Abstract
Pityriasis rosea is a dermatological disease with a well-documented clinical appearance, but less is known about causes and treatment. Bell's palsy is a neurological condition leading to acute idiopathic hemifacial paralysis. Recent studies indicate that human herpesvirus (HHV) 6–7 reactivation may be a contributing factor to both conditions. We report a case of the 2 concurrent diagnoses that supports a common contributing factor and suggests further awareness and research into the role HHV 6–7 may play in the aetiology of both conditions.
Background
Pityriasis rosea (PR) is a skin eruption that occurs without age, race or gender preference. It most commonly occurs in the Spring and Fall. Reported incidence is 158.9 cases/100 000 person-years in the community and ∼0.68/100 dermatological patients.1 2 It has been seen in multiple studies to follow a case-clustering pattern in communities, suggesting infectious aetiology, although other studies have shown no seasonal pattern.3 4 Classically, it begins with a 1–3 cm erythematous scaly ‘herald’ patch followed 2–3 weeks later by multiple erythematous macules and patches with a collarette scale distributed along skin cleavage lines on the trunk, proximal arms and face. The diagnosis is a clinical one, based on the distribution and qualities of the patches. The rash usually lasts 6–8 weeks.
PR has been associated with HHV 6–7 in multiple studies and reactivation of these viruses is a probable aetiology.5 Watanabe et al measured HHV 6–7 and CMV DNA using nested PCR in lesional skin, non-lesional skin, peripheral blood mononuclear cells, serum and saliva from patients with PR, patients with psoriasis and healthy controls (n=36). HHV 7 DNA was present in PR lesional skin (93%), non-lesional skin (86%), saliva (100%), peripheral blood mononucleocytes (83%) and serum (88%), but CMV was not detected in these tissues. Comparatively, psoriasis and healthy control patients had only rare positivity for HHV 6–7 in skin or serum samples. In situ hybridisation showed infiltrating mononuclear cells expressing HHV 6–7 mRNA in 100% of perivascular and 75% of periappendageal areas in patients with PR, but only 13% in psoriasis and healthy controls.6 Broccolo et al measured HHV 6–7 DNA load using PCR in plasma, peripheral blood mononuclear cells and skin biopsy tissue of patients with PR and compared them with patients with other dermatitis and healthy controls (n=30). HHV 6–7 DNA was detected in plasma of 17% and 39% of patients with PR, respectively, but none was found in control patient plasma. HHV 7 levels in peripheral blood mononuclear cells were higher in patients with PR than controls. HHV 6–7 antigens were found in PR skin biopsies (17% and 67%, respectively), but not in controls.7 There have been other studies on the topic that do not support this theory. The negative results may be caused by laboratory technique, such as using formalin on tissues or less-sensitive PCR methods. It may also be the case that HHV 6–7 reactivation is the cause of some PR cases, but not others. Overall, the literature supports HHV 6–7 infection as a potential cause of PR, but the true mechanism is not yet understood.
Bell's palsy is an acute onset hemifacial muscle weakness in the distribution of cranial nerve VII without a clear origin. It occurs in all ages and incidence has been reported as 15.2 cases/100 000 person-years.8 It usually resolves spontaneously within 3 weeks. Viral reactivation leading to potential inflammation in cranial nerves has been proposed as a contributing cause.9 10 Herpes simplex, varicella zoster and HHV 6 are commonly identified agents in multiple studies.11 A recent study demonstrates that patients with Bell's palsy have similar HSV and varicella levels, but significantly higher HHV-6 in saliva than controls. In addition, higher HHV-6 levels portend a slower recovery of facial paralysis.12 This evidence points in favour of a link between HHV-6 and Bell's palsy compared with other studied viruses, but more research needs to be performed to confirm these findings. Although Bell's palsy may be caused by a variety of factors, HHV 6–7 reactivation is a suspected contributor.
The statistical likelihood of contracting both diseases by random chance is very small. We calculated the coincidence to be ∼0.02415 cases/100 000 person-years by multiplying the reported incidences for each condition. A single aetiology manifesting as two separate pathological mechanisms could also explain the coincidence in this case. Since HHV 6–7 is named as a potential aetiology in both disease processes, it could be the culprit. This case potentially demonstrates a link between these two conditions and should prompt further inquiry into a shared viral source.
Case presentation
An Asian girl aged 10 years with no personal or family dermatological history and no neurological problems or recent illnesses developed a large scaly erythematous patch on her left lateral thigh. Two weeks later, multiple pink papules with a collarette scale appeared on her back, abdomen and face, sparing mucosa, scalp, palms and soles (figure 1).
Figure 1.
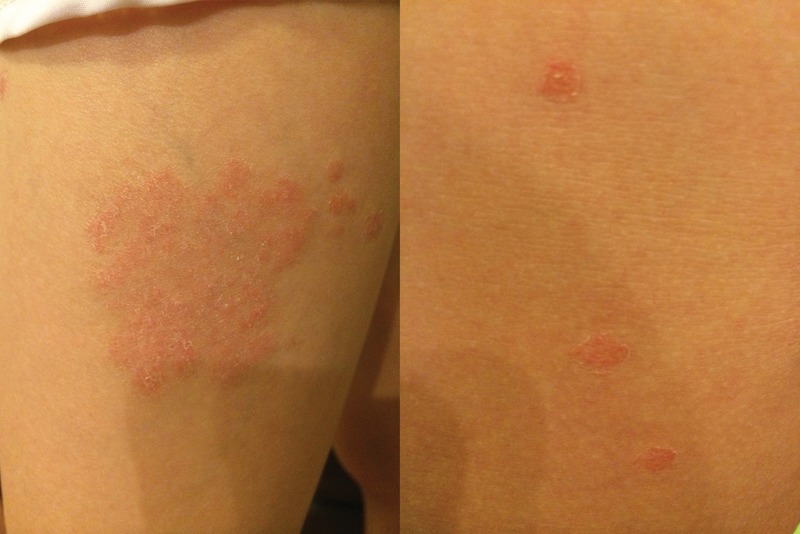
Pityriasis rosea early ‘herald patch’ and late widespread exanthema.
Five weeks from the onset of the rash, which still persisted, the patient developed a ‘funny feeling’ on her right face associated with a weak smile, squint and facial expression. She denied facial pain, swelling, recent fevers, personal history of cold sores and family history of neurological disease. Physical examination showed upper and lower right hemifacial paralysis with reduced facial expression (figure 2). At this time, she and her family pursued treatment by a dermatologist and she was diagnosed with concurrent PR and Bell's palsy.
Figure 2.
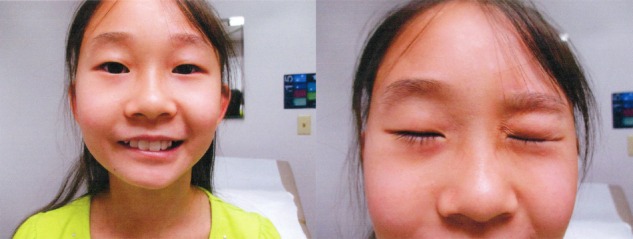
Bell's palsy facial hemiparesis when patient asked to 'smile' and 'squint'.
Investigations
None were taken. Both conditions can be diagnosed by clinical appearance alone.
Differential diagnosis
For the rash, differential diagnosis included psoriasis, seborrhoeic dermatitis, nummular eczema, irritant contact dermatitis and less likely secondary syphilis, but the distribution and classic appearance with scale, the ‘herald patch’ with a subsequent widespread rash, the duration of lesion presence and the lack of prior dermatological conditions or sexual contact made PR most likely. For the hemifacial paralysis, Bell's palsy was the leading diagnosis due to the lack of recent illness, inciting trauma, other neurological deficit, prior family or personal neurological disease and the eventual self-resolution of symptoms.
Treatment
Initially, her paediatrician prescribed hydrocortisone cream without significant improvement. Once she was diagnosed with both conditions, she was prescribed a 10-day course of acyclovir 400 mg TID and a 14-day tapering course of PO prednisone. Corticosteroids for acute Bell's palsy are recommended to improve long-term recovery in adults and children,13 14 and recent research shows moderate evidence that corticosteroid plus antiviral treatment increases complete recovery with no significant adverse events.15 In addition, there has been some evidence, although not yet conclusive, that acyclovir may reduce the duration and pruritus of PR, and a low dose (as prescribed here) is as effective as a higher dose in achieving outcomes.16 Given the low risk of side effects from acyclovir and the potential for added benefit, it was included in her treatment.
Outcome and follow-up
After 3 weeks, the rash improved and facial paralysis resolved. After 3 months, the rash had completely resolved and did not return (at the time of the 1-year follow-up).
Discussion
PR is a common disease in adults and children that may be associated with HHV 6–7 reactivation. A new classification scheme has recently been devised that delineates the differences between classic, relapsing, persistent, paediatric, pregnancy and PR-like eruptions, with a focus on the pathogenesis, distribution, frequency of herald patch, mucosal involvement, systemic symptoms, histopathology, mean duration and therapeutic options. Paediatric PR is notable for a shorter time lapse between herald patch and generalised eruption (4 days vs 2 weeks), shorter exanthema duration (16 vs 45 days), increased mucosal involvement and higher viral plasma HHV 6–7 levels, but a similar per cent of patients presenting with systemic symptoms.3 17 18 In children as well as adults, viral reactivation, rather than primary infection, is thought to be the likely aetiology. This is demonstrated by high-avidity IgG antibody titres and HHV 6–7 plasma viraemia during PR presentation. Nonetheless, the difference in presentation and duration of HHV 6–7 viraemia may indicate differences in pathogenesis pathways between the child and adult.17 18 Our patient's clinical picture is actually more fitting with the classic type, with a longer time lapse between herald patch and widespread exanthema (14 days), longer duration of exanthema (8 weeks) and lack of mucosal involvement.
This case represents a mechanistic and infectious association that merits further investigation. There is one other published case of PR associated with Bell's palsy from the 1950s, before the relationship with infection was identified. Recent research and this case of Bell's palsy associated with PR add more evidence that there may be a link between these two conditions through HHV 6–7 reactivation. Since both conditions are still undergoing research to determine infectious and inflammatory mechanisms, we encourage more studies of reactivation of HHV 6–7 as a common aetiology.
This case also sheds light on the surprising variety of possible presentations for this virus. For clinicians, it encourages a more thorough history-taking when presented with either disease and emphasises the importance of thinking broadly when concurrent illnesses such as these occur in a patient. In addition to further research on mechanisms, we advocate further epidemiological studies on concurrence rates of both diagnoses, the likelihood of clinicians to recognise them both and the impact this may have on treatment and on the individuals and families affected.
Patient's perspective.
Unfortunately unable to obtain.
Learning points.
Pityriasis rosea (PR) is potentially caused by reactivation of HHV 6–7 virus.
Recent evidence shows that Bell's palsy may also correspond to reactivation of HHV 6–7 virus.
Concurrent PR and Bell's palsy may be more common than expected by chance due to this shared causal mechanism.
Clinicians and researchers need to be more aware of this association between the diseases in the practice setting and for future research projects.
Footnotes
Contributors: MG and AM saw this patient in clinic and conceived the idea of writing it for publication. MG obtained photographs, data from the electronic medical record and written consent from the patient’s mother. VV performed the literature review for the paper, drafted the manuscript, formatted the photos and prepared the submission. MG and AM reviewed and edited the manuscript before the final submission. They both provided feedback to VV on interpretation of the literature, clinical diagnosis and discussion as well as edited the paper for grammatical and stylistic changes. The final version was approved by all three authors before submission.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chuang TY, Listrup DM, Perry HO et al. Pityriasis rosea in Rochester, Minnesota, 1969 to 1978. J Am Acad Dermatol 1982;7:80–9. 10.1016/S0190-9622(82)80013-3 [DOI] [PubMed] [Google Scholar]
- 2.Chuh AA, Molinari N, Sciallis G et al. Temporal case clustering in pityriasis rosea: a regression analysis on 1379 patients in Minnesota, Kuwait, and Diyarbakir, Turkey. Arch Dermatol 2005;141:767–71. 10.1001/archderm.141.6.767 [DOI] [PubMed] [Google Scholar]
- 3.Drago F, Broccolo F, Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol 2009;61:303–18. 10.1016/j.jaad.2008.07.045 [DOI] [PubMed] [Google Scholar]
- 4.Chuh A, Lee A, Zawar V et al. Pityriasis rosea—an update. Indian J Dermatol Venereol Leprol 2005;71:311–15. 10.4103/0378-6323.16779 [DOI] [PubMed] [Google Scholar]
- 5.Rebora A, Drago F, Broccolo F. Pityriasis rosea and herpesviruses: facts and controversies. Clin Dermatol 2010;28:497–501. 10.1016/j.clindermatol.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Kawamura T, Jacob SE et al. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol 2002;119:793–7. 10.1046/j.1523-1747.2002.00200.x [DOI] [PubMed] [Google Scholar]
- 7.Broccolo F, Drago F, Careddu AM et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol 2005;124:1234–40. 10.1111/j.0022-202X.2005.23719.x [DOI] [PubMed] [Google Scholar]
- 8.Morris AM, Deeks SL, Hill MD et al. Annualized incidence and spectrum of illness from an outbreak investigation of Bell's palsy. Neuroepidemiology 2002;21:255–61. doi:65645 [DOI] [PubMed] [Google Scholar]
- 9.Lazarini PR, Vianna MF, Alcantara MP et al. Herpes simplex virus in the saliva of peripheral Bell's palsy patients. Braz J Otorhinolaryngol 2006;72:7–11. 10.1016/S1808-8694(15)30026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abiko Y, Ikeda M, Hondo R. Secretion and dynamics of herpes simplex virus in tears and saliva of patients with Bell's palsy. Otol Neurotol 2002;23:779–83. 10.1097/00129492-200209000-00028 [DOI] [PubMed] [Google Scholar]
- 11.Glass GE, Tzafetta K. Bell's Palsy: a summary of current evidence and referral algorithm. Fam Pract 2014;31:631–42. 10.1093/fampra/cmu058 [DOI] [PubMed] [Google Scholar]
- 12.Turriziani O, Falasca F, Maida P et al. Early collection of saliva specimens from Bell's palsy patients: quantitative analysis of HHV-6, HSV-1, and VZV. J Med Virol 2014;86:1752–8. 10.1002/jmv.23917 [DOI] [PubMed] [Google Scholar]
- 13.Gronseth GS, Paduga R. Evidence-based guideline update: steroids and antivirals for Bell palsy: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012;79:2209–13. 10.1212/WNL.0b013e318275978c [DOI] [PubMed] [Google Scholar]
- 14.Arican P, Dundar NO, Gencpinar P et al. Efficacy of low-dose corticosteroid therapy versus high-dose corticosteroid therapy in bell's palsy in children. J Child Neurol 2016; [Epub ahead of print] 10.1177/0883073816668774 [DOI] [PubMed] [Google Scholar]
- 15.Sullivan F, Daly F, Gagyor I. Antiviral agents added to corticosteroids for early treatment of adults with acute idiopathic facial nerve paralysis (Bell Palsy). JAMA 2016;316:874–5. 10.1001/jama.2016.10160 [DOI] [PubMed] [Google Scholar]
- 16.Chuh A, Zawar V, Sciallis G et al. A position statement on the management of patients with pityriasis rosea. J Eur Acad Dermatol Venereol 2016;30:1670–81. 10.1111/jdv.13826 [DOI] [PubMed] [Google Scholar]
- 17.Drago F, Ciccarese G, Rebora A et al. Pityriasis rosea: a comprehensive classification. Dermatology 2016;232:431–7. 10.1159/000445375 [DOI] [PubMed] [Google Scholar]
- 18.Drago F, Ciccarese G, Broccolo F et al. Pityriasis rosea in children: clinical features and laboratory investigations. Dermatology 2015;231:9–14. 10.1159/000381285 [DOI] [PubMed] [Google Scholar]


