individuals with profound developmental delay who achieve few early skills, so have fewer skills to lose during the regression period; and an ‘atypical mild’ category in which individuals acquire language and hand use with mild or no delay, and do not experience regression in one of these domains. Ambulation in all participants was categorized in a binary (able to stand or walk, unable to stand or walk) and ordinal fashion (based on Clinical Severity Score). Other characteristics and comorbidities (e.g. breathing dysregulation) were characterized based on frequency during examination or using specific parameters. Socioeconomic status was categorized into ordinal groups based on comparison to the national median income. Interval of QTc on ECG was categorized into normal (≤450), borderline (451–470) and abnormal (>470). Growth parameters [height, weight, body mass index (BMI), head circumference] were categorized using both normative and Rett-specific z-scores (Cole et al., 1998; Kuczmarski et al., 2000; Tarquinio et al., 2012). The standard cut-offs of ±2 standard deviation (SD) (approximating second and 98th percentiles) were used for normative charts and more liberal cut-offs of −1.28 SD (10th percentile) and −0.67 SD (25th percentile) on Rett syndrome charts were tested, in keeping with recent recommendations (Leonard et al., 2013). Mutations of MECP2 in Rett syndrome were categorized in terms of average phenotypical severity: mild (R133C, R294X, R306C, and 3’ truncations) and severe (T158M, R168X, R255X, R270X, and large deletions) (Cuddapah et al., 2014).
Epilepsy was characterized in a binary fashion (those with seizures despite medical management; and those without seizures, with or without medication) and seizure severity was defined in an ordinal fashion based on seizure frequency (Table 1). Because no subjects experienced only a single seizure, seizures and epilepsy are used here interchangeably. Presence of seizures at a visit was reported if at least one seizure had occurred within the past 6-month interval (hereafter ‘active seizures’). Although definitions of pharmacoresistance have been proposed, no definitions of epilepsy intractability or remission have been universally accepted (Munger Clary and Choi, 2011). Therefore, remission was defined as a period of at least 6 months without a seizure (the shortest time between visits), and subjects were categorized based on pattern of remission and relapse (Supplementary Table 1) (Berg and Rychlik, 2015). Frequent seizures were defined as at least one seizure per month, and less frequent seizures were categorized as rare seizures. Terminal remission refers to seizure freedom for at least 6 months at completion of the study, and complete remission was defined as absence of seizures for at least 6 months without administration of anti-seizure drugs.
Table 1.
Percentage of seizure frequency and clinical diagnosis
| Diagnosis | n | Years in study (median, IQR) | No seizures in 6 months (%) | <Monthly (%) | <Weekly to monthly (%) | Weekly (%) | >Weekly (%) | Daily (%) |
|---|---|---|---|---|---|---|---|---|
| Classic Rett syndrome, female | 922 | 7.1, 4.3–8.5 | 68 | 7 | 8 | 6 | 8 | 3 |
| Atypical mild Rett syndrome, female | 78 | 5.5, 4.0–7.5 | 84 | 7 | 3 | 1 | 4 | 2 |
| Atypical severe Rett syndrome, female | 81 | 7.5, 4.8–8.5 | 66 | 10 | 5 | 6 | 10 | 3 |
| MECP2 mutation without Rett syndrome, female | 42 | 5.6, 3.3–8.1 | 86 | 1 | 1 | 3 | 8 | 2 |
| CDKL5, atypical severe Rett syndrome, female | 2 | 4.4–4.8 | 22 | 0 | 11 | 0 | 33 | 33 |
| Classic Rett syndrome, male | 1 | 4.4 | 80 | 20 | 0 | 0 | 0 | 0 |
| Atypical severe Rett syndrome, male | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 100 |
| MECP2 mutation without Rett syndrome, male | 20 | 4.4, 2.3–6.1 | 73 | 3 | 3 | 3 | 9 | 10 |
| MECP2 duplication, male | 28 | 4.8, 3.7–7.0 | 60 | 3 | 5 | 3 | 22 | 8 |
| MECP2 duplication, female | 8 | 5.5, 3.8–8.4 | 100 | 0 | 0 | 0 | 0 | 0 |
| Total | 1183 | 6.9, 4.2–8.4 | 70 | 7 | 7 | 6 | 8 | 3 |
Statistical analysis
Descriptive analyses were performed using mean ± SD and median and interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. Point prevalence (proportion with active seizures at a particular age), period prevalence (proportion with epilepsy over a certain period of time), and incidence of new onset seizure were calculated in those with Rett syndrome. Cumulative risk of developing epilepsy was calculated using the Kaplan-Meier, and the bivariate associations between cumulative risk and diagnoses, various clinical characteristics, and mutation type were tested using the log rank test. Logistic regression was used to assess the independent prognostic value for developing epilepsy based on various factors, including MECP2 mutation type, growth, ability to walk, scoliosis, breathing dysregulation, sleep disturbance, hand function, verbal and non-verbal language, frequency of stereotypies, muscle tone, reflexes, autonomic dysfunction, race, ethnicity, and socioeconomic status. The association of continuous variables (age, quality of life, number of hospitalizations or fractures, total scores on severity scales) with epilepsy was assessed by fitting to Cox proportional hazards models. Significant predictors of epilepsy in both logistic regression and Cox models were plotted as individual Kaplan-Meier curves. Statistical analyses were performed using SPSS and SAS (IBM Corp, 2013; SAS, 2016).
Human studies approval
Each site obtained and maintained Institutional Review Board (ethics) approval for the performance of this study, and all data were verified by the study clinician at time of exam and interview. Parental approval for study conduct and publication of results was obtained before entry into the study. The study has been registered with ClinicalTrials.gov: NCT00299312 since 3 March 2006.
Results
Overall, 1205 individuals were enrolled in the Rett Natural History Study; the final analysis focused on 778 with classic Rett syndrome followed longitudinally for up to 9.0 years (median 5.5 years, IQR 3.2–7.5 years) and a median of five visits each, for a total of 3939 person-years. Of the original 1205, diagnosis or date of birth could not be verified in 22. Due to scarcity, only summary statistics are provided for epilepsy in males with Rett syndrome or MECP2 mutation without Rett syndrome, participants with MECP2 duplication and the two individuals with atypical severe Rett syndrome due to CDKL5 mutation (Table 1). These individuals were excluded from regression analyses. The remaining cohort of 1123 female participants included 922 with classic Rett syndrome, 78 with atypical mild Rett syndrome, 81 with atypical severe Rett syndrome, and 42 with a pathogenic mutation in MECP2 but without clinical Rett syndrome. Median age of diagnosis in classic Rett syndrome was 2.7 years (IQR 2.0–4.1). Further details were published previously (Tarquinio et al., 2015b). Some subjects were only seen once (cross-sectional cases with retrospective data only), but most (87.7%, n = 985) were followed longitudinally, including 84.4% (n = 778) of those with classic Rett syndrome. None born after 1997 lived in a group home or institution. The proportion of those older than 18 years who lived in a group home was 7.3%, and in an institution was 1.2%. Fifty-two of the total cohort died before the end of the study (4.6%). Most deaths were due to cardiorespiratory issues, and survival for both classic and atypical Rett syndrome was >70% at 45 years (Tarquinio et al., 2015a).
Seizures in classic Rett syndrome
At recruitment, 55.2% (508/921) of parents reported a history of epilepsy, but only 29.9% (268/897) were having active seizures at the initial visit. During the period of study observation, the course of seizures was highly variable. Some subjects who had never had seizures developed seizures during the study, some with a history of seizures—who were in remission—relapsed, and some with active seizures experienced remission (Supplementary Table 1).
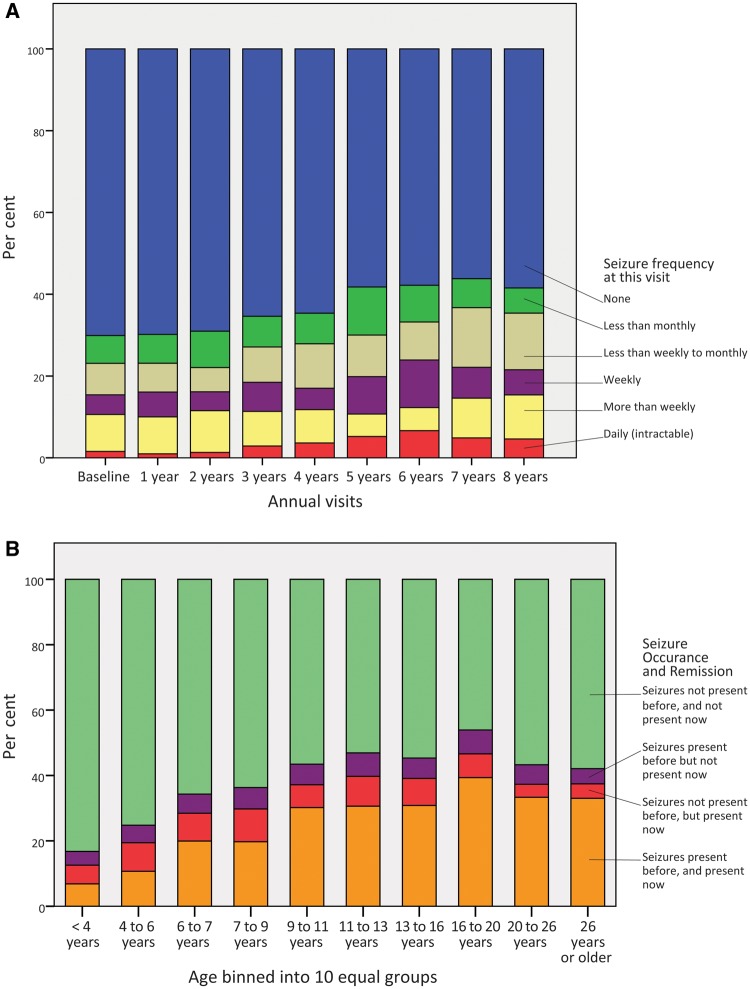
Among those who entered the study without a history of seizures, 32.3% (133/412) experienced their first seizure during the study. Although only 69.6% (642/922) of the total cohort ever experienced a seizure before or during the study, estimates of lifetime and age-specific proportions with epilepsy were higher when calculated using Kaplan-Meier survival statistics approached 90% (see below). Many became seizure-free by the end of the study, including both participants whose seizure onset predated the study and those whose seizures began during the study. Therefore, point prevalence of epilepsy at annual visits ranged from 29.8% to 43.8%. However, because many began experiencing seizures for the first time during the study, considering all observations, point prevalence increased gradually as the cohort aged [χ2(8, n = 4151) = 45.04, P < 0.001, Fig. 1A]. Daily seizures were uncommon at most visits. At recruitment, the most common category of seizures was ‘more than weekly,’ and at the end of the study less frequent seizures were more common; however, seizure severity was not significantly different among visits (P = 0.84). Seizure prevalence ranged by age group from 11.6% (30/258) in the youngest age group (<4 years) to a peak prevalence of 49.1% (78/159) in the 16 to <20 year age group, decreasing thereafter. Seizure occurrence and remission varied with age, and those in late teenage years had the highest proportion of persistent seizures (Fig. 1B).
Figure 1.
Seizure frequency, relapse, and remission in classic Rett syndrome. (A) Seizure frequency at each observation. (B) Proportion with active seizures based on age group.
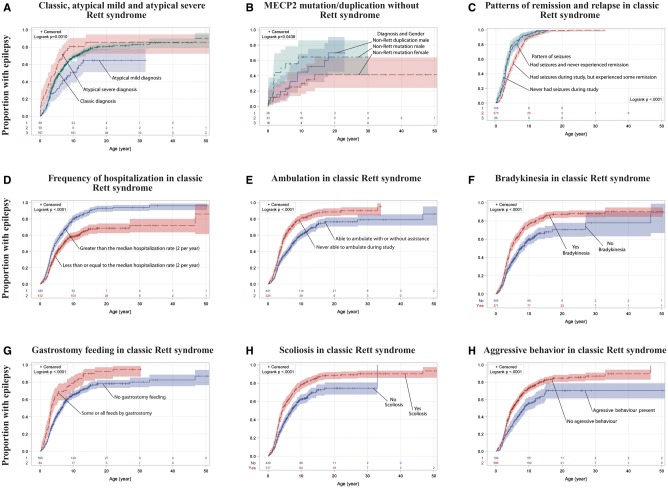
The age of first seizure onset in classic Rett syndrome ranged from immediately after birth to 46.8 years. Age of onset increased steeply during mid-childhood and then began to plateau: 25% had epilepsy by age 3.0 years, 50% by 5.9 years, and 75% by 13.2 years. The cumulative proportion of those who developed epilepsy approached 90% for classic Rett syndrome (Fig. 2A). Among the 778 participants followed longitudinally, 54.3% (423) had a history of seizures before the study, 56.9% (443) had seizures at some point during the observation period, and 17.1% (133) experienced their first seizure during the study. Length of observation was approximately 6 months longer in those who had active seizures at the beginning of the study but became seizure-free by the end of the study (mean = 7.1 years, SD = 2.0), compared to those who had active seizures at the beginning and end (mean = 6.4 years, SD = 2.4) and those who were seizure-free at the beginning and end of the study [mean = 6.4 years, SD = 2.3; F(3 775) = 2.78, P = 0.04]. As of the final visit, 38.9% (303) had active seizures.
Figure 2.
Cumulative incidence of epilepsy in Rett syndrome and MECP2-related disorders, and association with phenotypic characteristics. Cumulative proportion of epilepsy was significantly different between males and females who possessed a MECP2 mutation without clinical Rett syndrome (P < 0.05), but pairwise comparisons with MECP2 duplication were not significantly different (B). Cumulative probability was similar for individuals who had seizures before the study but were in long-term remission throughout the study (labelled ‘never had seizures during study’) and those who had seizures throughout the study without remission; however, individuals who had seizure remission alternating with relapse had onset of seizures significantly later in life (C, P < 0.001). Characteristics shown in D–I are all independently associated with cumulative incidence of epilepsy in classic Rett syndrome according to regression analysis, and categorical comparisons are depicted using the results of the log-rank test. For 108 (17%) of those with classic Rett syndrome and epilepsy, age of seizure onset prior to the study was not recalled; missing data ranged from 10 to 20% in other diagnoses above. Because of these missing data, bootstrap aggregating technique was used with 500 iterations simulating the cumulative proportion if only those with a known age of seizure onset were recruited, and an equivalent proportion of censored subjects was randomly removed from the survival analysis during each iteration. The number below curves indicates n in each category; colour bands indicate 95% confidence intervals.
Seizure semiology was captured at the initial visit, and among those with a history of epilepsy, semiology was almost evenly divided between focal-onset seizures (with or without secondary generalization) in 46.8% (226/483) and generalized onset seizures in 47.0% (227/483). Rarely other seizure types occurred exclusively, including myoclonic seizures in 1.7% (8/483), atonic seizures in 3.3% (16/483), and infantile spasms only in 1.2% (6/483). Seizure semiology was not significantly associated with age of onset of epilepsy or pattern of relapse and remission.
Somatic and head growth retardation were significantly associated with increased lifetime seizure prevalence. The Rett-specific z-scores were used, and individuals were categorized based on the median and upper and lower quartiles for age. Seizure prevalence was lower if height (65.7%, 295/499) or head circumference (67.5%, 326/483) was above the median compared to height (74.8%, 333/445, P = 0.003) or head circumference (73.4%, 303/413, P = 0.05) below the median. Although BMI was not associated with seizure prevalence, weight in the upper quartile trended to lower seizure prevalence (65.6%, 141/215) than weight in the lower quartiles (71.7%, 487/679, P = 0.09).
MECP2 mutation, diagnosis and seizures
Presence of a MECP2 mutation was not associated with a higher likelihood of seizures (69.8% in classic Rett syndrome with a mutation versus 74.2% without a mutation). When examined in isolation, specific type of MECP2 mutation was associated with prevalence of seizures among the most common 10 mutation types [χ2(9, n = 686) = 21.53, P = 0.01, Table 3]; however, seizure frequency was not associated with mutation type (Table 3). Yet, when either a logistic or Cox regression model was used to incorporate age and other clinical variables, mutation type was not independently associated with prevalence or frequency of seizures. The patterns of epilepsy prevalence, remission and relapse were not associated with MECP2 mutation, both when mutations were examined individually and based on mutation severity category. Paradoxically, among the mutations with lower epilepsy prevalence were two with more severe phenotype [i.e. R168X (n = 98), R270X (n = 54)].
Table 3.
MECP2 mutation types in classic Rett syndrome, seizure prevalence (cumulative) and seizure frequency (per cent averaged over all study visits)
| Type of MECP2 mutation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R106W | R133C | T158M | R168X | R255X | R270X | R294X | R306C | C-terminal truncation | Large deletion | Total | |
| Seizures present prior to end of study n (%) | 20 (76.9) | 28 (66.7) | 78 (78.0) | 61 (62.2) | 67 (77.0) | 31 (57.4) | 40 (71.4) | 34 (54.0) | 60 (75.9) | 54 (66.7) | 473 (69.0) |
| Never had seizures n (%) | 6 (23.1) | 14 (33.3) | 22 (22.0) | 37 (37.8) | 20 (23.0) | 23 (42.6) | 16 (28.6) | 29 (46.0) | 19 (24.1) | 27 (33.3) | 213 (31.0) |
| No seizures in 6 months (%) | 68 | 71 | 69 | 72 | 61 | 71 | 60 | 80 | 69 | 69 | 69 |
| <Monthly (%) | 4 | 9 | 7 | 5 | 11 | 7 | 5 | 5 | 8 | 7 | 7 |
| <Weekly to monthly (%) | 6 | 2 | 7 | 7 | 9 | 11 | 8 | 5 | 9 | 9 | 8 |
| Weekly (%) | 15 | 7 | 7 | 7 | 6 | 3 | 7 | 5 | 5 | 5 | 6 |
| >Weekly (%) | 6 | 9 | 9 | 6 | 10 | 6 | 15 | 4 | 8 | 8 | 8 |
| Daily (%) | 1 | 2 | 2 | 3 | 3 | 2 | 5 | 1 | 3 | 2 | 2 |
| Total n | 26 | 42 | 100 | 98 | 87 | 54 | 56 | 63 | 79 | 81 | 686 |
In contrast, seizure frequency and severity varied based on clinical diagnosis (Table 1). Seizures were more common in classic Rett syndrome (69.8%) compared to atypical mild Rett syndrome (55.1%, P = 0.006) and more common in atypical severe Rett syndrome (77.8%) compared to atypical mild Rett syndrome (P = 0.002), but seizure frequency in classic and atypical severe Rett syndrome was not significantly different. Males with MECP2 duplication, a small group of particular clinical interest, had higher point prevalence of seizures than any Rett syndrome group. However, the age of seizure onset was generally later in MECP2 duplication than in Rett syndrome, and the cumulative prevalence was only 70%.
Global Clinical Severity Scores and relation to epilepsy
In the 778 who participated in the longitudinal component of the study, global severity scores were higher in those with epilepsy. Median Clinical Severity Score (without including the seizure item) was 24.0 (IQR 19–29) in those with epilepsy and 19.5 (IQR 15–23) in those who never had seizures (U = 111 351, n1 = 262, n2 = 628, P < 0.001, r = 0.28). Because the Clinical Severity Score is a composite measure, we examined the individual severity components using logistic regression with presence of epilepsy as the dependent variable. Age at enrolment, frequency of hospitalizations, late age of diagnosis, hyperventilation, breath-holding, bradykinesia, scoliosis, ability to walk, and gastrostomy feeding were all independently associated with a higher odds of developing epilepsy, while aggressive behaviour was associated with a lower odds of epilepsy. Number of fractures and hyperreflexia trended to significant association. Child’s quality of life measures were incorporated into logistic regression, and the domains of physical role/social limitations, behaviour, and mental health were significantly associated with the presence of epilepsy (Table 2). Epilepsy was more common in those with a higher number of fractures. All of the individuals with a lifetime history of six or more fractures had epilepsy (n = 19), whereas only 69% of those with five or fewer fractures had epilepsy (n = 609, P = 0.03). When age of seizure onset was considered using Cox regression, frequency of hospitalizations, late age of diagnosis, bradykinesia, inability to walk, and gastrostomy feeding were independently associated with a higher hazard rate of developing epilepsy, while aggressive behaviour was associated with a lower hazard rate (Table 2). Independent predictors associated with epilepsy were examined individually using the log rank test, and the effect on the cumulative probability of developing epilepsy is detailed in Fig. 2D–I.
Table 2.
Characteristics associated with cumulative incidence of epilepsy (logistic regression as of last follow-up), and development of seizures adjusted for age of onset (Cox regression analysis)
| Variable | n for Logistic regression | OR | 95% CI | OR Sig. | n for Cox regression | HR | 95% CI | HR Sig. |
|---|---|---|---|---|---|---|---|---|
| Older age at recruitment | 866 | 1 | 1.0–1.1 | 0.013 | ||||
| Hospitalized frequently (>2 per year) | 866 | 2.7 | 1.8–3.9 | <0.001 | 769 | 1.6 | 1.3–1.9 | <0.001 |
| Diagnosed after 75th percentile | 866 | 2.3 | 1.5–3.7 | <0.001 | 769 | 1.3 | 1.1–1.6 | 0.047 |
| Diagnosed between median and 75th percentile | 866 | 2 | 1.1–3.6 | 0.015 | ||||
| Frequent hyperventilation | 866 | 1.8 | 1.2–2.7 | 0.003 | 769 | 1.2 | 0.9–1.5 | 0.055 |
| Frequent breath-holding | 866 | 1.9 | 1.1–3.3 | 0.022 | ||||
| Bradykinesia | 866 | 2.5 | 1.3–4.7 | 0.004 | 769 | 1.6 | 1.3–2.0 | <0.001 |
| Scoliosis | 866 | 1.6 | 1.1–2.4 | 0.016 | ||||
| Unable to ambulate | 866 | 1.6 | 1.1–2.5 | 0.022 | 769 | 1.4 | 1.1–1.7 | <0.001 |
| Severe dystonia | 769 | 1.3 | 1.0–1.6 | 0.024 | ||||
| Frequent stereotypies | 769 | 0.8 | 0.7–1.0 | 0.062 | ||||
| Gastrostomy feeding | 866 | 2 | 1.0–4.0 | 0.040 | 769 | 1.4 | 1.1–1.8 | 0.002 |
| Aggression | 866 | 0.5 | 0.3–0.8 | 0.009 | 769 | 0.6 | 0.5–0.9 | 0.003 |
| Role/social limitations – physical | 665 | 0.9 | 0.8–1.0 | 0.024 | ||||
| Behaviour | 665 | 1.5 | 1.1–2.0 | 0.009 | ||||
| Mental health | 665 | 0.8 | 0.5–1.0 | 0.023 | ||||
| Bone fractures | 866 | 1.5 | 0.9–2.6 | 0.091 | ||||
| Hyperreflexia | 866 | 1.4 | 1.0–2.1 | 0.062 | ||||
| Some verbal communication (babbling or words) | 769 | 0.9 | 0.7–1.0 | 0.101 |
Non-significant results or results that trended to significant association are in bold. Blank cells indicate that variable did not meet minimal P-value for inclusion in regression equation. CI = confidence interval; HR = hazard ratio; OR = odds ratio; Sig. = Significance of p-value.
Course of epilepsy in classic Rett syndrome
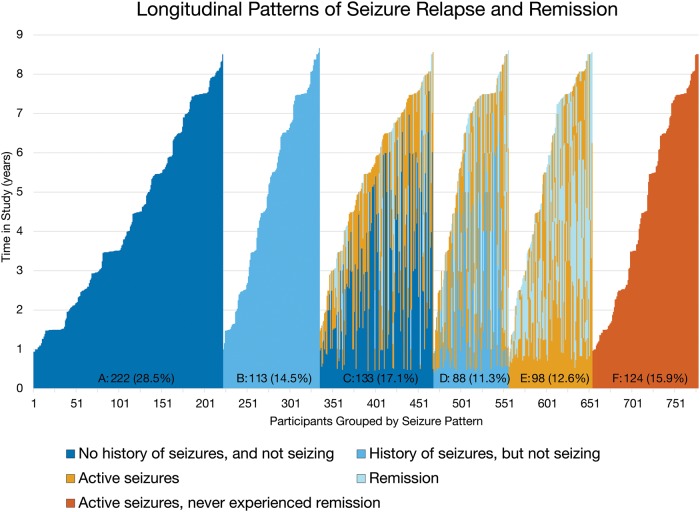
Patterns of seizure remission and relapse were determined in the 778 individuals followed prospectively, and six distinct groups emerged based on (i) having a history of seizures; (ii) seizures present at first study visit; and (iii) presence of relapse and remission (Fig. 3). Patterns of seizure relapse and remission varied over up to 9 years of follow-up, and three of these clusters (Groups C, D, and E) suffered from recurrent relapses and remissions, with as many as three remission/relapse cycles (Supplementary Table 1). To clarify, the seizure-free interval between visits will be 6 months longer than the period of remission, by definition. Although the average duration of remission (defined as at least 6 months free of seizures) was only 1.1 years, many experienced prolonged remission, as long as 7.5 years. Among those with active seizures during the course of the study who were followed for more than 2 years after seizure onset, 20.1% (89 of 442) had a 2-year remission, and among those followed for more than 5 years, 7.1% (24 of 340) experienced remission lasting 5 years or longer.
Figure 3.
Longitudinal patterns of seizure relapse and remission in classic Rett syndrome. Each column (total n = 778) represents one subject followed longitudinally for the duration of the study, represented by the y-axis (in years). Colour coding represents seizures observed at that period of the study (with or without active seizures). Groups labels and duration of follow-up (median, IQR) are as follows: A, never had seizures through terminal visit (3.5 years, 2.0–5.9 years); B, history of seizures prior to study, but never had seizures during the study (4.9 years, 2.2–7.0 years); C, no prior history of seizures, but began having seizures during the study (5.4 years, 3.8–6.6 years); D, prior history of seizures, not having seizures at beginning of study, but experienced seizure relapse (6.5 years, 3.9–7.0 years); E, having seizures at study onset but experienced remission during study (5.5 years, 3.1–7.1 years); F, having seizures at study onset and never experienced remission through terminal visit (4.0 years, 2.0–6.8 years).
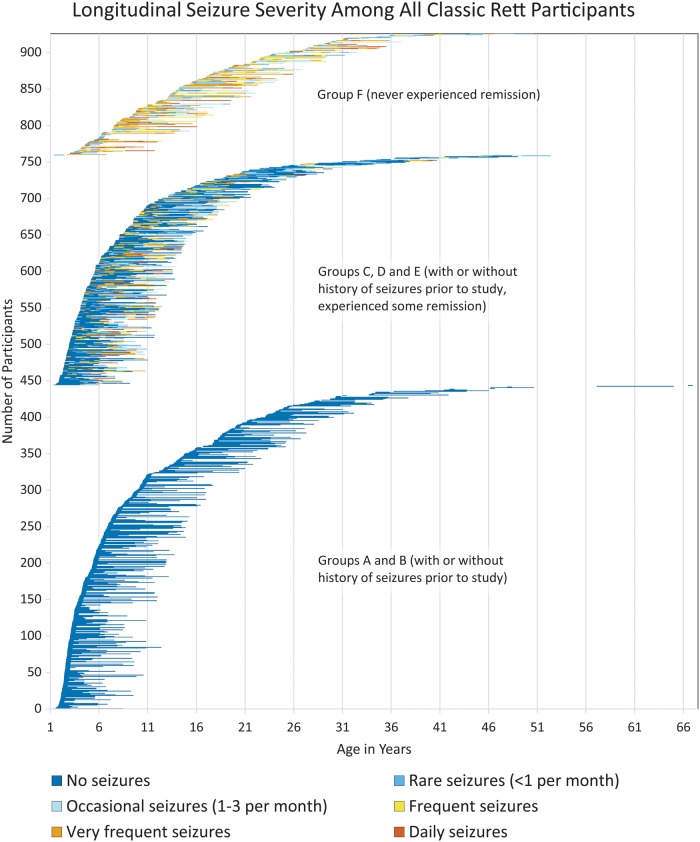
By integrating the patterns of seizure prevalence and frequency over the lifespan of individuals with Rett syndrome, fluctuations in seizure severity become clear (Fig. 4). This visual summary demonstrates that within the clusters of relapse/remission patterns, daily seizures are common but often resolve to less frequent seizures, even if remission does not occur. Moreover, although onset of epilepsy is rare in early childhood, seizures remit or relapse and worsen or lessen in frequency haphazardly over the lifespan. However, when one considers the age of onset of epilepsy, the patterns of remission and relapse are different depending on age of onset. Both those with lifelong intractable epilepsy without remission and those who experienced long-term remission began having seizures earlier (median 3 years), but those who had lifelong epilepsy with alternating relapse and remission began having seizures later (median 5 years, Fig. 2C). At last contact, 248 of those with a history of seizures achieved terminal remission, but only 74 (29.8%) of these were seizure-free OFF anti-seizure drugs. Among all those in the study who experienced seizures, this amounts to 11.5% with complete remission.
Figure 4.
Longitudinal seizure severity in classic Rett syndrome.
Accuracy of reporting
When we compared parental impression at the beginning of the study with physician diagnosis of seizures, physicians disagreed with parents 8% of the time; in 2.7% (24/896) of cases the physicians diagnosed spells as seizures that parents believed were not seizures, and in 3.7% (33/896) of cases, parents reported seizures that the physician deemed were non-epileptic in nature. Over the course of the study the percentage of disagreement was relatively stable. At the last visit, in 2.4% (18/761) of subjects, the physicians diagnosed seizure when parents reported no seizure, and for 3.7% (28/761) of subjects, their parents reported seizures that physicians diagnosed as non-epileptic.
Discussion
Rett syndrome is a rare and severe genetic encephalopathy affecting roughly 1:10 000 females born each year. Rare disease networks, such as the Rett Natural History Study, are an essential resource for amassing the number of subjects necessary to provide a robust understanding of the natural history of these rare and extremely serious disorders. This understanding is a prerequisite to planning and performing therapeutic trials to improve outcomes. In this longitudinal study of Rett syndrome, we found that, although the cumulative risk of developing epilepsy is high (∼90% over the lifespan), the course of seizure occurrence and remission is strikingly variable. Some subjects never attained even moderate seizure control, whereas in others, seizures completely remitted and they were able to withdraw medication treatment. The most common scenarios, however, reflect a remitting and relapsing tendency, waxing and waning over the lifespan. Notably, active epilepsy was most common in late adolescence; although no evidence exists to support this, one could hypothesize this is due to hormonal changes.
A number of cohort studies have examined overall health in patients with Rett syndrome, and have found high overall prevalence of a history of epilepsy. The Dutch cohort includes data on 53 individuals collected in 2007, and 37 of these had follow-up data in 2012 (Halbach et al., 2013). Among these, 76% had a history of seizures, but 54% of those with seizures had been seizure-free at some point for at least 3 months. Over time, both improvement and deterioration occurred, although parents indicated epilepsy stabilized between 20–30 years of life. The Italian cohort included surveys from 84 subjects, 82% of whom had a history of seizures (Vignoli et al., 2012). Seizure onset varied widely from 1 to 16 years, and a high proportion suffered drug-resistant frequent seizures (36%). The large Australian study (423 subjects) combined data from the Australian database and an international cohort, and found a history of seizures in only 45% (Anderson et al., 2014). Again, drug-resistant epilepsy was present in 36% of those with a history of seizures who were taking anti-seizure drugs.
In our study, we were able to identify important key features associated with the development of seizures and with the course and severity of epilepsy over time. Seizure severity, defined based on frequency of seizures, is associated with Rett syndrome diagnosis (typical versus atypical) and overall disease severity, but only weakly associated with MECP2 mutation. Most patients with Rett syndrome enter remission for at least a brief period of time on standard treatment strategies, although they often follow a waxing and waning course. Notably, persistent drug resistant epilepsy without remission is not the most common pattern, only affecting 16% of those with classic Rett syndrome, a figure less than half that of the Italian and Australian studies.
This study is the first to characterize the pattern of remission and relapse of seizures in Rett syndrome. Although many participants had no seizures through the course of the study, the majority (Groups C, D, E in Fig. 3) suffered frequent relapses and remissions, often long in duration. Average remission duration is relatively short, and remission and relapse can occur across the lifespan. Despite this frequent fluctuation, some experience longer remissions; indeed, a substantial group experienced remission lasting 5 years or longer. This pattern of frequent remission and relapse is similar to that seen in other forms of childhood-onset epilepsy (Berg and Rychlik, 2015). Compared to the low proportion of those with childhood-onset epilepsy who never experience remission (5%), a much greater proportion of those with Rett syndrome never undergo prolonged remission (15.9%). Alternately, compared to the 33% of those highlighted by Berg and Rychlik (2015) with ‘smooth-sailing’ childhood epilepsy, only 20% of those with Rett syndrome and epilepsy fall into this prolonged remission category. Therefore a person with Rett syndrome has a 43% chance of either not having epilepsy or having epilepsy with little seizure burden
The three groups identified in Fig. 4, those with no seizures for the duration of the study, those who fluctuated between relapse and remission of seizures, and those who endured epilepsy without remission, have clear differences in age of onset. This raises the issue of different processes driving the clinical patterns of epilepsy in Rett syndrome. The mechanisms of epilepsy in Rett syndrome, based on MECP2 deficit, generally support an imbalance of excitation and inhibition, and have been speculated to include multiple specific neuronal cell types, as well as non-cell-autonomous effects of glial cells. Neuronal cell types implicated include forebrain excitatory pyramidal neurons, and inhibitory interneurons, including the somatostatin positive type, and some results are in direct conflict between studies (Zhang et al., 2014; Calfa et al., 2015; Ito-Ishida et al., 2015). Other publications have found no clear difference in neuronal excitability in Mecp2-null mice (McLeod et al., 2013). In summary, the neurobiology of epilepsy in Rett syndrome is poorly understood, and longitudinal neurophysiological studies in animal models are lacking (Benke, 2014). Variability in the animal models is not surprising given the variability of the clinical phenotype in humans. This study is the first to suggest distinct patterns of Rett syndrome epilepsy phenotype in individuals followed longitudinally. Whether these distinct patterns align with the neurobiological differences seen in animal models of Rett syndrome should be the subject of future research, as this may drive phenotype-specific treatments.
Other notable observations include that length of observation was significantly greater for those who experienced remission in Rett syndrome. This suggests that longer observation is associated with eventually achieving at least transient seizure freedom in Rett syndrome, and contrasts with CDKL5 syndrome in which seizures often persist inexorably despite heroic seizure management efforts (Muller et al., 2015). However, this distinction may be partially accounted for by selection bias. In all likelihood, children with more severe epilepsy are likely not to participate in the study due to medical fragility, or to discontinue participation, as the study did not offer any treatment. The association between growth and seizure prevalence (i.e. greater height and weight are associated with lower seizure prevalence) is intriguing, particularly because growth is a modifiable risk factor in Rett syndrome. While the source of the association remains unknown, individuals with Rett syndrome often exhibit a secondary mitochondrial dysfunction, and hypothalamic abnormalities have been reported in MECP2-deficient mice (Eeg-Olofsson et al., 1990; Coker and Melnyk, 1991; Ben-Shachar et al., 2009; Garcia-Rudaz et al., 2009; Grosser et al., 2012; Torres-Andrade et al., 2014). Recent evidence supports the association between mitochondrial dysfunction and epilepsy, and nutritional intervention has been a fundamental component of managing mitochondrial disease (Yuen and Sander, 2011; Kim do et al., 2015). Adequate nutrition, by gastrostomy tube when needed, can improve somatic growth in Rett syndrome markedly (Motil et al., 2009), and the association between growth and epilepsy raises the possibility that early nutritional intervention could decrease seizure prevalence in Rett syndrome. Notably, the proportion of seizures in different categories was markedly different when standard growth curves were used compared to Rett-specific curves; due to the confounding association with age, older individuals have both higher seizure prevalence and lower z-scores on standard charts. The Rett-specific z-scores eliminate the confounding effect of age, and estimate the true association between growth and seizure prevalence.
Methyl-CpG-binding protein 2 (MECP2) mutation and duplication are associated with a wide phenotypic variation. Therefore, estimates of the phenotype–genotype association between epilepsy in Rett syndrome and a specific mutation in MECP2-related disorders are largely dependent on sample size and composition. Most studies have found that epilepsy prevalence is lower in late truncating deletions (Cardoza et al., 2011; Nissenkorn et al., 2015). These same studies, however, have found conflicting associations between epilepsy prevalence and point mutations, with one reporting a higher prevalence of epilepsy in R306C mutations compared to R133C, and the other study finding the opposite association. Others have found T158M, R255X and large deletions to confer a higher likelihood of seizures (Bao et al., 2013). The large sample size presented in our study conflicts with these prior reports with respect to phenotype–genotype association; we found that if different mutations alter epilepsy risk at all, this effect is small.
Other factors also influenced prevalence of seizures in Rett syndrome. Notably, those with aggressive behaviour have a lower frequency of seizures. While this may seem counterintuitive, the findings of the quality of life study from the Rett natural history study demonstrate that negative behaviours are often associated with lower overall clinical severity (Lane et al., 2011). However, in this cohort, aggressive behaviour was associated with a lower risk for epilepsy independent of other clinical severity factors.
Anecdotally, seizure frequency is thought to decrease in adolescence among Rett syndrome clinicians, yet estimates may reflect cross-sectional design or bias. Because all phenotype–genotype studies have been cross-sectional, associations between epilepsy and genetic mutation could be influenced by sampling error. Additionally, many studies mix the concepts of seizure frequency and medication use into a combined severity score, rather than separately documenting seizure frequency and medication use as we did. Although our previous report found onset after age 20 to be rare, the current study, using Kaplan-Meier technique, found ∼8% of individuals have onset of epilepsy after age 20 years. Moreover, fluctuations in seizure severity continue throughout adolescence and adulthood.
Because EEG is almost universally abnormal after age 3 in Rett syndrome, experts typically do not prescribe anti-seizure medications for patients without clear epileptic seizures, despite an abnormal EEG. Due to the wide variety of behaviours seen in Rett syndrome, ‘spells’ of uncertain significance should be investigated with long-term video EEG or ambulatory EEG prior to starting medications. An examination of 82 long-term video EEG studies, lasting from 8 to 120 h, found that although parents of 42% of the 55 individuals with a history of seizures identified events typical of their child’s seizure, EEG showed no epileptiform correlate to the clinical behaviour (Glaze et al., 1998). Although all patients had abnormal EEGs, only 16% had electro-clinical seizures, including both focal and generalized events, during recording. The non-epileptic spells seen in this study consisted of staring, laughing, pupillary dilatation, breathing dysregulation, as well as a variety of motor activities. EEG during sleep is generally abnormal after regression, and can consist of slowing and rare epileptiform discharges, or even of a continuous spike-and-slow-wave pattern reminiscent of an epileptic encephalopathy. However, unlike epileptic encephalopathy, no evidence supports treatment of these features to normalize the EEG, as this has not historically resulted in cognitive improvement. As parents and physicians disagree whether spells are seizures as frequently as 8% of the time, we view this diagnostic distinction as critically important. Especially when spells include the features of non-epileptic paroxysms listed in Glaze (2005), video-EEG should be used to confirm spells before prescribing treatment. Diagnosis should not rely on interictal abnormalities, which are present in all children with Rett syndrome after age 3, whether or not seizures are present (Glaze, 2005). Notably, many of the anti-seizure medications can impact non-epileptic behaviours such as stereotypies and breathing dysfunction (Kumandas et al., 2001; Goyal et al., 2004). In such cases, treatment response can reinforce an incorrect diagnosis—relapse may prompt trial of other anti-seizure drugs with more serious side effects that do not improve these behaviours.
The Rett Natural History Study cohort represents 10–20% of the entire US Rett syndrome population; therefore this should be the most accurate estimate of epilepsy prevalence in Rett syndrome available. The present is the largest longitudinal investigation of seizures and epilepsy in Rett syndrome. Nonetheless, the study has some limitations that include the absence of systematic EEG documentation of seizures. All efforts were made to include EEG findings when obtaining clinical history and diagnosing epilepsy, but source documentation was not compiled for statistical analysis. Additionally, this study does not address physician prescribing practices or the efficacy of specific anti-seizure medications. Based on previous publications, medication use is largely based on regional prescribing practices rather than efficacy or adverse effects (Bao et al., 2013; Pintaudi et al., 2015). Although cases and retrospective studies document efficacy of specific medications in Rett syndrome, no systematic study has demonstrated superiority of a specific medication or class of medications to treat epilepsy in Rett syndrome (Haas et al., 1986; Kumandas et al., 2001; Goyal et al., 2004; Specchio et al., 2010). No specific medications are contraindicated in Rett syndrome; however, the issue of bone health is an important consideration when prescribing anti-seizure drugs. One study found increased risk of fracture in Rett syndrome associated with valproate use, whereas another study demonstrated that low vitamin D levels, common in Rett syndrome, were not associated with anti-seizure drug use (Leonard et al., 2010; Motil et al., 2011). In summary, when seizures are concluded to be epileptic in origin and anti-seizure drugs are initiated, the side effect profiles of medications should be considered carefully, and medications, which can cause behavioural issues (e.g. levetiracetam), anorexia and nephrolithiasis (e.g. topiramate), QT interval prolongation (e.g. felbamate), or marked sedation (e.g. benzodiazepines), should be used with caution. As in other neurological disorders, medication choice in Rett syndrome should be based on consideration of semiology, EEG characteristics, and risk/benefit with respect to adverse effects, and video-EEG should be used liberally to confirm the epileptogenic nature of events.
Complete remission OFF medications can be achieved in Rett syndrome, although the majority who are seizure-free remain on medications. Future directions include efforts to assess medication efficacy in Rett syndrome, and to define a Rett epilepsy profile characterized by age of onset, seizure type, EEG character, and specific treatment responsiveness.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the guidance of Gary Cutter regarding statistical analysis, and the help of Marianne Smith at Offset Prep with preparation of figures.
Funding
Supported by NIH U54 grants RR019478 (NCRR) and HD061222 (NICHD), and IDDRC grant HD38985 (NICHD), funds from the International Rett Syndrome Foundation and Civitan International Research Center. The Angelman, Rett and Prader-Willi Consortium [U54 RR019478 (NCRR) and HD061222 (NICHD)] is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
References
- Anderson A, Wong K, Jacoby P, Downs J, Leonard H. Twenty years of surveillance in Rett syndrome: what does this tell us?. Orphanet J Rare Dis 2014; 9: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Downs J, Wong K, Williams S, Leonard H. Using a large international sample to investigate epilepsy in Rett syndrome. Dev Med Child Neurol 2013; 55: 553–8. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet 2009; 18: 2431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke TA. What you seize is what you get: do we yet understand epilepsy in rett syndrome?. Epilepsy Curr 2014; 14: 283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia 2015; 56: 40–8. [DOI] [PubMed] [Google Scholar]
- Calfa G, Li W, Rutherford JM, Pozzo-Miller L. Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus 2015; 25: 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoza B, Clarke A, Wilcox J, Gibbon F, Smith PE, Archer H, et al. Epilepsy in Rett syndrome: association between phenotype and genotype, and implications for practice. Seizure 2011; 20: 646–9. [DOI] [PubMed] [Google Scholar]
- Coker SB, Melnyk AR. Rett syndrome and mitochondrial enzyme deficiencies. J Child Neurol 1991; 6: 164–6. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998; 17: 407–29. [PubMed] [Google Scholar]
- Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia 1993; 34: 592–6. [DOI] [PubMed] [Google Scholar]
- Cuddapah VA, Pillai RB, Shekar KV, Lane JB, Motil KJ, Skinner SA, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J Med Genet 2014; 51: 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeg-Olofsson O, al-Zuhair AG, Teebi AS, Daoud AS, Zaki M, Besisso MS, et al. Rett syndrome: a mitochondrial disease?. J Child Neurol 1990; 5: 210–4. [DOI] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005; 46: 470–2. [DOI] [PubMed] [Google Scholar]
- Garcia-Rudaz C, Deng V, Matagne V, Ronnekleiv OK, Bosch M, Han V, et al. FXYD1, a modulator of Na,K-ATPase activity, facilitates female sexual development by maintaining gonadotrophin-releasing hormone neuronal excitability. J Neuroendocrinol 2009; 21: 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze DG. Neurophysiology of Rett syndrome. J Child Neurol 2005; 20: 740–6. [DOI] [PubMed] [Google Scholar]
- Glaze DG, Percy AK, Skinner S, Motil KJ, Neul JL, Barrish JO, et al. Epilepsy and the natural history of Rett syndrome. Neurology 2010; 74: 909–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze DG, Schultz RJ, Frost JD. Rett syndrome: characterization of seizures versus non-seizures. Electroencephalogr Clin Neurophysiol 1998; 106: 79–83. [DOI] [PubMed] [Google Scholar]
- Goyal M, O’Riordan MA, Wiznitzer M. Effect of topiramate on seizures and respiratory dysrhythmia in Rett syndrome. J Child Neurol 2004; 19: 588–91. [DOI] [PubMed] [Google Scholar]
- Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B, et al. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis 2012; 48: 102–14. [DOI] [PubMed] [Google Scholar]
- Haas RH, Rice MA, Trauner DA, Merritt TA, Opitz JM, Reynolds JF. Therapeutic effects of a ketogenic diet in rett syndrome. Am J Med Genet 1986; 25(S1): 225–46. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol 2002; 6: 293–7. [DOI] [PubMed] [Google Scholar]
- Halbach NS, Smeets EE, Steinbusch C, Maaskant MA, van Waardenburg D, Curfs LM. Aging in Rett syndrome: a longitudinal study. Clin Genet 2013; 84: 223–9. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS statistics for windows. 22nd edn Armonk, NY: IBM Corp; 2013. [Google Scholar]
- Ito-Ishida A, Ure K, Chen H, Swann JW, Zoghbi HY. Loss of MeCP2 in Parvalbumin-and Somatostatin-expressing neurons in mice leads to distinct Rett syndrome-like phenotypes. Neuron 2015; 88: 651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Christodoulou J, Leonard H. Seizures in Rett syndrome: an overview from a one-year calendar study. Eur J Paediatr Neurol 2007; 11: 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim do Y, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol 2015; 78: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data 2000; 314: 1–27. [PubMed] [Google Scholar]
- Kumandas S, Caksen H, Ciftci A, Ozturk M, Per H. Lamotrigine in two cases of Rett syndrome. Brain Dev 2001; 23: 240–2. [DOI] [PubMed] [Google Scholar]
- Lane JB, Lee HS, Smith LW, Cheng P, Percy AK, Glaze DG, et al. Clinical severity and quality of life in children and adolescents with Rett syndrome. Neurology 2011; 77: 1812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard H, Downs J, Jian L, Bebbington A, Jacoby P, Nagarajan L, et al. Valproate and risk of fracture in Rett syndrome. Arch Dis Child 2010; 95: 444–8. [DOI] [PubMed] [Google Scholar]
- Leonard H, Ravikumara M, Baikie G, Naseem N, Ellaway C, Percy A, et al. Assessment and management of nutrition and growth in Rett syndrome. J Pediatr Gastroenterol Nutri 2013; 57: 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod F, Ganley R, Williams L, Selfridge J, Bird A, Cobb SR. Reduced seizure threshold and altered network oscillatory properties in a mouse model of Rett syndrome. Neuroscience 2013; 231: 195–205. [DOI] [PubMed] [Google Scholar]
- Motil KJ, Barrish JO, Lane J, Geerts SP, Annese F, McNair L, et al. Vitamin D deficiency is prevalent in girls and women with Rett syndrome. J Pediatr Gastroenterol Nutri 2011; 53: 569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motil KJ, Morrissey M, Caeg E, Barrish JO, Glaze DG. Gastrostomy placement improves height and weight gain in girls with Rett syndrome. J Pediatr Gastroenterol Nutri 2009; 49: 237–42. [DOI] [PubMed] [Google Scholar]
- Muller A, Helbig I, Jansen C, Bast T, Guerrini R, Jahn J, et al. Retrospective evaluation of low long-term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5-related epilepsy. Eur J Paediatr Neurol 2015; 20: 147–51. [DOI] [PubMed] [Google Scholar]
- Munger Clary H, Choi H. Prognosis of intractable epilepsy: is long-term seizure freedom possible with medical management?. Curr Neurol Neurosci Rep 2011; 11: 409–17. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol 2010; 68: 944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissenkorn A, Levy-Drummer RS, Bondi O, Renieri A, Villard L, Mari F, et al. Epilepsy in Rett syndrome–lessons from the Rett networked database. Epilepsia 2015; 56: 569–76. [DOI] [PubMed] [Google Scholar]
- Pintaudi M, Calevo MG, Vignoli A, Baglietto MG, Hayek Y, Traverso M, et al. Antiepileptic drugs in Rett syndrome. Eur J Paediatr Neurol 2015; 19: 446–52. [DOI] [PubMed] [Google Scholar]
- SAS Institute 9.4 edn The SAS system for Windows. Release 9.4 Cary, NC: SAS Institute Inc.; 2016. [Google Scholar]
- Specchio N, Balestri M, Striano P, Cilio MR, Nardello R, Patane S, et al. Efficacy of levetiracetam in the treatment of drug-resistant Rett syndrome. Epilepsy Res 2010; 88: 112–17. [DOI] [PubMed] [Google Scholar]
- Steffenburg U, Hagberg G, Hagberg B. Epilepsy in a representative series of Rett syndrome. Acta Paediatr 2001; 90: 34–9. [DOI] [PubMed] [Google Scholar]
- Tarquinio DC, Hou W, Neul JL, Kaufmann WE, Glaze D, Motil K, et al. The changing face of survival in Rett syndrome and MECP2-related disorders. Pediatr Neurol 2015a; 53: 402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquinio DC, Hou W, Neul JL, Lane JB, Barnes KV, O’Leary HM, et al. Age of diagnosis in Rett syndrome: patterns of recognition among diagnosticians and risk factors for late diagnosis. Pediatr Neurol 2015b; 52: 585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquinio DC, Motil KJ, Hou W, Lee HS, Glaze DG, Skinner SA, et al. Growth failure and outcome in Rett syndrome: specific growth references. Neurology 2012; 79: 1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Andrade R, Moldenhauer R, Gutierrez-Bertin N, Soto-Covasich J, Mancilla-Medina C, Ehrenfeld C, et al. The increase in body weight induced by lack of methyl CpG binding protein-2 is associated with altered leptin signalling in the hypothalamus. Exp Physiol 2014; 99: 1229–40. [DOI] [PubMed] [Google Scholar]
- Vignoli A, La Briola F, Peron A, Turner K, Savini M, Cogliati F, et al. Medical care of adolescents and women with Rett syndrome: an Italian study. Am J Med Genet Part A 2012; 158A: 13–8. [DOI] [PubMed] [Google Scholar]
- Yuen AW, Sander JW. Impaired mitochondrial energy production: the basis of pharmacoresistance in epilepsy. Med Hypotheses 2011; 77: 536–40. [DOI] [PubMed] [Google Scholar]
- Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci 2014; 34: 2754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.