There are conflicting data on the origin and progression of neurodegeneration in semantic variant primary progressive aphasia (svPPA). Collins et al. identify a left temporal pole region with consistent atrophy in two patient cohorts. The region’s connectivity in healthy adults predicts the localization and magnitude of distributed atrophy in svPPA.
Keywords: atrophy, resting state connectivity, primary progressive aphasia, semantic dementia, temporal lobe
Abstract
A wealth of neuroimaging research has associated semantic variant primary progressive aphasia with distributed cortical atrophy that is most prominent in the left anterior temporal cortex; however, there is little consensus regarding which region within the anterior temporal cortex is most prominently damaged, which may indicate the putative origin of neurodegeneration. In this study, we localized the most prominent and consistent region of atrophy in semantic variant primary progressive aphasia using cortical thickness analysis in two independent patient samples (n = 16 and 28, respectively) relative to age-matched controls (n = 30). Across both samples the point of maximal atrophy was located in the same region of the left temporal pole. This same region was the point of maximal atrophy in 100% of individual patients in both semantic variant primary progressive aphasia samples. Using resting state functional connectivity in healthy young adults (n = 89), we showed that the seed region derived from the semantic variant primary progressive aphasia analysis was strongly connected with a large-scale network that closely resembled the distributed atrophy pattern in semantic variant primary progressive aphasia. In both patient samples, the magnitude of atrophy within a brain region was predicted by that region’s strength of functional connectivity to the temporopolar seed region in healthy adults. These findings suggest that cortical atrophy in semantic variant primary progressive aphasia may follow connectional pathways within a large-scale network that converges on the temporal pole.
Introduction
The semantic variant of primary progressive aphasia (svPPA) is a devastating, ultimately fatal, neurodegenerative disease characterized by the progressive loss of semantic memory (Warrington, 1975; Snowden et al., 1989; Hodges et al., 1992; Hodges and Patterson, 2007; Gorno-Tempini et al., 2011). A wealth of neuroimaging studies have identified an extensive pattern of asymmetric bilateral cortical and subcortical neurodegeneration in svPPA, which is most prominent in the left anterior temporal cortex and encompasses a large portion of the temporal lobes (reviewed in Whitwell and Josephs, 2012; Diehl-Schmid et al., 2014). As svPPA progresses, the pattern of atrophy observed becomes more distributed, and envelops inferior frontal, anterior insular, anterior cingulate, posterior temporal, and parietal cortex (Czarnecki et al., 2008; Brambati et al., 2009, 2015; Rohrer et al., 2009), as well as the ventral striatum and amygdala. SvPPA is usually considered to be a subset of individuals with semantic dementia (Adlam et al., 2006), which has a heterogeneous clinical presentation including impairments in language, visual knowledge, multimodal semantic memory, and social cognition and behavioural regulation (Chan et al., 2009; Josephs et al., 2009). Whereas a proportion of patients meeting the criterion for semantic dementia (∼30%) present initially with atrophy that is more pronounced in the right than left anterior temporal lobes (Chan et al., 2009; Josephs et al., 2009), svPPA is always associated with early atrophy that is most pronounced in the left anterior temporal lobe. Here, we chose to focus on patients meeting the criteria for svPPA (Gorno-Tempini et al., 2011), which emphasizes predominant semantic language impairment and left anterior temporal atrophy.
The localization of the most prominent neurodegeneration (as measured by atrophy or hypometabolism) in svPPA, which may indicate the putative origin of neurodegeneration, is a subject of debate. While some studies have localized the area of greatest degenerative change to the anterior fusiform or inferior temporal gyrus (Seeley et al., 2009), others consider the temporal pole (Mummery et al., 2000; Galton et al., 2001; Davies et al., 2009; Rohrer et al., 2009), entorhinal cortex (Chan et al., 2001), or perirhinal cortex (La Joie et al., 2014) to be the most prominent region of neurodegeneration. Although these discrepancies might be the result of biological variability between samples, they may also arise from differences in the neuroimaging analysis techniques used, which include voxel-based (VBM; Mummery et al., 2000; Gorno-Tempini et al., 2004; Seeley et al., 2009), manual region of interest tracing (Chan et al., 2001; Galton et al., 2001), cortical thickness analyses (Rohrer et al., 2009), or voxel-based analysis of hypometabolism obtained using 18Fluorodeoxyglucose (FDG-PET; La Joie et al., 2014). No studies have attempted to assess the reliability of effects across multiple samples of patients with svPPA.
Beyond the anterior temporal cortex, a distributed set of brain regions undergoes neurodegeneration in svPPA. Support for the hypothesis that neurodegenerative diseases selectively target large-scale networks identifiable in the healthy brain (Mesulam, 2000; Buckner et al., 2005; Seeley et al., 2009) has come from studies demonstrating considerable overlap between the topography of atrophy in patients with neurodegenerative diseases and that of distributed neural networks identified in healthy subjects using resting state functional connectivity (Seeley et al., 2009; Zhou et al., 2012; La Joie et al., 2014) or diffusion-weighted imaging (Raj et al., 2012). With regard to networks relevant to svPPA, a recent study from our lab has identified the anterior temporal cortex as a point of convergence for four large-scale sensory, association, and paralimbic functional-anatomic brain networks. In svPPA, physiological abnormalities appear to extend all the way back to posterior primary visual and auditory sensory cortex (Guo et al., 2013). These previous findings demonstrating the functional heterogeneity (Ding et al., 2009; Pascual et al., 2015) and distributed connectivity (Guo et al., 2013) of the anterior temporal lobes underscore the importance of identifying the precise focal point of neurodegeneration in svPPA and the intrinsic connectivity of this region in the healthy brain. Knowledge of the putative site of origin of neurodegeneration in svPPA and its connectivity will further elucidate whether principles governing the organization of large-scale functional networks provide insights into the distributed atrophy patterns in svPPA.
In the present study we used cortical thickness analysis and surface-based inter-subject registration to precisely localize the point of maximal atrophy in svPPA using an unbiased whole-cortex approach. We expanded upon previous analyses of focal atrophy in svPPA by investigating the reliability of the localization of this focal atrophy point in two independent samples, examining both the maximal atrophy point for each of the two sample groups versus older healthy controls, as well as the overlap of the maximal atrophy point between individual subjects. We then used resting state functional connectivity MRI (rs-fcMRI) in healthy young adults to assess the intrinsic network connectivity of this region. We predicted that the point of maximal atrophy in svPPA (i) would be replicable across independent patient samples and across a series of individual patients; and (ii) would be interconnected with an intrinsic neural network in healthy adults whose topography mimics the topography of distributed atrophy in svPPA. The latter finding would support previous observations that the topography of regional atrophy in patients with neurodegenerative diseases respects the topography of large-scale networks in the healthy brain and improve our understanding of disease onset and progression in svPPA. We further predicted, as a novel test of the network neurodegeneration hypothesis, that the strength of connectivity of every region within the large-scale healthy network to the putative onset site would predict the magnitude of that region’s atrophy in svPPA. Such a finding would extend previous tests of the network neurodegeneration hypothesis (Zhou et al., 2012) by further supporting the notion that progressive neurodegeneration in svPPA follows the topography of connectional architecture and prioritizes the strongest connections from the site of origin.
Materials and methods
Participants
Control groups
The sample for the cortical thickness analysis included 30 older adults (15 females, age mean = 65.06, SD = 6.18, range = 52–80 years) who underwent detailed clinical and cognitive assessments and were determined to be cognitively normal. The sample for the rs-fcMRI analysis included 89 young adults [45 females, age mean = 22.4, standard deviation (SD) = 3.34, range = 18–33 years]. Our choice of a young adult healthy control group for the rs-fcMRI analysis was motivated by the fact that the topography of large-scale intrinsic networks is largely preserved across age, and recent work (Grothe et al., 2016) has shown that intrinsic networks identified in healthy young adults recapitulate the patterns of pathology, hypometabolism, and atrophy in neurodegenerative disease. All control participants were right-handed native English speakers with normal or corrected-to-normal vision, and no history of neurological or psychiatric disorders. All participants gave written informed consent in accordance with the institutional review board of the Massachusetts General Hospital and Partners Healthcare System Human Research Committee.
Patients
Two independent samples of patients with svPPA were included in this study. The first patient sample was recruited from an ongoing longitudinal study being conducted in the Massachusetts General Hospital (MGH) Frontotemporal Disorders Unit. The MGH sample included 16 patients with svPPA (nine females, age mean = 64.01, SD = 7.37, range = 53–78 years). The second patient sample was recruited from the Memory and Aging Center at the University of California San Francisco (UCSF). The UCSF sample included 28 svPPA patients (18 females, age mean = 63.84, SD = 8.1, range = 49–80 years). All participants in both samples gave written informed consent, and the institutional review boards at MGH/Partners and UCSF approved the relevant studies. All patients with svPPA in both samples (Tables 1 and 2) were evaluated using a structured clinical assessment performed by a behavioural neurologist and speech pathologist, as previously described (Sapolsky et al., 2010; Wilson et al., 2014).
Table 1.
Demographic and clinical data for the MGH svPPA patient sample
| Case | Age | Age of onset | Gender | Education | CDR | MMSE | BNT (%) | CBS Naming (%) | CBS WPM (%) | PPT (%) | WAB SS | WAB Rep (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | 47 | M | 20 | 0.5 | 20 | 3% | 27% | 63% | 84% | ||
| 2 | 72 | 62 | F | 18 | 0.5 | 23 | 3 | 83 | 9 | 90 | ||
| 3 | 70 | 65 | F | 12 | 0.5 | - | 59 | 73 | 6 | 69 | ||
| 4 | 59 | 54 | F | 12 | 0.5 | 25 | 13 | 63 | 68 | 8 | ||
| 5 | 79 | 74 | M | 18 | 0.5 | 19 | 33 | 91 | 81 | 10 | 96 | |
| 6 | 58 | 55 | M | 16 | 1.0 | 28 | 50 | 50 | 8 | |||
| 7 | 71 | 66 | M | 12 | 0.5 | 19 | 69 | 94 | 10 | |||
| 8 | 61 | 57 | M | 14 | 0.5 | 24 | 20 | 53 | 10 | |||
| 9 | 70 | 62 | F | 18 | 0.5 | 25 | 18 | 19 | 83 | 8 | ||
| 10 | 63 | 61 | F | 16 | 0.5 | 6 | ||||||
| 11 | 55 | 49 | F | 16 | 0.5 | 24 | 7 | 58 | 75 | 85 | ||
| 12 | 53 | 49 | F | 18 | - | - | 7 | 75 | 6 | |||
| 13 | 62 | 60 | F | 13 | 1.0 | 21 | 42 | 71 | 78 | |||
| 14 | 61 | 57 | M | 16 | 0.5 | 24 | 3 | 19 | 54 | 93 | ||
| 15 | 61 | 57 | F | 16 | 0.0 | 27 | 27 | |||||
| 16 | 62 | 58 | M | 20 | 0.0 | 28 | 57 | 94 | 100 | 10 | 98 |
Empty cells indicate missing data.
BNT = Boston Naming Task; CBS Naming = Cambridge Semantic Battery Naming Task; CBS WPM = Cambridge Semantic Battery Word Picture Matching Task; CDR = global score on the Clinical Dementia Rating Scale; MMSE = global score on the Mini-Mental Status Examination; PPT = Pyramids and Palm Trees; WAB Rep = Western Aphasia Battery Repetition Test; WAB SS = Western Aphasia Battery Spontaneous Speech (0 = severely impaired, 10 = normal).
Table 2.
Demographic and clinical data for UCSF svPPA patient sample
| Case | Age | Age of onset | Gender | Education | CDR | MMSE | BNT (%) | PPVT | WAB AWRT (%) | PPT (%) | WAB SS | WAB Rep (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | 53 | M | 12 | 0.5 | 23 | 13 | 100 | ||||
| 2 | 75 | 72 | F | 16 | 1 | 22 | 67 | 62 | 10 | 98 | ||
| 3 | 62 | 60 | M | 18 | 0.5 | 3 | 87 | |||||
| 4 | 61 | 57 | M | 18 | 0.5 | 28 | 27 | 100 | 9 | 82 | ||
| 5 | 71 | 68 | F | 16 | 0 | 28 | 33 | 38 | 94 | |||
| 6 | 65 | 60 | M | 17 | 0.5 | 16 | 0 | 100 | 100 | 8 | 70 | |
| 7 | 56 | 54 | F | 22 | 0 | 30 | 47 | 92 | 10 | 96 | ||
| 8 | 73 | 72 | F | 16 | 0 | 19 | 7 | |||||
| 9 | 68 | 66 | F | 19 | 0.5 | 23 | 6 | 50 | ||||
| 10 | 67 | 60 | F | 14 | 1 | 7 | 13 | 100 | 52 | 60 | ||
| 11 | 58 | 56 | M | 16 | 0.5 | 29 | 20 | 87 | 92 | 9 | 100 | |
| 12 | 62 | 57 | F | 14 | 0.5 | 19 | 0 | 72 | 56 | 9 | 96 | |
| 13 | 62 | 58 | F | 14 | 0.5 | 8 | 13 | 93 | 83 | 6 | 60 | |
| 14 | 60 | 53 | F | 18 | 0.5 | 26 | 13 | 19 | 85 | 65 | 10 | 95 |
| 15 | 80 | 71 | M | 18 | 1 | 13 | 25 | 85 | 56 | 7 | 97 | |
| 16 | 56 | 50 | M | 12 | 0.5 | 20 | 7 | 38 | 100 | 71 | 9 | 84 |
| 17 | 64 | 52 | F | 12 | 0.5 | 28 | 20 | 63 | 100 | 65 | 9 | 93 |
| 18 | 65 | 61 | M | 16 | 0.5 | 29 | 40 | 69 | 100 | 85 | 10 | 94 |
| 19 | 61 | 57 | F | 11 | 1 | 30 | 53 | 25 | 97 | 87 | ||
| 20 | 64 | 59 | M | 13 | 0.5 | 26 | 13 | 25 | 58 | 67 | 10 | 97 |
| 21 | 67 | 56 | M | 20 | 1 | 12 | 13 | 31 | 98 | 8 | 73 | |
| 22 | 49 | 48 | M | 12 | 0.5 | 26 | 27 | 75 | 100 | 88 | 9 | 81 |
| 23 | 72 | 67 | M | 16 | 0.5 | 30 | 67 | 69 | 100 | 92 | 9 | 100 |
| 24 | 66 | 60 | F | 19 | 1 | 26 | 67 | 69 | 95 | 90 | 10 | 93 |
| 25 | 55 | 53 | M | 14 | 1 | 27 | 27 | 56 | 95 | 9 | 89 | |
| 26 | 74 | 72 | F | 0.5 | 22 | 7 | 38 | 81 | 9 | 89 | ||
| 27 | 57 | 46 | F | 18 | 1 | 28 | 33 | 96 | ||||
| 28 | 61 | 57 | M | 18 | 1 | 15 | 7 |
Empty cells denote missing data.
BNT = Boston Naming Task; CBS Naming = Cambridge Semantic Battery Naming Task; CBS WPM = Cambridge Semantic Battery Word Picture Matching Task; CDR = global score on the Clinical Dementia Rating Scale; MMSE = global score on the Mini-Mental Status Examination; PPT = Pyramids and Palm Trees; WAB Rep = Western Aphasia Battery Repetition Test; WAB SS = Western Aphasia Battery Spontaneous Speech (0 = severely impaired, 10 = normal).
Patients were diagnosed with semantic variant PPA based on recent guidelines (Gorno-Tempini et al., 2011). A diagnosis of PPA required progressive deterioration of speech and/or language functions, and that deficits be largely restricted to speech and/or language for at least the first 2 years of the illness (Mesulam, 2003). All patients included here had semantic language impairments and cortical atrophy that was most prominent in the left anterior temporal lobes upon first assessment. Visual inspection of a clinical MRI ruled out other causes of focal brain damage and in all cases provided imaging support for the diagnosis (anterior temporal atrophy).
Image acquisition
Massachusetts General Hospital sample
Imaging data for the MGH patient sample were collected on a 3 T Magnetom Tim Trio system at MGH (Siemens), using a 12-channel phased-array head coil. Structural data for the MGH patient sample were acquired using a standard T1-weighted 3D MPRAGE sequence that varied slightly across patients. Nine patients were scanned using the following parameters: repetition time/echo time/flip angle = 2.53 s/3.48 ms/12°, resolution = 1.0 m isotropic. Three patients were scanned using the following parameters: repetition time/echo time/flip angle = 2.53 s/1.61 ms/12°, resolution = 1.0 m isotropic. Three patients were scanned using the following parameters: repetition time/echo time/flip angle = 2.30 s/2.98 ms/16°, resolution = 1.0 m isotropic. One patient was scanned using the following parameters: repetition time/echo time/flip angle = 2.53 s/6.8 ms/16°, resolution = 1.0 m isotropic.
University of California San Francisco sample
Structural data for the UCSF patient sample were acquired on a 1.5 T Magnetom VISION system at UCSF (Siemens), using a standard quadrature head coil. For each patient a T1-weighted image of the entire brain was obtained using a MPRAGE sequence (repetition time/echo time/flip angle = 10 s/4 ms/15°, 1.0 × 1.0 mm2 in-plane resolution, 1.5 mm slab thickness).
Healthy controls
Imaging data for both healthy adult samples were collected on a 3 T Magnetom Tim Trio system at MGH (Siemens), using a 12-channel phased-array head coil. Structural data for the older control sample (n = 30) were acquired using one of two T1-weighted 3D MPRAGE sequences (repetition time/echo time/flip angle = 2.30 s/2.98 ms/16°, or repetition time/echo time/flip angle = 2.53 s/3.45 ms/12°, resolution = 1.0 mm isotropic for both sequences). Structural data for the young control sample (n = 89) were also acquired using a T1-weighted 3D MPRAGE sequence (repetition time/echo time/flip angle = 2.2 s/1.54 ms/7° resolution = 1.2 mm isotropic). Functional MRI data for the young control sample were acquired for 6 mins during rest using a gradient-echo, echo-planar sequence with the following parameters: repetition time = 3000 ms; echo time = 30 ms; flip angle = 85°, 47 slices; voxel resolution: 3 mm isotropic. During the rs-fcMRI run participants were directed to keep their eyes open and to remain as still as possible.
Data processing and statistical analysis
Cortical thickness analyses
Cortical reconstructions of the T1-weighted images were performed using the FreeSurfer analysis suite version 5.3.0 (Supplementary Fig. 1). Two independent investigators checked every coronal slice of the MGH svPPA patient sample after manual edits were made to ensure that the newly formed white matter and pial surfaces respected the topography of the underlying anatomy. The scans of the healthy controls did not require manual editing. This boundary was then used by the deformable surface algorithm to identify the pial surface (Fischl and Dale, 2000) for each subject. Cortical thickness was measured by calculating the distance between the white matter and pial surfaces at ∼160 000 points (vertices) in each cerebral hemisphere. Each subject’s reconstructed brain was then morphed and registered to an average spherical space that optimally aligned gyral and sulcal features, thus enabling the accurate matching of cortical locations among individuals across the entire cortical surface. Individual thickness measures were mapped into this new space. A Gaussian kernel of 10 mm full-width at half-maximum was applied to the subjects’ cortical thickness maps before further analyses.
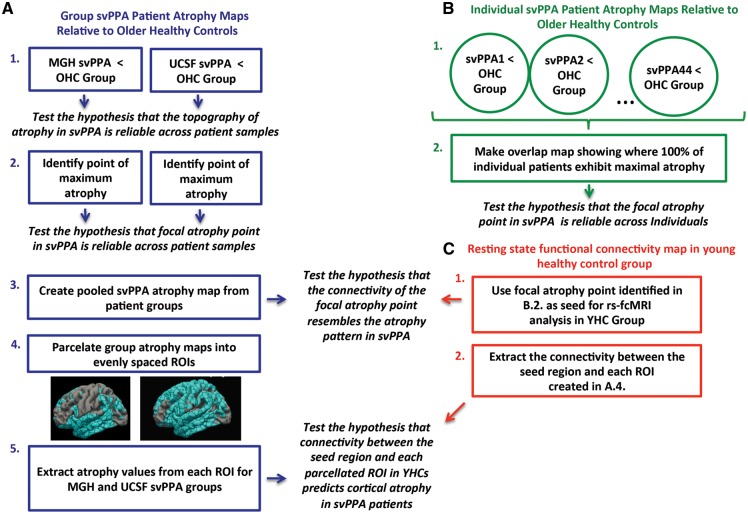
Cortical thickness group comparisons were performed using a 2-class general linear model (GLM) for the effect of clinical status, as implemented in FreeSurfer. Several group analyses were performed: one comparing each patient sample (MGH and UCSF) to the older healthy control group, one comparing the pooled (MGH and UCSF combined) svPPA patient sample to the older healthy control group, and one comparing each individual patient to the entire older healthy control group (Fig. 1).
Figure 1.
Schematic representation of study design. Three independent analysis streams were used to test hypotheses (displayed in italics) about the topography and point of focal atrophy in svPPA. The steps used in each analysis stream are numbered and enclosed by squares. Arrows point to the specific hypothesis tested in each analysis step. (A) Analyses performed on group maps of cortical atrophy in the MGH and UCSF svPPA samples. (B) Analyses performed on individual maps of cortical atrophy across both svPPA patient samples. (C) Analyses performed on rs-fcMRI map derived from a young healthy control (YHC) group. OHC = older healthy control group; ROI = region of interest.
Resting state functional connectivity MRI analyses
All rs-fcMRI data were first preprocessed using the following steps: removal of the first four functional volumes to allow for T1-equilibration effects, slice timing correction (SPM2, Wellcome Department of Cognitive Neurology, London, UK), head motion correction using rigid-body transformation in three translations and three rotations (FMRIB, Oxford, UK), spatial normalization to standard (MNI-152) atlas space, resampling to 2 mm isotropic voxels, spatial smoothing with a 6 mm full-width at half-maximum Gaussian kernel, and low-pass temporal filtering to remove frequencies >0.08 Hz. Sources of spurious variance and their temporal derivatives were removed from the preprocessed data using linear regression. These sources of spurious variance included the six parameters derived from head-motion correction, the signal averaged over the whole brain, the average signal from a region located deep in white matter, and the average signal from ventricular CSF. A 4 mm spherical seed region (region of interest) was generated centred on the focal svPPA atrophy point identified in the cortical thickness analysis. A correlation map for this seed was generated by computing the Pearson’s product moment correlation ‘r’ between every voxel in the brain and the average mean signal time course of the voxels within this sphere. Positive values in this map were then converted to z-scores using Fisher’s r-to-z transformation for comparison with a pooled cortical atrophy map derived from both svPPA patient samples. In keeping with previous work (Pascual et al., 2015) we chose a liberal threshold (z > 0.04) that has been frequently used in rs-fcMRI studies because it provides results that are similar to the results obtained with independent component analysis (Van Dijk et al., 2010). Non-parametric permutation testing (5000 permutations) was performed on the resulting z-score maps using FSL Randomise (Winkler et al., 2014), correcting for multiple comparisons using the threshold-free cluster enhancement (TFCE) technique [statistical threshold was set at P < 0.05, family-wise error (FWE) corrected]. All rs-fcMRI results were projected to the pial surface for comparison with the results of the cortical thickness analyses.
Relating connectivity to atrophy
First, the entire cortex of the average spherical surface was microparcellated into 642 regions of interest of similar area (mean = 255.2 vertices, SD = 2.49 vertices) per hemisphere, without adhering to any anatomical boundaries. Only regions of interest that had at least 70% of their vertices within the atrophy mask (binarized at P < 0.01) for each sample and at least one vertex with a rs-fcMRI z-value > 0.04 were used in further analysis, resulting to a total of 209 regions of interest for the MGH sample and 275 for the UCSF sample. The mean cortical thickness for each region of interest (averaged across all vertices within the region of interest) was calculated for each patient sample using the appropriate sample-specific atrophy mask. Mean cortical thickness for all regions of interest in both patient sample atrophy masks was also calculated for the older healthy control group. The relative percentage of atrophy in each region of interest was then calculated for each patient sample separately using the following formula: [1 − (mean patient cortical thickness / mean older healthy control group cortical thickness)] × 100.
The average normalized (z) rs-fcMRI of each sample-specific region of interest with the focal atrophy point was also extracted from the young healthy control group rs-fcMRI map. The Pearson’s product-moment correlation coefficient ‘r’ was then computed between the rs-fcMRI of each region of interest in young healthy control groups and cortical atrophy in each region of interest for each patient group. The region of interest containing the temporal pole seed for the rs-fcMRI map was excluded from both correlation analyses to avoid artificially inflating the estimated connectivity of this region. As an additional control we repeated both analyses using partial correlations controlling for the Euclidean distance (in MNI space) between the centre of each region of interest, and the centre of the temporal pole seed region.
Results
The focal point of cortical atrophy in svPPA is localized to the left temporal pole
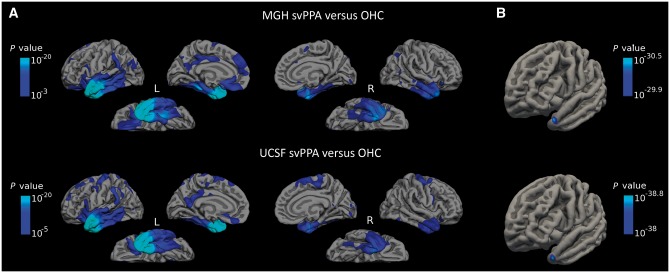
Both svPPA patient samples demonstrated substantial cortical atrophy relative to healthy controls that was—while most prominent in the left anterior temporal cortex—distributed throughout portions of left medial and lateral temporal cortex, left anterior insula, left ventromedial prefrontal and orbitofrontal cortex, left anterior and posterior cingulate, and left inferior parietal lobule (Fig. 2A); the right anterior temporal cortex was also consistently atrophied, although generally to a much lesser degree than the left. Visual inspection of the maps revealed more widespread cortical atrophy in the UCSF patient sample (than in the MGH sample) that extended into the left and right superior frontal gyrus. This likely reflects the difference in sample size (28 versus 16) and resultant differences in statistical sensitivity between the two patient groups. To localize the most atrophied point in both patient samples, we imposed a threshold on each group map that included vertices with atrophy P-values in the top 98% (min 10−30 and 10−38 for the MGH and UCSF samples, respectively). This stringent thresholding localized a global maxima for each patient group that was located in area TG of the left temporal pole (Ding et al., 2009; Pascual et al., 2015) that was strikingly similar across both patient groups (MNI coordinates for the MGH sample: −42, 16, −30, UCSF sample: −42, 16, −30, Fig. 2B).
Figure 2.
Cortical atrophy is localized in a highly consistent fashion in MGH and UCSF svPPA samples, and is most prominent in the temporal pole. (A) Relative to healthy controls, svPPA patients from the MGH sample (top row) and UCSF sample (bottom row) displayed an asymmetric pattern of cortical atrophy that was most prominent in the left anterior temporal cortex. Due to differences in the sample size of each patient group, different colour-scale values were used to allow optimal visualization of the localization of atrophy. (B) The maximal point of cortical atrophy in both patient groups was localized in the left temporal pole, consistent with area TG. OHC = older healthy control group.
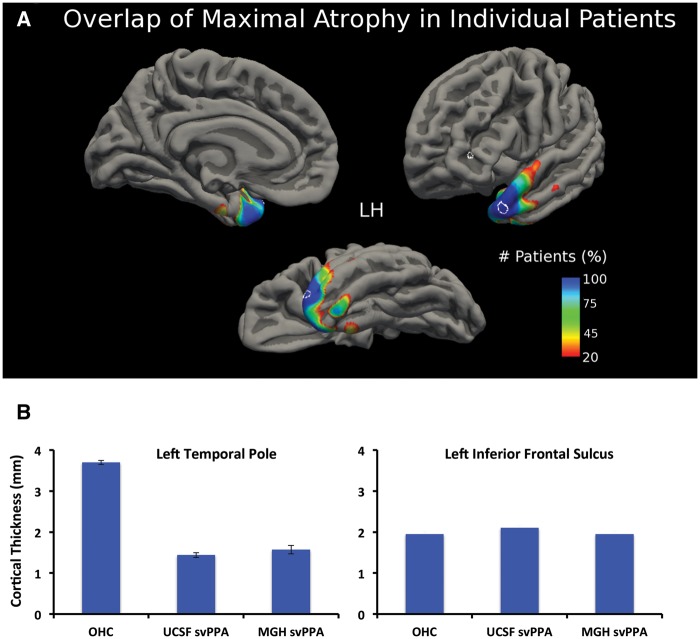
The focal point of cortical atrophy is highly reliable across individual patients
Individual patterns of cortical atrophy can vary substantially within patients of the same diagnosis, and do not necessarily mirror atrophy patterns identified at the group level. Thus, in the present study we assessed the similarity of atrophy patterns in single patients by computing the vertex-wise difference in cortical thickness of each of the 44 patients with svPPA against the group of older adult healthy controls. Each of the resulting single-subject maps was then binarized to include only the top 1% of P-values. These maps were then combined creating a heat-map displaying—for each vertex—the percentage of patients with this region as their most atrophic point (Fig. 3A). Remarkably, the maximal atrophic point (top 1% of P-values) for 100% of the patients with svPPA was located in a small region of the left lateral temporal pole (MNI coordinates: −43, 15, −29) and in a small region of the left medial temporal pole (MNI coordinates: −27, 8, −36). Other regions, such as the rostral fusiform gyrus or the rostral tip of the temporal pole were affected in at least 75% of patients. The region identified in the lateral temporal pole that was affected maximally in 100% of patients overlaps entirely with the focal atrophy point identified in our previous analysis of focal atrophy in each patient group (Fig. 2B). Cortical thickness in this region did not differ significantly between the two patient populations (P = 0.27, Fig. 3B), but was much lower in patients relative to controls (P = 1.01 × 10−42). Notably, average thickness in the patient group was 1.49 mm, while in controls it was 3.7 mm, a loss of >50% of the cortical mantle in patients. An additional analysis of mean cortical thickness in a control region in the inferior frontal sulcus, which was selected because it falls outside of the typical pattern of cortical atrophy in patients with svPPA (reviewed in Diehl-Schmid et al., 2014), revealed no difference between patients and controls (P = 0.25).
Figure 3.
Cortical atrophy in each individual svPPA patients overlaps with the same area of the left temporal pole identified in the group analysis. (A) A heat map based on individual patients atrophy maps revealed that 100% of patients had their maximal atrophy point (top 1% of cortical atrophy) in the same region of the left temporal pole, just lateral to the tip (circled; MNI coordinates: x = −42, y = 17, z = −31) and a small area at the medial region (MNI coordinates: x = −27, y = 7, z = −36). (B) Relative to controls, cortical thickness was substantially lower in patients in this focal atrophic point of the left temporal pole (thickness of this region in patients is less than half of that of controls). In contrast, a control region placed in the left inferior frontal sulcus did not differ in thickness between patients and controls (also circled in A). OHC = older healthy control group.
The distributed cortical connectivity of the healthy temporal pole resembles the distributed atrophy pattern in svPPA
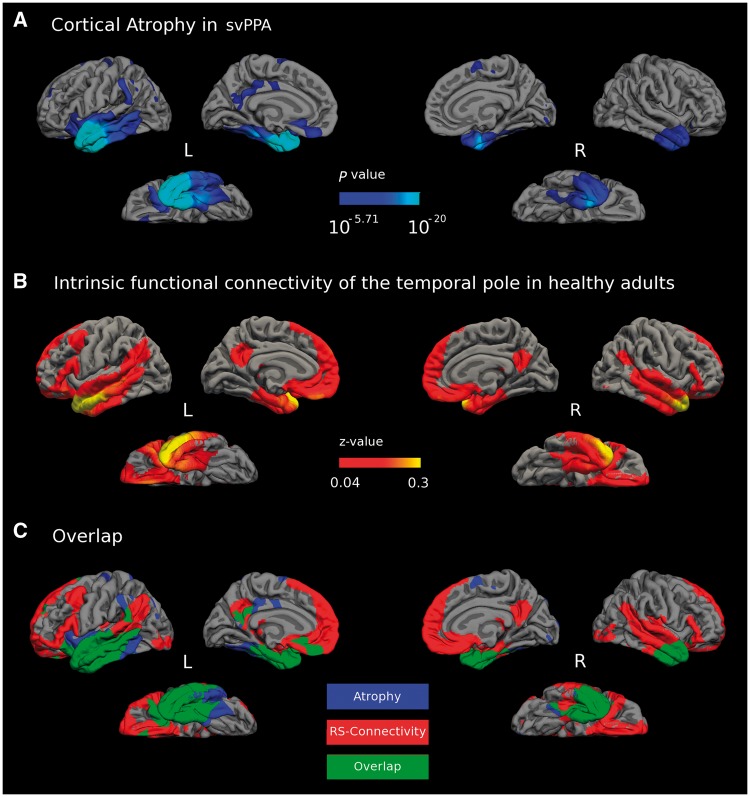
Investigating the distributed cortical connectivity of the tip of the temporal pole—the focal point of atrophy in svPPA—revealed a network of brain regions in healthy young adults (threshold at P < 0.05, FWE corrected) that enveloped 64.88% of the atrophy map identified in our cortical thickness analysis of the combined svPPA patient group (Fig. 4C). This connectivity pattern was present in portions of the bilateral anterior temporal cortices (parahippocampal gyrus, fusiform gyrus, inferior and middle temporal gyrus); frontal cortices (orbitofrontal cortex, medial, ventrolateral, and dorsolateral prefrontal cortex); inferior parietal lobule (angular gyrus and supramarginal gyrus); precuneus and posterior cingulate cortex. The area of focal atrophy also displayed functional connectivity with several subcortical brain regions, including the bilateral amygdala and hippocampi (data not shown). This network of brain regions is strikingly similar to a functional-anatomic network that our lab recently dubbed the default-semantic network (Pascual et al., 2015), which is interconnected with areas TG and TE of the temporal pole, and encompasses much of the default mode network (Buckner et al., 2008) and several regions implicated in semantic cognition (Binder et al., 2009). The earliest available MRI scan was used for the cortical thickness analysis of each svPPA patient included here. Thus, one possibility is that brain regions that are functionally connected with the temporal pole focal atrophy point, but not yet damaged in this sample, may be regions vulnerable to atrophy at later stages of the disease.
Figure 4.
Convergence of healthy large-scale network connectivity and atrophy in svPPA. (A) Pooled cortical atrophy map, including patients from both the MGH and UCSF samples (n = 44, FDR corrected P < 0.0001). (B) The temporal pole area of greatest atrophy in svPPA (Fig. 1B) was used as a seed to generate a resting-state (RS) functional connectivity map of a large-scale intrinsic connectivity network in healthy adults (FWE corrected P < 0.05). (C) The topography of these two maps shows substantial overlap (green) between the large-scale intrinsic temporal pole network in healthy individuals (binarized at z > 0.04, only including vertices that survived an FWE correction for multiple comparisons P < 0.05) (red) and cortical atrophy in the pooled patient sample (FDR corrected P < 0.0001, blue).
The strength of healthy connectivity within the large-scale intrinsic temporal pole network predicts the magnitude of cortical atrophy in svPPA
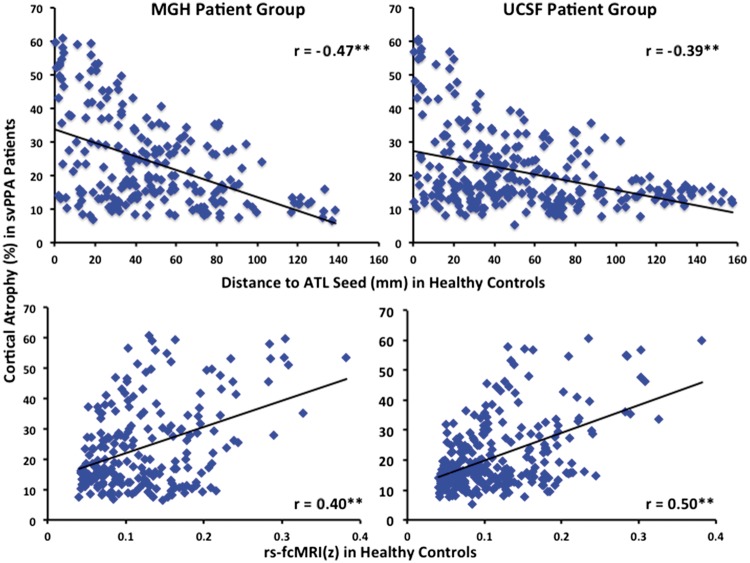
We computed the Pearson’s product-moment correlation coefficient ‘r’ between (i) the per cent of cortical atrophy in each microparcellated region of interest for each of the two svPPA patient samples; and (ii) the average normalized rs-fcMRI value (z) of each region of interest with the temporal pole seed region in young healthy control groups (Fig. 5A). In both patient samples a moderate-to-strong correlation was observed between the magnitude of cortical atrophy in a region of interest, and that region of interest’s rs-fcMRI with the temporal pole seed region in young healthy control groups (MGH: r = 0.4, P < 0.001; UCSF: r = 0.5, P < 0.001). We additionally computed Pearson’s ‘r’ between (i) the per cent of cortical atrophy in each region of interest for each svPPA sample; and (ii) the Euclidean distance (in MNI space) between each region of interest and the temporal pole seed region. In both patient samples a moderate-to-strong negative correlation was observed between the magnitude of cortical atrophy in a region of interest, and that region of interest’s Euclidean distance to the temporal pole seed (MGH: r = −0.47, P < 0.001; UCSF: r = −0.39, P < 0.001). The absolute values of the correlation coefficients between the magnitude of atrophy in each region of interest and that region of interest’s Euclidean distance to the seed, or rs-fcMRI with the seed, did not differ significantly in either patient group (MGH: z = 0.85, P = 0.39; UCSF: z = 1.68, P = 0.09). Furthermore, in each patient sample there was no significant correlation between the Euclidean distance of each region of interest to the temporal pole seed, and the rs-fcMRI of each region of interest with the temporal pole seed in young healthy control groups, suggesting that each measure might be contributing unique variance to the magnitude of atrophy in each region of interest in svPPA (MGH: r = −0.02, P = 0.74; UCSF: r = −0.11, P = 0.06).
Figure 5.
Strength of connectivity of distributed cortical regions with the temporal pole in healthy adults predicts magnitude of atrophy within distributed cortical regions in svPPA patients. Within each region of interest the Euclidean distance to the temporal pole (top), and the strength of its functional connectivity with the temporal pole in young healthy adults (bottom) predicted the magnitude of cortical atrophy in both svPPA patient cohorts (**P < 0.001). The region of interest containing the temporal pole seed for the rs-fcMRI map was excluded from all correlation analyses.
We conducted a series of partial correlation analyses to assess the unique contributions of temporopolar rs-fcMRI and Euclidean distance to the magnitude of atrophy in each region of interest. In both patient samples the effect of rs-fcMRI remained significant after controlling for the Euclidean distance (in MNI space) between each region of interest and the temporal pole seed (MGH: r = 0.47, P < 0.001; UCSF: r = 0.5, P < 0.001). Additionally, in both samples the effect of Euclidean distance remained significant after controlling for rs-fcMRI with the temporal pole seed (MGH: r = −0.52, P < 0.001; UCSF: r = −0.38, P < 0.001). We interpret these results to indicate that, if atrophy in svPPA is assumed to progress linearly, it progresses in a manner dictated by both the strength of connections with the site of origin and physical distance from the site of origin.
Discussion
Although the most prominent neurodegeneration in svPPA is unquestionably within the anterior temporal cortex, the precise localization of its epicentre has been a subject of debate. Using cortical thickness analysis and cortical surface-based registration in two independent samples of patients with svPPA, we found that the point of maximal cortical atrophy—likely the initial neurodegenerative lesion—is localized reliably in the left temporal pole, slightly dorsolateral to the tip. We further investigated the reliability of focal atrophy across individual patients and found that 100% of individuals in both svPPA samples had one of their maximally atrophic points in the same temporopolar region. Beyond the anterior temporal cortex, patients with svPPA exhibit less prominent neurodegeneration in distributed cortical and subcortical regions (Mummery et al., 2000; Rosen et al., 2002; Gorno-Tempini et al., 2004; Brambati et al., 2009; Rohrer et al., 2009). As predicted, the focal atrophy point identified in our cortical thickness analysis was interconnected with a network of brain regions in healthy adults that closely resembled the distributed cortical atrophy pattern of svPPA patients, replicating previous findings (Seeley et al., 2009). Furthermore, the magnitude of cortical atrophy within a given brain region in both samples of svPPA patients was predicted by that region’s connectivity strength and Euclidean distance to the temporal pole in healthy adults. This provides additional support for the hypothesis that progressive neurodegeneration in svPPA follows connectional pathways within a large-scale neural network that is connected to the temporal pole.
Reliability of focal atrophy in svPPA
Prior work aiming to localize the most prominent degenerative change in svPPA has produced a variety of results, including the anterior fusiform or inferior temporal gyrus (Seeley et al., 2009), temporal pole (Mummery et al., 2000; Galton et al., 2001; Davies et al., 2009; Rohrer et al., 2009), entorhinal cortex (Chan et al., 2001), and perirhinal cortex (La Joie et al., 2014). This variability likely reflects differences in the neuroimaging analysis technique used and biological variability between patient samples. Here, using cortical thickness analysis we identified a point of focal atrophy that was consistent across 44 individual patients and two neuroimaging centres. Previous analyses of neurodegeneration in svPPA that were conducted in volume space, using either VBM or PET, may have lacked the spatial precision necessary to identify a reliable point of maximal abnormality (Iaccarino et al., 2015). Even when conducted at high spatial resolutions, single volumetric units (voxels) can contain segments of grey and white matter depending on thresholding and image acquisition parameters. Furthermore, single voxels may cut across gyral/sulcal boundaries limiting the degree to which individual subjects can be aligned according to cortical anatomy. The use of cortical thickness analyses enabled us to detect the most prominent area of cortical atrophy with sub-millimetre precision without interference from cortical folding. Furthermore, we used surface-based intersubject registration, which optimally aligns gyral and sulcal features across individual subjects and enables the precise matching of morphologically homologous cortical locations while reducing geometric distortion (Fischl et al., 1999; Fischl and Dale, 2000). The pathologically thin temporopolar cortex in svPPA—less than half its typical thickness—can readily be seen in post-mortem tissue and likely reflects substantial cell loss in cortical grey matter. The reliability of the localization of this effect, our experience that many patients exhibit prominent atrophy at initial clinical presentation, and the fact that the temporal pole does not usually undergo atrophy in normal ageing (Bakkour et al., 2013) offer the opportunity to consider this measure as a biomarker that could be used in the in-depth evaluation of patients presenting with mild symptoms of memory, language, or obvious semantic impairment.
An important caveat of the current research is that it is currently unclear whether regions with greater cortical atrophy are necessarily those that begin deteriorating first. Longitudinal (Rogalski et al., 2011, 2014) and cross-sectional (Rohrer et al., 2009) studies have shown that atrophy is apparent in the left temporal pole early in svPPA; however, some of these patients exhibit fairly widespread atrophy by the time they reach clinical attention and receive a diagnosis. In the present sample, 10 patients received an MRI within 2 years of symptom onset (Supplementary Fig. 2). Individual atrophy maps for each of these patients revealed left temporopolar atrophy that was most prominent in the focal atrophy region identified with our larger sample. Furthermore, the magnitude of cortical thinning across the cortical mantle outside the anterior temporal lobe was predicted by disease duration to a much greater extent than in the temporal pole region of interest (Supplementary Fig. 3) supporting our speculation that the atrophic process may have completed in this region by the time these patients reach clinical attention. However, identifying a true origin of progressive atrophy in any neurodegenerative disease will necessitate the identification of patients even earlier, before atrophy has begun to spread throughout the cortex. At present, it is not clear how to identify svPPA patients earlier, since genetic or environmental risk factors are not known.
Functional implications of focal temporopolar atrophy in svPPA
Patients with svPPA usually exhibit impaired semantic memory across all modalities and object categories (Bozeat et al., 2000; Hodges et al., 2000; Lambon Ralph et al., 2007; Luzzi et al., 2007; Goll et al., 2010; Piwnica Worms et al., 2010), although initially have preserved performance for concepts with rich auditory or motor characteristics (Hoffman et al., 2012). Previous work has associated diffuse atrophy in the left anterior temporal lobe with semantic processing deficits in semantic dementia (Mummery et al., 2000). More recently, it has been shown that atrophy in a region of interest in the left temporal pole predicts semantic impairment in primary progressive aphasia (Sapolsky et al., 2010). However, when a whole-brain regression is used, semantic memory impairments in patients with semantic dementia are best predicted by focal hypometabolism in the bilateral anterior fusiform gyrus (also known as perirhinal cortex) (Mion et al., 2010). Thus, one possibility is that atrophy begins in the temporal pole but that semantic deficits manifest when the damage encroaches on a semantic hub in the anterior fusiform/perirhinal cortex (Mion et al., 2010).
The selectivity of the cognitive impairments and cortical atrophy in svPPA are often cited in support of hub models of semantic memory. According to this account, modality-specific sensory information is integrated into multi-modal semantic representations in the rostral temporal poles (Patterson et al., 2007; Lambon Ralph et al., 2010; Baron and Osherson, 2011; Rice et al., 2015a). It should be noted that this view is contentious, with other researchers arguing that there is no centralized semantic hub, and instead that semantic memory is subserved by a distributed network of sensory and motor brain regions (Martin, 2007; Binder et al., 2009). Regardless, a large body of functional neuroimaging work has implicated the temporal poles in semantic processing (Damasio et al., 2004; Patterson et al., 2007; Visser et al., 2010). Several laterality differences have been observed, with the left and right temporal poles being relatively more involved in tasks using stimuli that are verbal versus non-verbal (Damasio et al., 2004; Butler et al., 2009; Mion et al., 2010; Gainotti, 2012, 2015; Rice et al., 2015a, b) or non-social versus social (Olson et al., 2007; Simmons and Martin, 2009; Wong and Gallate, 2012; Olson et al., 2013). These specializations are graded (Rice et al., 2015a, b), and may be driven in part by the differential connectivity of the left and right temporal poles with the extended language network in the left hemisphere, and with the extended face-processing network in the right hemisphere (Pyles et al., 2013; Hurley et al., 2015). Early atrophy of the left temporal pole in svPPA appears sufficient to produce semantic impairments for verbal stimuli (Mesulam et al., 2013). The spread of atrophy to the right temporal pole and other nodes within this network likely contribute to the behavioural and social symptoms that are often observed as svPPA progresses (Mummery et al., 2000; Rosen et al., 2002; Seeley et al., 2005; Chan et al., 2009; Rohrer and Warren, 2010; Irish et al., 2014).
Intrinsic network architecture and neurodegeneration in svPPA
Our results suggest that neurodegeneration in svPPA may originate in the left temporal pole and then preferentially targets brain regions with the strongest connections to the temporal pole. The hub architecture of the temporal pole may render it more vulnerable to initial neurodegeneration due to its high network centrality, which is hypothesized to facilitate activity-dependent ‘wear and tear’ (Buckner et al., 2009). Consistent with this hypothesis, a previous study showed that cortical areas with greater network flow were more likely to constitute neurodegenerative disease-critical epicentres (Zhou et al., 2012). The same study revealed that functional path distance to syndrome-specific epicentres predicted distributed cortical atrophy in multiple neurodegenerative disorders. These results are consistent with our finding that the strength of connectivity—in addition to physical distance—between the temporal pole and distributed cortical regions in healthy adults predicted the magnitude of cortical atrophy in patients with svPPA, supporting the concept that the spread of neurodegenerative pathology is guided in part by the connectivity of the point of origin.
In addition to the default-semantic network, area TG in the rostral temporal pole is heavily interconnected with the rest of the anterior temporal lobes (Pascual et al., 2015). The connectivity of this region with other anterior temporal lobe regions may facilitate the spread of neurodegeneration across independent intrinsic networks and account for the cortical atrophy observed in both svPPA patient samples outside of the default-semantic network. Thus, it is possible that the atrophy observed in both patient samples in the insula and mid-cingulate may be partially attributable to the connectivity of these regions with area TA, which is located posterior and adjacent to area TG, is intrinsically connected to TG, and is interconnected with a network of brain regions important for auditory, somatosensory, and affective processing. This account is speculative, and further work using more refined neural network measures, such as graph theory (Bullmore and Sporns, 2009) or stepwise-connectivity (Sepulcre et al., 2012), is necessary to advance our understanding of how cortical atrophy may progress within and across intrinsic neural networks.
Relating functional connectivity to white-matter pathways
Converging evidence has suggested that several neurodegenerative diseases may progress along white matter fibre tracts connecting large-scale networks (Englund et al., 1988; Villain et al., 2008; Kuczynski et al., 2010). Supporting this hypothesis, laboratory evidence has shown that disease proteins can travel through local and long-range pathways via transynaptic spread (Frost et al., 2009; Palop and Mucke, 2010; Sanders et al., 2014). Although the relationship between the strength of functional connectivity and underlying white matter integrity is still being examined (Honey et al., 2009; Van Den Heuvel et al., 2009; Hermundstad et al., 2013), the results of our rs-fcMRI analysis are broadly consistent with previously reported patterns of white matter degeneration in svPPA (Acosta-Cabronero et al., 2011; Agosta et al., 2012; Iaccarino et al., 2015). In patients with svPPA, degeneration has been observed in white matter fibres projecting from the rostral temporal lobes into the arcuate, uncinate, and inferior longitudinal fasciculi (Agosta et al., 2010, 2012, 2013a; Acosta-Cabronero et al., 2011). Based on this previous work, and the results reported here, we speculate that selective atrophy in svPPA may originate in the left temporal pole and then proceed posteriorly to envelop the lateral temporal cortices and angular gyrus via the arcuate fasciculus, the orbitofrontal cortices via the uncinate fasciculus, and ventral temporal cortices via the inferior longitudinal fasciculus. Importantly, the dorsal part of temporal pole has been demonstrated to be interconnected with the angular and supramarginal gyri of the inferior parietal lobule via the middle longitudinal fascicle (Makris et al., 2009, 2013a, b). This long association connection between the superior temporal pole and parietal regions may play an important role in word and sentence comprehension, as has recently been suggested by Mesulam et al. (2015).
Although atrophy in svPPA is more prominent in the left hemisphere, substantial atrophy also occurs in the right temporal pole (Rohrer et al., 2009; Rogalski et al., 2011; Brambati et al., 2015). The right and left temporal poles have strong intrinsic functional connectivity and are structurally connected via the anterior commissure (Makris et al., 1999; Catani et al., 2002; Catani and Thiebaut de Schotten, 2008). Whether the anterior commissure is a conduit for the spread of pathogenic protein forms between the left and right temporal poles in svPPA remains an open question. To date, no studies have reported altered microstructural changes in the anterior commissure in svPPA patients, possibly due to the small size of this fibre bundle, which in part makes it difficult to delineate from other pathways that converge in the temporal poles (Patel et al., 2010).
Limitations and future directions
There is variability in the cortical atrophy exhibited by patients with svPPA, with some patients having more involvement of lateral language or ventral visual semantic circuits and others having more involvement of medial paralimbic/affective networks. Additionally, some patients with semantic dementia (∼30%) present initially with greater atrophy in the right anterior temporal lobe, and these patients often have greater social-emotional deficits along with prosopagnosia (Chan et al., 2009; Hodges et al., 2010; Irish et al., 2013). Patient-specific neurodevelopmental factors likely contribute to the selective vulnerability of neural regions and networks to progressive degeneration (Rogalski et al., 2008; Spreng et al., 2010; Miller et al., 2013), and may account for some of the variability observed in the atrophy patterns of patients with svPPA. In our patient sample, selective atrophy in the left temporal pole was remarkably consistent across individuals but much more variable in other brain regions. Additional work with larger sample sizes could help elucidate the patient-specific factors that drive the distribution of neuronal loss in svPPA.
Here we used the focal atrophy point in svPPA to investigate the functional connectivity of this region in a large group of young healthy adults. This same approach has been used in several previous studies (Seeley et al., 2009; Zhou et al., 2012; La Joie et al., 2014) and has proven useful for explicating intrinsic neural networks that are robustly expressed in the population. However, there is some variability in neural network topology across individuals (Mueller et al., 2013), and intrinsic neural networks may selectively reorganize in patients with neurodegenerative diseases (Xie and He, 2011; Agosta et al., 2013b, 2014). An interesting avenue for future research will be to investigate the degree to which individual differences in the connectivity of the temporal poles (or other disease-related focal atrophy points) predict the progressive spread of neurodegeneration within a single patient. The results reported here suggest a close correspondence between functional connectivity and progressive atrophy, and we expect an even closer correspondence will be observed when individual differences in connectivity are taken into account.
Funding
This work was supported by the following National Institutes of Health Grants: R21 NS077059, R01 NS050915, P01 AG005134. This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number(s) S10RR023401, S10RR023043, S10RR021110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- rs-fcMRI
resting-state functional connectivity MRI
- svPPA
semantic variant primary progressive aphasia
References
- Acosta-Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, Williams GB, et al. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain 2011; 134 (Pt 7): 2025–35. [DOI] [PubMed] [Google Scholar]
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin?. Brain 2006; 129 (Pt 11): 3066–80. [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Canu E, Cappa SF, Magnani G, Franceschi M, et al. Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang 2013a; 127: 157–66. [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Valsasina P, Canu E, Meani A, Marcone A, et al. Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol Aging 2014; 35: 2646–55. [DOI] [PubMed] [Google Scholar]
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain 2010; 133 (Pt 1): 286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Sala S, Valsasina P, Meani A, Canu E, Magnani G, et al. Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 2013b; 81: 134–43. [DOI] [PubMed] [Google Scholar]
- Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex 2012; 22: 2705–14. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage 2013; 76: 332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron SG, Osherson D. Evidence for conceptual combination in the left anterior temporal lobe. Neuroimage 2011; 55: 1847–52. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 2009; 19: 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 2000; 38: 1207–15. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Amici S, Racine CA, Neuhaus J, Miller Z, Ogar J, et al. Longitudinal gray matter contraction in three variants of primary progressive aphasia: a tenser-based morphometry study. Neuroimage Clin 2015; 8: 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging 2009; 30: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 2009; 29: 1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, Larossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005; 25: 7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 186–98. [DOI] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. The neural correlates of verbal and non-verbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol 2009; 22: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in Vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002; 17: 77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut De Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44: 1105–32. [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain 2009; 132: 1287–98. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol 2001; 49: 433–42. [PubMed] [Google Scholar]
- Czarnecki K, Duffy JR, Nehl CR, Cross SA, Molano JR, Jack CR Jr, et al. Very early semantic dementia with progressive temporal lobe atrophy: an 8-year longitudinal study. Arch Neurol 2008; 65: 1659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Serano MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition 2004; 92: 179–229. [DOI] [PubMed] [Google Scholar]
- Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR. The neural basis of semantic memory: evidence from semantic dementia. Neurobiol Aging 2009; 30: 2043–52. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Onur OA, Kuhn J, Gruppe T, Drzezga A. Imaging frontotemporal lobar degeneration. Curr Neurol Neurosci Rep 2014; 14: 489–89. [DOI] [PubMed] [Google Scholar]
- Ding S-L, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic and pathological markers. J Comp Neurol 2009; 514: 595–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer’s type. Biochemical and neuropathological correlates. Brain 1988; 111 (Pt 6): 1425–39. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001; 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem 2009; 284: 3546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. The format of conceptual representations disrupted in semantic dementia: a position paper. Cortex 2012; 48: 521–9. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non-verbal representations?. Neurosci Biobehav Rev 2015; 51: 296–312. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 2001; 57: 216–25. [DOI] [PubMed] [Google Scholar]
- Goll JC, Crutch SJ, Loo JHY, Rohrer JD, Frost C, Bamiou DE, et al. Non-verbal sound processing in the primary progressive aphasias. Brain 2010; 133: 272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe MJ, Teipel SJ, Alzheimer’s Disease Neuroimaging Initiative. Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Hum Brain Mapp 2016; 37: 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Gorno-Tempini ML, Gesierich B, Henry M, Trujillo A, Shany-Ur T, et al. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain 2013; 136 (Pt 10): 2979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, Freeman S, et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci USA 2013; 110: 6169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Bozeat S, Lambon Ralph MA, Patterson K, Spatt J. The role of conceptual knowledge in object use evidence from semantic dementia. Brain 2000; 123 (Pt 9): 1913–25. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, Mcmonagle P, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain 2010; 133: 300–6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007; 6: 1004–14. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Brain 1992; 115: 1783–806. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Ralph MaL. The degraded concept representation system in semantic dementia: damage to pan-modal hub, then visual spoke. Brain 2012; 135 (Pt 12): 3770–80. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity. Proc Natl Acad Sci USA 2009; 106: 2035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, Mesulam MM. Asymmetric connectivity between the anterior temporal lobe and the language network. J Cogn Neurosci 2015; 27: 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino L, Crespi C, Della Rosa PA, Catricala E, Guidi L, Marcone A, et al. The semantic variant of primary progressive aphasia: clinical and neuroimaging evidence in single subjects. PLoS One 2015; 10: e0120197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014; 137 (Pt 4): 1241–53. [DOI] [PubMed] [Google Scholar]
- Irish M, Kumfor F, Hodges JR, Piguet O. A tale of two hemispheres: contrasting socioemotional dysfunction in right- versus left-lateralised semantic dementia. Dementia E Neuropsychologia 2013; 7: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009; 73: 1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczynski B, Targan E, Madison C, Weiner M, Zhang Y, Reed B, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement 2010; 6: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pélerin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer’s and semantic dementia-targeted networks. Neuron 2014; 81: 1417–28. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain 2007; 130: 1127–37. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci USA 2010; 107: 2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007; 45: 1823–31. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 1999; 9: 18–45. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 2009; 19: 777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Preti MG, Asami T, Pelavin P, Campbell B, Papadimitriou GM, et al. Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Struct Funct 2013a; 218: 951–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Preti MG, Wassermann D, Rathi Y, Papadimitriou GM, Yergatian C, et al. Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging Behav 2013b; 7: 335–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Ann Rev Psychol 2007; 58: 25–45. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A plasticity-based theory of the pathogenesis of Alzheimer’s disease. Ann N Y Acad Sci 2000; 924: 42–52. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia-A language-based dementia. N Engl J Med 2003; 349: 1535–42. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain 2015; 138 (Pt 8): 2423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 2013; 136: 601–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 2013; 136: 3461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain 2010; 133: 3256–68. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, et al. Individual variability in functional connectivity architecture of the human brain. Neuron 2013; 77: 586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000; 47: 36–45. [PubMed] [Google Scholar]
- Olson IR, Mccoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes : a review and theoretical framework. Soc Cogn Affect Neurosci 2013; 8: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 2007; 130 (Pt 7): 1718–31. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 2010; 13: 812–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, et al. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex 2015; 25: 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MD, Toussaint N, Charles-Edwards GD, Lin JP, Batchelor PG. Distribution and fibre field similarity mapping of the human anterior commissure fibres by diffusion tensor imaging. Magma 2010; 23: 399–408. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007; 8: 976–87. [DOI] [PubMed] [Google Scholar]
- Piwnica Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex 2010; 46: 761–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles JA, Verstynen TD, Schneider W, Tarr MJ. Explicating the face perception network with white matter connectivity. PLoS One 2013; 8: e61611–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron 2012; 73: 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Hoffman P, Lambon Ralph MA. Graded specialization within and between the anterior temporal lobes. Ann N Y Acad Sci 2015a; 1359: 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Lambon Ralph MA, Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: an ALE meta-analysis of 97 functional neuroimaging studies. Cereb Cortex 2015b; 25: 4374–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 2011; 76: 1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014; 83: 1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol 2008; 65: 244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci 2010; 293: 35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 2009; 72: 1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 2002; 58: 695–701. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002; 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. Neuron 2004; 14: 721–730. [DOI] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, Devos SL, Sharma AM, Mirbaha H, Li A, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 2014; 82: 1271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, et al. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology 2010; 75: 358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology 2005; 64: 1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Yeo TB, Liu H, Johnson KA. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci 2012; 32: 10649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc 2009; 15: 645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia : a form of circumscribed atrophy. Behav Neurol 1989; 2: 167–82. [Google Scholar]
- Spreng RN, Rosen HJ, Strother S, Chow TW, Diehl-Schmid J, Freedman M, et al. Occupation attributes relate to location of atrophy in frontotemporal lobar degeneration. Neuropsychologia 2010; 48: 3634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 2009; 30: 3127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010; 103: 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, De La Sayette V, Mézenge F, Landeau B, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci 2008; 28: 6174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci 2010; 22: 1083–94. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol 1975; 27: 635–57. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA. Recent advances in the imaging of frontotemporal dementia. Curr Neurol Neurosci Rep 2012; 12: 715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Demarco AT, Henry ML, Gesierich B, Babiak M, Mandelli ML, et al. What role does the anterior temporal lobe play in sentence-level processing? Neural correlates of syntactic processing in semantic PPA. J Cogn Neurosci 2014; 26: 970–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res 2012; 1449: 94–116. [DOI] [PubMed] [Google Scholar]
- Xie T, He Y. Mapping the Alzheimer’s brain with connectomics. Front Psychiatry 2011; 2: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.