Abstract
Diabetes can affect many parts of the body and is associated with serious complications. Oxidative stress is a major contributor in the pathogenesis of diabetic complications and bilirubin has been shown to have antioxidant effects. The number of studies on the effect of bilirubin on the risk of diabetic complications has increased, but the results are inconsistent. Thus, we performed a meta-analysis to determine the relationship between bilirubin concentration and the risk of diabetic complications, and to investigate if there was a dose-response relationship. We carried out an extensive search in multiple databases. A fixed or random-effects model was used to calculate the pooled estimates. We conducted a dose-response meta-analysis to analyze the association between these estimates. A total of 132,240 subjects from 27 included studies were analyzed in our meta-analysis. A negative nonlinear association between bilirubin concentration and the risk of diabetic complications was identified (OR: 0.77, 95% CI: 0.73–0.81), with a nonlinear association. We also found that there was a negative association between bilirubin concentration and the risk of diabetic nephropathy, diabetic retinopathy and diabetic neuropathy. The results of our meta-analysis indicate that bilirubin may play a protective role in the occurrence of diabetic complications.
Diabetes Mellitus (DM) is an important non-communicable disease, and is a serious threat to human health and global economies. Due to population ageing, urbanization and lifestyle changes, the number of people with DM has increased sharply in both developed and developing countries1. The International Diabetes Federation (IDF) reported that the number of individuals with DM was estimated to be 382 million in 2013, and predicted that almost 600 million people would develop this disease by 2035. In the United States, 29.1 million people had DM in 20142, and the United Health Group predicted that approximately half of all Americans would have prediabetes or diabetes by 20203. Approximately 99% of all diabetic patients (28.9 million people aged 20 years or older) have DM. Recently, the burden of DM was shown to be in developing countries rather than developed countries1,4. The IDF predicted that by 2035, ten countries would have the largest diabetes populations, including four Asian countries–China, India, Indonesia, and Japan—And Asia would be the center of the DM epidemic4.
DM has serious effects on many parts of the body, such as the eyes, kidneys, limbs and nervous system. Therefore, diabetic patients can develop many microvascular complications, such as blindness, kidney failure, and lower-limb disorders leading to amputation. From 2005 to 2008, 4.2 million diabetic patients aged 40 years or older developed diabetic retinopathy (DR), which is caused by damage to the small blood vessels in the retina1. Diabetic nephropathy (DN) is the leading cause of chronic renal failure and end-stage renal disease5. In 2011, 49,677 Americans began treatment for kidney failure due to diabetes6. Approximately 73,000 diabetic patients aged 20 years or older underwent non-traumatic lower-limb amputations6. Diabetic neuropathy is defined as the presence of symptoms and/or signs of peripheral nerve dysfunctions after exclusion of other causes. It is a heterogeneous condition that encompasses a wide range of peripheral nerve dysfunction and its development may be attributed to diabetes per se or to factors associated with the disease1. DM and its complications had become major public health challenge of the 21st century.
As one of the most highly conserved groups of non-polar molecules, bilirubin has been shown to have antioxidant and anti-inflammatory effects7,8. Bilirubin belongs to a phylogenetically old superfamily of tetrapyrrolic compounds, and is the end product of heme catabolism. Cyclic tetrapyrrole heme is divided into biliverdin, carbon monoxide (CO), and ferrous iron (Fe2+) due to the effects of heme oxygenase (HO), which is an enzyme system comprised of two forms: HO-1 and HO-2. Biliverdin is subsequently reduced to bilirubin by biliverdin reductase. Bilirubin concentration increased as higher HO-1 expression increases9. An animal study had found that higher HO-1 expression had a protective effect on many diseases10. Several studies on the HO-1 pathway have focused on various diseases such as cardiovascular disease, DM and diabetic complications, and evaluated the association between bilirubin concentration and these diseases11,12,13. Initially, the studies on bilirubin were primarily focused on cholestasis, especially in newborns14. In recent years, studies on bilirubin found that it played a protective role in vascular diseases. In a prospective study, it was found that the risk of ischemic stroke in the highest bilirubin group was lower than that in the lowest bilirubin group after adjustment for multiple confounding factors (OR: 0.66, 95% confidence interval (CI): 0.49–0.89)15. Additionally, Inoguchi et al. demonstrated that the prevalence of vascular complications was lower in diabetic patients with Gilbert’s syndrome than that in diabetic patients with normal bilirubin concentration16.
Long-term hyperglycemia leads to the generation of reactive oxygen species, mainly by mitochondria. The reaction process occurs in endothelial cells, and led to vascular complications. In diabetic patients, bilirubin may exhibit its potent antioxidant property by inhibiting lipid peroxidation and attenuating low-density lipoprotein (LDL) oxidation16. In a new review published in 2016, the authors found that mildly elevated bilirubin concentration had protective effect on an array of diseases (such as cardiovascular disease and diabetes) associated with increased oxidative stress, targeting elevated bilirubin metabolism could be considered a potential therapeutic approach to ameliorate a variety of conditions17. The relationship between bilirubin metabolism and occurrence, development and prognosis of disease has become a research focus. In recent years, the number of studies on the relationship between bilirubin concentration and the risk of diabetic complications has increased, some studies have indicated that high bilirubin concentration has protective effect on diabetic complications18,19,20,21, but some studies did not find this relationship22,23. Therefore, it was very necessary to analyze the relationship between bilirubin concentration and the risk of diabetic complications using the meta-analysis method. We also carried out a dose-response meta-analysis to provide a more accurate assessment on the relationship between bilirubin concentration and the risk of diabetic complications.
Results
Search results and study characteristics
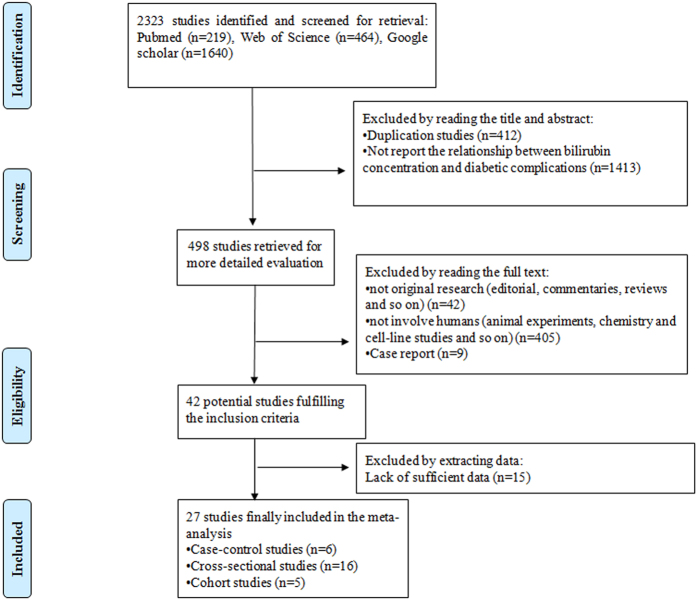
Potential studies (2323) were identified from three electronic databases (MEDLINE: 219, Web of Science: 464, and Google scholar: 1640) using search strategy. After reading the title and abstract, we found that 412 studies were duplicates and 1413 studies did not report the relationship between bilirubin concentration and the risk of diabetic complications. Four hundred and ninety-eight studies were read in full, and 471 studies were excluded for various reasons. In total, 27 studies7,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43 satisfied the inclusion criteria for our meta-analysis. A flow chart of the screening process is shown in Fig. 1.
Figure 1. The flow chart of screening progress in our meta-analysis.
Table 1 shows the details of the included studies in our meta-analysis. The 27 studies were from five countries (one study was carried out in Australia7, thirteen in China18,19,24,26,27,29,30,33,34,35,40,43, one in India28, six in Japan20,23,25,31,36,42 and six in Korea21,22,37,38,39,41) and included five cohort studies7,19,23,31,36, sixteen cross-sectional studies20,21,22,25,30,32,33,34,35,37,38,39,40,41,42,43 and six case-control studies18,24,26,27,28,29. Eleven studies assessed DN19,22,23,24,25,29,31,32,33,40,43, twelve assessed DR18,19,25,26,27,28,30,34,35,36,40, two assessed diabetic peripheral neuropathy (DPN)39,40, two assessed diabetic arterial stiffness (DAS)21,43, one assessed diabetic coronary heart disease (DCH)43, one assessed diabetic ischemic stroke (DIS)43, one assessed diabetic carotid atherosclerosis (DCA)42, one assessed diabetic obstructive coronary artery disease (DOCAD)38, one assessed diabetic cardiovascular autonomic neuropathy (DCAN)37 and one assessed diabetic amputations (DA)7. All the ORs in the included studies were adjusted for the greatest number of potential confounders.
Table 1. Details of the included studies in our meta-analysis.
| Author | Year | Country | Design | Source of subjects | Number of subjects | Outcome | Odds ratio (95% CI) | Adjustment for covariates | Quality of study |
|---|---|---|---|---|---|---|---|---|---|
| Liu, Miao43 | 2016 | China | cross-sectional | Hospital-based | 1839 | DN | 0.79 (0.67,0.87) | age, education, marital status, current smoking, current drinking, physical activity ≥0.5 h/day, BMI, hypertension, dyslipidemia, treatment and control status of diabetes | 10 |
| DR | 0.30 (0.22,0.40) | age, education, marital status, current smoking, current drinking, physical activity ≥0.5 h/day, BMI, hypertension, dyslipidemia, treatment and control status of diabetes | |||||||
| DAS | 0.51 (0.41,0.57) | age, education, marital status, current smoking, current drinking, physical activity ≥0.5 h/day, BMI, hypertension, dyslipidemia, treatment and control status of diabetes | |||||||
| DCH | 0.59 (0.43,0.76) | age, education, marital status, current smoking, current drinking, physical activity ≥0.5 h/day, BMI, hypertension, dyslipidemia, treatment and control status of diabetes | |||||||
| DIS | 0.82 (0.76,0.87) | age, education, marital status, current smoking, current drinking, physical activity ≥0.5 h/day, BMI, hypertension, dyslipidemia, treatment and control status of diabetes | |||||||
| Wang, Jing19 | 2016 | China | cohort | Population-based | 2958 | DN | 0.74 (0.56,0.98) | age, gender, central obesity, education, smoking status, drinking status, physical activity, SBP, TG, HDL-C, use of medications (hypotensor, lipid-lowering), liver function (ALP, AST and ALT), FBG, use of antidiabetic, duration of diabetes, use of aspirin | 11 |
| Ryuichi, Kawamoto42 | 2016 | Japan | cross-sectional | Hospital-based | 374 | DCA | 0.46 (0.23,0.93) | age, gender, BMI, smking status, SBP, DBP, Antihepertensive medication, TG, HDL-C, LDL-C, Antidyslipidemic medication, FBG, insulin therapy, eGFR, Uric acid, AST, GGT | 9 |
| Jiang, Pijun24 | 2016 | China | case-control | Hospital-based | 561 | DN | 0.91 (0.83,1.00) | not list specifically | 7 |
| Jaechan, Leem38 | 2015 | Korea | cross-sectional | Hospital-based | 460 | DOCAD | 0.86 (0.81,0.92) | age, gender, BMI, duration of diabetes, hypertension, SBP, DBP, current smoking, HbA1C, LDL-C, HDL-C, TG, DR, DN, eGFR, current use of insulin and a statin, AST, ALT and alcohol intake | 11 |
| Zhang, Dan18 | 2015 | China | case-control | Hospital-based | 553 | DR | 0.91 (0.88,094) | age, gender, duration of diabetes, BMI, HbA1C, LDL-C, TG, SUA and SBP | 8 |
| Chen, Fang26 | 2015 | China | case-control | Hospital-based | 237 | DR | 0.85 (0.78,0.94) | age, duration of diabetes, FBG, Arteriosclerosis, SBP, UAER | 8 |
| Wei, Wei27 | 2015 | China | case-control | Hospital-based | 100 | DR | 0.84 (0.72,0.99) | not list specifically | 7 |
| Risa, Sekioka20 | 2015 | Japan | cross-sectional | Hospital-based | 674 | DR | 0.92 (0.89,0.96) | not list specifically | 8 |
| Eun Sook, Kim39 | 2015 | Korea | cross-sectional | Hospital-based | 1207 | DPN | 0.63 (0.40,0.99) | age, gender, BMI, duration of diabetes, drinking and smoking status, history of cardiovascular disease, HbA1c, SBP, ALT, hyperlipidemia, eGFR, use of insulin and antihypertensive agents, autonomic neuropathy, DR, and albuminuria | 11 |
| Hamamoto S25 | 2015 | Japan | cross-sectional | Hospital-based | 523 | DR | 0.92 (0.87,0.97) | age, gender, smoking status | 10 |
| DN | 0.87 (0.82,0.93) | age, gender, smoking status | |||||||
| Apoorva, Dave28 | 2015 | India | case-control | Hospital-based | 80 | DR | 0.84 (0.71,0.99) | not list specifically | 7 |
| Wang, Ru40 | 2015 | China | cross-sectional | Hospital-based | 5961 | DR | 0.48 (0.34,0.68) | age, gender, BMI, duration of diabetes, drinking and smoking status, HbA1C, FBG, ALT, AST, TC, TG and drug treatment | 11 |
| DN | 0.38 (0.31,0.46) | age, gender, BMI, duration of diabetes, drinking and smoking status, HbA1C, FBG, ALT, AST, TC, TG and drug treatment | |||||||
| DPN | 0.62 (0.53,0.73) | age, gender, BMI, duration of diabetes, drinking and smoking status, HbA1C, FBG, ALT, AST, TC, TG and drug treatment | |||||||
| Cai, Junwei29 | 2015 | China | case-control | Hospital-based | 102 | DN | 0.82 (0.69,0.99) | age, BMI, duration of diabetes, SBP, DBP, ALT, FBG, HbA1C, FIN, TG, TC, HDL-C, LDL-C | 7 |
| Syeda Sadia NAJAM30 | 2014 | China | cross-sectional | Population-based | 1761 | DR | 0.55 (0.33,0.91) | age, gender, current smoking, drinking status, postprandial plasma glucose, HbA1c, DBP, TC, TG, LDL-C and GGT | 11 |
| Kiwako Toya23 | 2014 | Japan | cohort | Hospital-based | 1418 | DN (microalbuminuria) | 0.96 (0.91,1.02) | age, gender, use of renin–angiotensin–aldosterone system | 11 |
| DN (microalbuminuria) | 0.86 (0.77,0.95) | age, gender, use of renin–angiotensin–aldosterone system | |||||||
| Tsuyoshi Mashitani31 | 2014 | Japan | cohort | Hospital-based | 957 | DN | 0.41 (0.16,1.04) | age, gender, BMI, duration of diabetes, follow-up time, SBP, drinking and smoking status, HbA1c, UACR, and use of RAS inhibitors and statins and hemoglobin levels. | 11 |
| Eun Sook Kim21 | 2014 | Korea | cross-sectional | Hospital-based | 1711 | DAS | Male:1.35 (0.59,3.07) | age, BMI, duration of diabetes, drinking and smoking status, history of CVD, HbA1c, SBP, DBP, ALT, TC, TG, HDL-C, eGFR, use of insulin, ACEi/ARB, statin, retinopathy and albumin-to-creatinine ratio. | 11 |
| DAS | Female:0.32 (0.16,0.65) | age, BMI, duration of diabetes, drinking and smoking status, history of CVD, HbA1c, SBP, DBP, ALT, TC, TG, HDL-C, eGFR, use of insulin, ACEi/ARB, statin, retinopathy and albumin-to-creatinine ratio. | |||||||
| J. O. Chung37 | 2013 | Korea | cross-sectional | Hospital-based | 2291 | DCAN | 0.36 (0.21,0.63) | age, gender, BMI, smoking habits, AST, ALT, hypertension, hyperlipidaemia, HbA1c, diabetes duration, retinopathy and nephropathy | 11 |
| K. H. Chan7 | 2013 | Australia | cohort | Hospital-based | 9795 | DA | 0.50 (0.27,0.95) | age, height, smoking status, GGT, HbA1c, and history of previous PAD, non-PAD CVD, amputation or diabetic skin ulcer, neuropathy, nephropathy and diabetic retinopathy, as well as trial treatment allocation | 11 |
| Luo, Yajing32 | 2013 | China | cross-sectional | Hospital-based | 246 | DN | 091 (0.83,0.99) | duration of diabetes, SBP, HbA1C, FBG, 2hPG, TC, TG | 11 |
| Lai, Jie33 | 2013 | China | cross-sectional | Hospital-based | 435 | DN | 0.78 (0.74,0.83) | duration of diabetes, SBP, FBG, SUA, Lp (a), hs-CRP, LDL-C, HbA1C | 11 |
| Seong-Woo Choi41 | 2012 | Korea | cross-sectional | Population-based | 690 | HbA1c ≥6.5% | 0.40 (0.20,0.80) | age, gender, abdominal circumstance, smoking, diabetic duration, hypertension, CCVD history, HDL-C, LDL-C, TG, fasting glucose, eGFR, AST, ALT and GGT. | 11 |
| Miho Yasuda36 | 2011 | Japan | cohort | Population-based | 1672 | DR* | 0.25 (0.09,0.72) | age, gender, 2hPG, SBP, TC, HDL-C, GGT, history of cardiovascular disease, smoking habits, and alcohol intake. | 11 |
| DR | 0.39 (0.12,1.30) | age, gender, 2hPG, SBP, TC, HDL-C, GGT, history of cardiovascular disease, smoking habits and alcohol intake. | |||||||
| Su, Zhiyan35 | 2010 | China | cross-sectional | Hospital-based | 664 | DR | 0.91 (0.88,0.94) | age, gender, duration of diabetes, BMI, WHR, HbA1C, LDL-C, TG, UA, SBP | 11 |
| Jia, Yumei34 | 2010 | China | cross-sectional | Hospital-based | 1062 | DR | 0.91 (0.87,0.96) | age, gender, duration of diabetes, TC, TG, HDL-C, LDL-C | 11 |
| Seung Seok Han22 | 2010 | Korea | cross-sectional | Population-based | 93909 | DN | Male:0.88 (0.64,1.19) | age, BMI, hypertension, TC, TG, HDL- C, and hepatic markers including AST, ALT, alkaline phosphatase, and GGT | 11 |
| DN | Female:0.68 (0.43,1.08) | age, BMI, hypertension, TC, TG, HDL- C, and hepatic markers including AST, ALT, alkaline phosphatase, and GGT |
Note: HbA1C: hemoglobin A1c; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglycerides; HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol; SUA: serum uric acid; eGFR: estimated glomerular filtration rate; ALP: alkaline phosphatase; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: γ-glutamyl transpeptidase; 2hPG: 2-hour post-load plasma glucose; *DR in high blood sugar condition.
Table 2 shows the basic characteristics of the subjects in the included studies. A total of 132,240 subjects were included in the studies. The study population in each study ranged from 80 to 93,909 and the proportion of males ranged from 32.61% to 100.00%. The mean age of the study population ranged from 48.70 to 87.40 years. The mean BMI of the study population ranged from 21.40 to 26.60 kg/m2. The proportion of smokers in the study population ranged from 2.60% to 48.07%, and the proportion of alcohol drinkers in the study population ranged from 7.80% to 51.85%. The mean of duration of DM ranged from 6.14 to 15.19 years.
Table 2. The basic characteristics of the subjects in the included studies.
| Study | Male (%) | Age (Year) | BMI (kg/m2) | Duration of diabetes (Year) | Smokers (%) | Alcohol drinkers (%) |
|---|---|---|---|---|---|---|
| Liu, Miao (2016) | 100 | 87.40 | 24.7 | n/r | 2.60 | 7.80 |
| Wang, Jing (2016) | 45.54 | 64.07 | n/r | n/r | 28.74 | 26.17 |
| Ryuichi, Kawamoto (2016) | 45.20 | 80.00 | 21.40 | n/r | 24.60 | n/r |
| Jiang, Pijun (2016) | 50.27 | 60.97 | 23.56 | n/r | n/r | n/r |
| Jaechan, Leem (2015) | 62.17 | 63.00 | 25.07 | 13.34 | 23.04 | n/r |
| Zhang, Dan (2015) | 49.01 | 59.00 | 24.81 | 10.80 | n/r | n/r |
| Chen, Fang (2015) | 56.54 | 58.71 | 24.89 | 9.58 | 35.44 | n/r |
| Wei, Wei (2015) | n/r | 59.93 | n/r | 7.93 | 0.00 | n/r |
| Risa, Sekioka (2015) | 66.17 | 64.70 | 25.50 | 13.90 | 48.07 | n/r |
| Eun Sook, Kim (2015) | 47.80 | 55.83 | 25.00 | 6.93 | 21.13 | 38.44 |
| Hamamoto S (2015) | 59.66 | 60.50 | 24.70 | 12.20 | n/r | n/r |
| Apoorva, Dave (2015) | n/r | 53.93 | n/r | 7.94 | n/r | n/r |
| Wang, Ru (2015) | 60.14 | 54.13 | 26.60 | 9.92 | 28.38 | 27.38 |
| Cai, Junwei(2015) | 47.06 | 67.22 | 25.41 | 12.48 | n/r | n/r |
| Syeda Sadia NAJAM(2014) | 42.87 | 61.17 | 26.23 | n/r | 20.22 | 10.34 |
| Kiwako Toya(2014) | 58.18 | 59.00 | 23.70 | 14.00 | n/r | n/r |
| Kiwako Toya (2014) | 60.16 | 61.00 | 25.00 | 15.00 | n/r | n/r |
| Tsuyoshi Mashitani (2014) | 63.01 | 67.10 | 24.80 | 14.10 | 20.38 | n/r |
| Eun Sook Kim (2014)-Male | n/r | 55.20 | 24.60 | 7.00 | 38.04 | 56.63 |
| Eun Sook Kim (2014)-Female | n/r | 58.80 | 25.10 | 8.10 | 5.09 | 17.59 |
| J. O. Chung (2013) | 64.99 | 59.01 | 24.38 | 9.24 | 17.55 | 32.52 |
| K. H. Chan (2013) | 61.72 | 62.00 | n/r | n/r | 9.41 | n/r |
| Luo, Yajing (2013) | 47.97 | 62.05 | 26.60 | 10.76 | 28.86 | n/r |
| Lai, Jie (2013) | 54.02 | 63.10 | 25.29 | 15.19 | n/r | n/r |
| Seong-Woo Choi(2012) | 32.61 | 68.20 | 24.50 | 8.90 | 15.36 | n/r |
| Miho Yasuda (2011)* | 53.35 | 64.17 | 23.93 | 6.14 | 20.87 | 51.85 |
| Miho Yasuda (2011) | n/r | n/r | n/r | n/r | n/r | n/r |
| Su, Zhiyan (2010) | 49.25 | 59.70 | 24.90 | 9.17 | 32.83 | n/r |
| Jia, Yumei (2010) | 53.77 | 58.16 | n/r | 7.33 | n/r | n/r |
| Seung Seok Han (2010)-Male | n/r | n/r | n/r | n/r | n/r | n/r |
| Seung Seok Han (2010)-Female | n/r | n/r | n/r | n/r | n/r | n/r |
*DR in high blood sugar condition; n/r: not reported.
We assessed 13 biochemical indices which may be related to the occurrence of diabetic complications. Table 3 shows these biochemical indices in the subjects included in the studies.
Table 3. The biochemical indicators of the subjects in the included studies.
| Study | FBG (mmol/L) | HbA1C (%) | Hypertension (%) | SBP (mmHg) | DBP (mmHg) | Dyslipidaemia (%) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | SUA (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu, Miao (2016) | 7.0 | n/r | 67.7 | 133.3 | 72.6 | 38.7 | 4.90 | 1.80 | 1.90 | 2.50 | n/r |
| Wang, Jing (2016) | 8.23 | n/r | 45.30 | 131.3 | 77.27 | n/r | 5.27 | n/r | 1.37 | 3.10 | n/r |
| Ryuichi, Kawamoto (2016) | n/r | n/r | 9.10 | 136.00 | 74.00 | n/r | n/r | 0.90 | 1.42 | 2.75 | n/r |
| Jiang, Pijun (2016) | 10.41 | 9.33 | n/r | 134.78 | 74.17 | n/r | 4.69 | 2.03 | 1.17 | 2.70 | 307.72 |
| Jaechan, Leem (2015) | n/r | n/r | 54.13 | 132.91 | 78.00 | n/r | 4.53 | 1.37 | 1.27 | 2.83 | n/r |
| Zhang, Dan (2015) | n/r | 8.81 | n/r | 135.32 | 113.07 | n/r | n/r | 1.64 | 1.22 | 3.22 | n/r |
| Chen, Fang (2015) | 9.78 | 9.98 | 50.63 | 129.95 | 77.87 | n/r | 4.64 | 2.36 | 1.06 | n/r | n/r |
| Wei, Wei (2015) | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Risa, Sekioka (2015) | n/r | 9.13 | 73.89 | n/r | n/r | 77.15 | n/r | n/r | n/r | n/r | n/r |
| Eun Sook, Kim (2015) | n/r | 8.44 | n/r | 129.90 | 78.39 | 31.07 | n/r | 2.01 | 1.19 | 2.65 | n/r |
| Hamamoto S (2015) | n/r | 9.60 | n/r | 132.69 | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Apoorva, Dave (2015) | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Wang, Ru (2015) | 8.18 | 8.45 | n/r | 136.22 | 79.68 | n/r | 4.65 | 1.98 | 1.10 | 2.88 | n/r |
| Cai, Junwei (2015) | 9.61 | 7.86 | n/r | 125.55 | 76.06 | n/r | 6.11 | 2.05 | 1.05 | 2.71 | n/r |
| Syeda Sadia NAJAM (2014) | 7.01 | 6.50 | 45.32 | 149.11 | 84.16 | n/r | 5.50 | 1.68 | 1.27 | 3.29 | n/r |
| Kiwako Toya (2014) | n/r | 8.00 | n/r | 132.00 | 76.00 | n/r | n/r | n/r | 1.42 | n/r | n/r |
| Kiwako Toya (2014) | n/r | 8.30 | n/r | 139.00 | 76.00 | n/r | n/r | n/r | 1.32 | n/r | n/r |
| Tsuyoshi Mashitani (2014) | n/r | 7.60 | n/r | 134.20 | 74.50 | n/r | 5.00 | 1.70 | 1.50 | 2.90 | 321.30 |
| Eun Sook Kim (2014)-Male | n/r | 8.20 | n/r | 129.00 | 79.70 | n/r | 4.59 | 2.64 | 1.14 | 2.50 | n/r |
| Eun Sook Kim (2014)-Female | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| J. O. Chung (2013) | 8.38 | 8.52 | 68.66 | 127.22 | 77.23 | 64.82 | 4.68 | 1.80 | 1.20 | 2.80 | n/r |
| K. H. Chan (2013) | n/r | n/r | 56.58 | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Luo, Yajing (2013) | 9.87 | 8.93 | n/r | 149.53 | 81.57 | n/r | 5.13 | 2.03 | n/r | 3.05 | 289.55 |
| Lai, Jie (2013) | 9.31 | 8.10 | n/r | 136.27 | n/r | n/r | 4.76 | 1.85 | 1.22 | 3.00 | 328.39 |
| Seong-Woo Choi (2012) | 7.60 | 7.40 | 64.64 | 130.30 | 72.00 | n/r | 5.01 | 2.06 | 1.23 | 2.85 | n/r |
| Miho Yasuda (2011)* | 6.21 | 5.37 | 58.49 | 135.29 | 81.96 | n/r | 5.48 | n/r | 1.70 | n/r | n/r |
| Miho Yasuda (2011) | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Su, Zhiyan (2010) | n/r | 9.22 | n/r | 134.70 | 80.00 | n/r | 4.95 | 1.66 | 1.20 | 3.19 | 308.77 |
| Jia, Yumei (2010) | 9.31 | 9.38 | n/r | n/r | n/r | n/r | 4.98 | 2.13 | 1.51 | 2.70 | n/r |
| Seung Seok Han (2010)-Male | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
| Seung Seok Han (2010)-Female | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r |
*DR in high blood sugar condition; n/r: not reported.
We assessed the quality of included studies using the quality assessment tool44, which was based on a modified Newcastle-Ottawa Quality Assessment Scale (NOS) assessment tool. The quality scores of the included studies are shown in Table 1. The quality scores ranged from seven to eleven and sixteen studies scored eleven points. Eight studies were assessed as moderate quality, and nineteen studies were assessed as high quality.
Bilirubin and the risk of diabetic complications
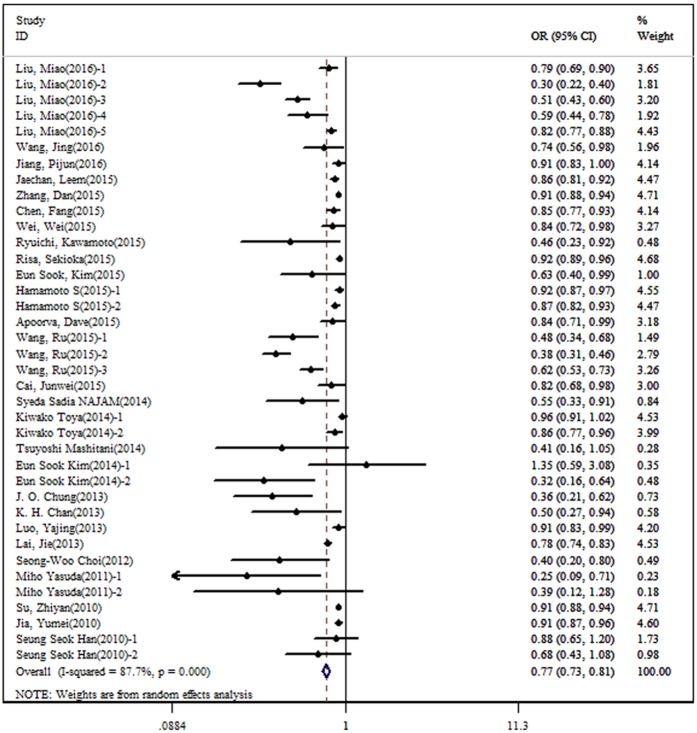
During the process of extracting data, we found that Liu et al.43 studied several diabetic complications, including DN, DR, DAS, DCH, DIS. Hamamoto et al.25 studied DR and DN, Wang et al.40 studied DR, DN and DPN, Kiwako et al.23 studied microalbuminuria and macroalbuminuria, Eun et al.21 studied DAS by sex, Miho et al.36 studied DR in different blood sugar level, and Seung et al.22 studied DN by sex. Therefore, we extracted 38 sets of statistical data from 27 studies. A negative association between bilirubin concentration and the risk of diabetic complications was observed (OR: 0.77, 95% CI: 0.73–0.81), with high heterogeneity (I2 = 87.7%, P < 0.001) (Fig. 2). In order to determine the source of heterogeneity, continuous variables were used in meta-regression, and we found that FBG and HbA1C may be the source of heterogeneity (P value for meta-regression on FBG was = 0.009; P value for meta-regression on HbA1C was = 0.014). Furthermore, we used subgroup analysis with dichotomous variables, and the results also showed that there was a significant association between bilirubin concentration and the risk of diabetic complications. The overall and subgroup results are shown in Table 4.
Figure 2. The forest plot on the association between bilirubin concentration and the risk of diabetic complications.
Table 4. The pooled ORs on the association between bilirubin concentration and the risk of diabetic complications.
| No. of study data | Model for meta-analysis | OR (95%CI) | I2 (%) | P for heterogeneity | |
|---|---|---|---|---|---|
| Overall | 38 | R | 0.77 (0.73, 0.81) | 87.7 | <0.001 |
| Subgroup Analysis | |||||
| Country | |||||
| China | 19 | R | 0.73 (0.68, 0.79) | 92.4 | <0.001 |
| Korea | 8 | R | 0.65 (0.49, 0.84) | 72.0 | 0.001 |
| Japan | 9 | R | 0.90 (0.85, 0.95) | 62.1 | 0.007 |
| Else | 2 | R | 0.71 (0.44, 1.14) | 59.1 | 0.118 |
| Number | |||||
| <2000 | 30 | R | 0.82 (0.79, 0.86) | 84.4 | <0.001 |
| ≥2000 | 8 | R | 0.57 (0.45, 0.71) | 78.4 | <0.001 |
| Design | |||||
| Case-control | 5 | F | 0.90 (0.87, 0.93) | 0.0 | 0.453 |
| Cross-sectional | 26 | R | 0.73 (0.68, 0.78) | 90.7 | <0.001 |
| Cohort | 7 | R | 0.78 (0.65, 0.93) | 70.8 | 0.002 |
| Source of subjects | |||||
| Hospital-based | 31 | R | 0.78 (0.74, 0.82) | 89.4 | <0.001 |
| Population-based | 7 | F | 0.63 (0.49, 0.81) | 41.7 | 0.113 |
| FBG | |||||
| <9.00 | 13 | R | 0.53 (0.43, 0.64) | 90.5 | <0.001 |
| ≥9.00 | 6 | R | 0.86 (0.81, 0.92) | 74.3 | 0.002 |
| HbA1C | |||||
| <9.00 | 16 | R | 0.70 (0.63, 0.79) | 90.6 | <0.001 |
| ≥9.00 | 7 | F | 0.91 (0.89, 0.93) | 0.0 | 0.622 |
| NOS | |||||
| Moderate quality | 8 | F | 0.90 (0.87, 0.93) | 21.8 | 0.257 |
| High quality | 30 | R | 0.72 (0.67, 0.77) | 89.7 | <0.001 |
R, random-effects model, F: fix-effects model.
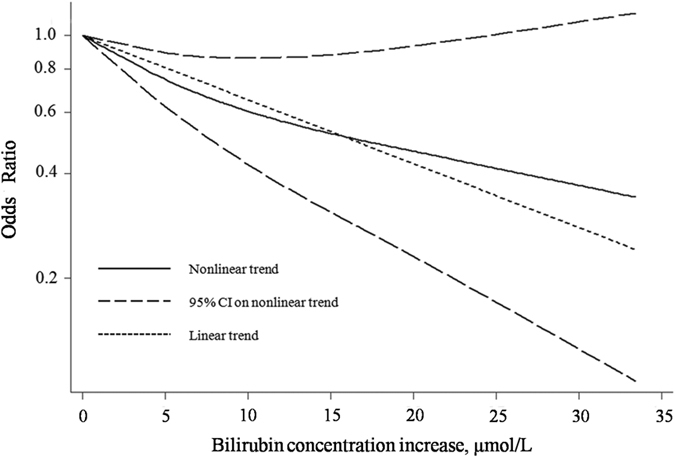
In the dose-response analysis, we found that 23 sets of statistical data from 13 of the included studies were suitable. A nonlinear association between bilirubin concentration and the risk of diabetic complications was found (P < 0.001). A degree of heterogeneity was observed (P = 0.032). Figure 3 shows the dose–response association between bilirubin concentration and the risk of diabetic complications.
Figure 3. The dose-response association between bilirubin concentration and the risk of diabetic complications.
In the sensitivity analysis, we omitted one study at a time, and the pooled OR ranged from 0.76 (95% CI: 0.72–0.80) to 0.79 (95% CI: 0.76–0.83). The results of the sensitivity analysis showed that the results for the effects of bilirubin on diabetic complications were robust.
Both the Begg’s and Egger’s tests showed that publication bias existed in the comparison between bilirubin concentration and the risk of diabetic complications (P value for Begg’s test was <0.001, and P value for Egger’s test was <0.001). Thus, we performed the trim and fill method to identify and correct the asymmetry of the funnel plot, and the results showed a negative association between bilirubin concentration and the risk of diabetic complications (OR: 0.77, 95% CI: 0.73–0.81, P value for heterogeneity was <0.001).
Bilirubin and the risk of DN
We extracted 13 statistical data sets from 11 studies on DN. We also found that there was a negative association between bilirubin concentration and the risk of DN (OR: 0.79, 95% CI: 0.72–0.87), with high heterogeneity (I2 = 88.1%, P < 0.001). We also used subgroup analysis with dichotomous variables and the results show that there was a significant association between bilirubin concentration and the risk of DN. The overall and subgroup results are shown in Table 5. The results of the sensitivity analysis also show that the overall result for the effects of bilirubin on DN were robust, and when we omitted one study at a time, the pooled OR ranged from 0.78 (95% CI: 0.70–0.86) to 0.86 (95% CI: 0.81–0.91).
Table 5. The pooled ORs on the association between bilirubin concentration and risk of DN and DR.
| The pooled ORs on the association between bilirubin concentration and risk of DN | The pooled ORs on the association between bilirubin concentration and risk of DR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of study data | Model for meta-analysis | OR (95%CI) | I2 (%) | P for heterogeneity | No. of study data | Model for meta-analysis | OR (95%CI) | I2 (%) | P for heterogeneity | |
| Overall | 13 | R | 0.79 (0.72, 0.87) | 88.1 | <0.001 | 13 | R | 0.84 (0.79, 0.89) | 85.3 | <0.001 |
| Subgroup Analysis | ||||||||||
| Country | ||||||||||
| China | 7 | R | 0.75 (0.64, 0.87) | 91.6 | <0.001 | 8 | R | 0.80 (0.73, 0.87) | 90.2 | <0.001 |
| Korea | 2 | F | 0.81 (0.63, 1.05) | 0.0 | 0.363 | 0 | — | — | — | — |
| Japan | 4 | R | 0.89 (0.82, 0.97) | 67.5 | 0.026 | 4 | R | 0.89 (0.81, 0.93) | 62.6 | 0.046 |
| Number | ||||||||||
| <2000 | 9 | R | 0.86 (0.81, 0.92) | 75.2 | <0.001 | 12 | R | 0.86 (0.81, 0.91) | 84.0 | <0.001 |
| ≥2000 | 4 | R | 0.63 (0.41, 0.99) | 89.1 | <0.001 | 1 | — | 0.48 (0.34, 0.68) | — | — |
| Design | ||||||||||
| Case-control | 2 | F | 0.89 (0.82, 0.97) | 1.0 | 0.315 | 3 | F | 0.89 (0.85, 0.94) | 22.8 | 0.274 |
| Cross-sectional | 7 | R | 0.74 (0.64, 0.86) | 91.5 | <0.001 | 8 | R | 0.81 (0.75, 0.89) | 90.1 | <0.001 |
| Cohort | 4 | R | 0.87 (0.76, 0.99) | 66.0 | 0.032 | 2 | F | 0.30 (0.14, 0.66) | 0.0 | 0.581 |
| Source of subjects | ||||||||||
| Hospital-based | 10 | R | 0.80 (0.72, 0.88) | 90.9 | <0.001 | 10 | R | 0.85 (0.81, 0.91) | 87.1 | <0.001 |
| Population-based | 3 | F | 0.78 (0.64, 0.94) | 0.0 | 0.589 | 3 | F | 0.46 (0.30, 0.71) | 0.0 | 0.392 |
| FBG | ||||||||||
| <9.00 | 3 | R | 0.61 (0.37, 0.99) | 94.7 | <0.001 | 4 | F | 0.40 (0.28, 0.56) | 55.8 | 0.079 |
| ≥9.00 | 4 | R | 0.85 (0.78, 0.94) | 75.8 | 0.006 | 2 | F | 0.89 (0.84, 0.95) | 37.7 | 0.205 |
| HbA1C | ||||||||||
| <9.00 | 7 | R | 0.76 (0.65, 0.89) | 93.7 | <0.001 | 4 | R | 0.56 (0.34, 0.93) | 86.7 | <0.001 |
| ≥9.00 | 2 | F | 0.88 (0.84, 0.93) | 0.0 | 0.433 | 5 | F | 0.91 (0.89, 0.93) | 0.0 | 0.643 |
| NOS | ||||||||||
| Moderate quality | 2 | F | 0.89 (0.82, 0.97) | 1.0 | 0.315 | 5 | F | 0.91 (0.88, 0.93) | 3.7 | 0.386 |
| High quality | 11 | R | 0.78 (0.70, 0.87) | 89.8 | <0.001 | 8 | R | 0.72 (0.63, 0.82) | 90.9 | <0.001 |
R, random-effects model, F: fix-effects model.
Bilirubin and the risk of DR
We extracted 13 statistical data sets from 12 studies on DR. We found that there was a negative association between bilirubin concentration and the risk of DR (OR: 0.84, 95% CI: 0.79–0.89), with moderate heterogeneity (I2 = 85.3%, P < 0.001). We also used subgroup analysis with dichotomous variables and the results show that there was a significant association between bilirubin concentration and the risk of DR. The overall and subgroup results are shown in Table 5. The results of the sensitivity analysis also show that the overall result for the effects of bilirubin on DR were robust, and when we omitted one study at a time, the pooled OR ranged from 0.81 (95% CI: 0.76–0.88) to 0.89 (95% CI: 0.86–0.92).
Bilirubin and the risk of diabetic neuropathy
In this study, diabetic neuropathy included DPN and DCAN. We found that there was a negative association between bilirubin concentration and the risk of diabetic neuropathy (OR: 0.56, 95% CI: 0.43–0.74), with moderate heterogeneity (I2 = 43.3%, P = 0.172). As the number of studies on diabetic neuropathy was small, subgroup and sensitivity analyses were not performed.
Discussion
To the best of our knowledge, this is the first meta-analysis to determine the association between bilirubin concentration and the risk of diabetic complications. We included 27 studies involving 132,240 subjects from five countries. Eleven studies assessed DN, twelve assessed DR, three assessed diabetic neuropathy, and five assessed other complications. The quality of the included studies was relatively high, and more than two-thirds of studies were rated 11 points in our quality assessment. Our results indicated that there was a negative association between bilirubin concentration and the risk of diabetic complications (OR: 0.77, 95% CI: 0.73–0.81), and similar results were also found for DN (OR: 0.79, 95% CI: 0.72–0.87), DR (OR: 0.84, 95% CI: 0.79–0.89) and diabetic neuropathy (OR: 0.56, 95% CI: 0.43–0.74). The dose–response relationship between bilirubin concentration and the risk of diabetic complications was non-liner. Bilirubin showed a protective effect on the risk of diabetic complications.
Bilirubin has potent antioxidant properties, which were first reported in 195412. Due to its antioxidant capacity, bilirubin is oxidized to biliverdin, which is immediately reduced by biliverdin reductase to bilirubin11. It suppressed the oxidation of lipids and lipoproteins, especially low-density lipoprotein cholesterol45. Compared with the water-soluble antioxidants (such as glutathione), bilirubin has been shown to be more effective at protecting lipids from oxidation11. In addition, bilirubin also has anti-inflammatory properties. The vivo study on subjects with Gilbert’s syndrome had found that there was a negative association between serum bilirubin concentration and soluble forms of CD40 ligand and P-selectin46. Several studies found a negative association between serum bilirubin concentration and C-reactive protein13,47,48. In cardiovascular diseases, several studies found that a negative association between bilirubin concentration and the risk of both coronary and peripheral atherosclerotic disease49,50,51, and a meta-analysis which involved 15000 subjects, demonstrated that increased bilirubin concentration could reduce the risk of cardiovascular diseases52.
Diabetes can affect many parts of the body and is associated with serious complications, such as heart disease, stroke, blindness, kidney failure, and lower-limb disorders leading to amputation. Oxidative stress is a major contributor in the pathogenesis of diabetic complications53. Kumar et al. showed that serum bilirubin concentration was negatively correlated with the level of oxidative stress and positively correlated with the levels of antioxidative enzyme such as superoxide dismutase, catalase, and glutathione peroxidase54. Therefore, bilirubin may protect against diabetic complications. However, to date, the mechanism of diabetic complications is still unclear. Possible mechanisms underlying the association between bilirubin and diabetic complications are as follows: high blood sugar can lead to mitochondrial superoxide overproduction in endothelial cells of both large and small vessels, five major pathways, which included polyol pathway flux, increased formation of advanced glycation end products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms, and overactivity of the hexosamine pathway, can be activated by increased superoxide production. These five pathways are involved in the pathogenesis of diabetic complications. Bilirubin not only can inhibit lipid peroxidation and attenuate LDL oxidation, but also reduce the levels of reactive oxygen species. Several studies have analyzed the association between bilirubin concentration and the risk of diabetic complications (such as DN, DR and other diabetic vascular complications)19,38,43,44.
For example, in DN, Hamamoto et al.25 carried out across-sectional study on 523 diabetic patients in Japan. They found that lower bilirubin concentration was an independent risk factor for DN. In DR, Syeda et al.30 performed a population-based cross-sectional study on 1761 diabetic patients aged ≥40 years in China. The subjects were assigned to quartiles based on serum total bilirubin concentration. The results showed that the prevalence of DR in the entire study population was 9.6%. The prevalence of DR was significantly lower in the highest quartile compared with the other three quartiles. After adjustment for multiple confounding factors, diabetic patients in the highest quartile were less likely to suffer from DR than patients in the lowest quartile. Chung et al.37 also performed a cross-sectional study in patients with diabetic neuropathy with regard to cardiovascular autonomic neuropathy. The results of this study showed that serum total bilirubin concentration was significantly lower in subjects with cardiovascular autonomic neuropathy. Serum total bilirubin levels were significantly associated with cardiovascular autonomic neuropathy after adjustment for multiple confounders. However, the results on the association between bilirubin concentration and the risk of diabetic complications were inconsistent. Therefore, it was necessary to systematically analyze the relevant studies, and determine their relationship and the key factors that may influence the results of the studies.
In order to ensure that the results of our meta-analysis were both reliable and credible, we carried out the following ways: First, we extracted 38 results on the association between bilirubin concentration and the risk of diabetic complications from the included 27 studies, which helped us to understand the effects of bilirubin on the various complications of DM. The ORs which were extracted from the included studies, were adjusted to take into account the common confounders (such as gender, age and BMI), this ensured the stability of the results of our meta-analysis. Second, the standardized NOS was not suitable for assessing the effects of bilirubin on diabetic complications, therefore, we modified the NOS to satisfy our assessment as the included studies were evaluated more accurately, and this ensured the credibility of the results of our meta-analysis. The included studies did not have a low quality assessment rating, and the results of subgroup analysis were similar to the overall results. Third, we not only analyzed the association between bilirubin concentration and the risk of diabetic complications, but also determined the dose-response relationship, and found that there was a nonlinear association between bilirubin concentration and the risk of diabetic complications. We further analyzed the data for DN and DR. The results were similar to the overall results. This ensured the reliability of the results of our meta-analysis. Fourth, the potential confounders that were extracted contained dichotomous and continuous variables, thus we respectively carried out subgroup analysis and meta-regression, to analyze the effects of the potential confounders on the results of our meta-analysis. We found that FBG and HbA1C may be the source of heterogeneity. Blood glucose control reduced the risk of diabetic complications, especially microvascular (such as eye, kidney and nerve) disease1. These results suggested that FBG and HbA1C must be considered confounders which were adjusted in later studies on the association between bilirubin concentration and the risk of diabetic complications.
Although we attempt to ensure that our results were reliable and credible, there were some limitations in our study. First, because the authors of this article did not have access to EMBASE, we could not directly search the relevant articles in this database. To make up for this limitation as much as possible, we searched the relevant articles in Google Scholar. If we found the relevant article in Google Scholar, we purchased the article or sought help online. Second, the source of the subjects in 80% of included studies was hospital-based, these studies may have a certain degree of selection bias as no randomization was performed. We performed subgroup analysis on the source of subjects, and found a negative association between bilirubin concentration and the risk of diabetic complications, and I2 in the population-based group was lower than 50%. Third, publication bias was observed, and in order to eliminate this, the trim and fill method was used, and a negative association between bilirubin concentration and the risk of diabetic complications was observed. Fourth, we found that only one study had been carried out in Australia, and the other studies were carried out in Asia. This might lead to selection bias due to race. There was a significant difference in the association between bilirubin concentration and the risk of DA in the Australian study, these results suggested that bilirubin may have an impact on the risk of diabetic complications not only in Asian populations, but also in populations from other counties. We also found that there were only five cohort studies, which accounted for merely 18% of the included studies. Thus, large-sample, long-term cohort studies are needed to validate our results, especially studies from countries outside Asia.
In summary, we collected literature that studied the association between bilirubin concentration and the risk of diabetic complications. Our meta-analysis indicated that higher bilirubin concentration reduced the risk of diabetic complications. This meta-analysis was the first comprehensive quantitative assessment of bilirubin on diabetic complications, suggested that bilirubin may be used as a biomarker of the occurrence of diabetic complications.
Research Design and Methods
Search Strategy and Selection Criteria
An extensive search strategy was carried out in multiple databases by October 27, 2016 (MEDLINE, Web of Science, and Google Scholar). In order to collect the relevant articles as many as possible, we set the following key words: bilirubin and (“retinopathy” or “nephropathy” or “neuropathy” or “vascular complication”). We also reviewed reference lists from all the relevant original research and review articles to identify additional possibly eligible studies. There is no language restriction.
Inclusion and exclusion criteria
According to the inclusion criteria, the possibly relevant articles which had been collected were independently assessed by three reviewers (Xiaomei Wu, Bo Zhu and Yang Yang). Any disagreements were solved by discussion.
The diagnostic criterion for DM, prediabetes and hyperglycemia was used by the World Health Organization (WHO) 1999 criteria55 or American Diabetes Association (ADA) 2010 guidelines56. On the basis of these diagnostic criterions, DN was defined as urinary albumin excretion ≥30 mg/day (or ≥30 μg/g creatinine), and contained microalbuminuria (urinary albumin excretion between 30 and 300 mg/day or between 30 and 300 μg/g creatinine) and macroalbuminuria (urinary albumin excretion >300 mg/day or >300 μg/g creatinine)57. DR was evaluated by an ophthalmologist using fundoscopy exam and contained normal, non-proliferative and proliferative types. Non-proliferative DR showed one or more of the following symptoms: microaneurysm, haemorrhage, exudates, or microvascular abnormalities; proliferative DR showed the generation of new vessels and fibrosis. DPN was defined as patient reporting changes in sensations using the Michigan Neuropathy Screening Instrument (MNSI). DOCAD was defined as ≥50% diameter stenosis in at least one coronary artery. DCAN was assessed by analyzing heart rate response during deep breathing, lying-to-standing and the Valsalva manoeuvre and evaluating blood pressure response to standing58. DAS was defined as pulse wave velocity (PWV) ≥1745 cm/s21. DA were defined as those above the ankle and minor amputations as those below the ankle7. DCH was defined as having been diagnosis one of the following diseases: stable angina, unstable angina, myocardial infarction43. DIS was the result of a disturbance of the cerebral circulation which leads ultimately to cell death43. DCA was defined as IMT ≥1.0 mm or plaque lesion42.
The included studies in our meta-analysis met the following criteria: (1) the study should investigate the relationship between bilirubin concentration and the risk of diabetic complications; (2) the study should contain the odds ratio (OR) with 95% confidence intervals (CIs) on the relationship between bilirubin concentration and the risk of diabetic complications, or had provided available data to calculate the corresponding estimate; (3) if the results of more than two study came from the same population, the latest or highest-quality result was adopted.
The studies were excluded from our meta-analysis if the study did not investigate the relationship between bilirubin concentration and the risk of diabetic complications. The exclusion criteria also contained: (1) the study was not original research, for example editorial, commentary and review; (2) subjects in the study did not involve humans, for example animal experiments, chemistry and cell-line studies; (3) individual case reports; (4) the study did not provide sufficient data to calculate the corresponding estimate on the relationship between bilirubin concentration and the risk of diabetic complications.
Data extraction and conversion
The included studies were independently read in detail by two reviewers (Bo Zhu and Xiaomei Wu). Two reviewers extracted the information by a table, which was prepared using Microsoft Excel 2007. The information extracted included the following: author, year of publication, country; study design, source of subjects, number of subjects and baseline risk factors (body mass index (BMI), Duration of diabetes, Smoking and alcohol consumption), outcome and its definition, biochemical indexes (fasting blood glucose (FBG), hemoglobin A1c (HbA1C), hypertension, systolic blood pressure (SBP), diastolic blood pressure (DBP), dyslipidaemia, total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C) and serum uric acid (SUA)), the results of statistical analysis. In order to reduce the effects of confounding factors on the pooled estimates, we preferred to extract and analyze the adjusted estimates rather than unadjusted estimates. When the studies only provided categories of bilirubin concentration, the results of the highest category were selected for analysis. For studies, which presented several estimates adjusted for different number of potential confounders, the estimate that adjusted for the most number of potential confounders was selected for analysis.
The units of bilirubin concentration were often used as μmol/L and mg/dL. In order to make unit consistency in all included studies, we converted mg/dL to μmol/L with multiplying by 17.1.
Quality assessment
The quality of final included studies was independently assessed by two reviewers (Bo Zhu and Xiaomei Wu). There was no standardized quality assessment tool on assessing the effects of bilirubin on diabetic complications, so NOS was modified to satisfy our requirement according to previous study44. Six aspects were used to evaluate the quality of the included study, as follows: (1) sample representativeness; (2) sample size; (3) outcome definition and measurement; (4) comparability of results; (5) outcome assessment and (6) statistical methods. The score of study quality ranged from zero to eleven. The study with ten or more points was regard as “high quality”, the study with seven to nine points was regard as “moderate quality”, and otherwise, the study was regarded as “low quality”. The quality assessment tool is shown in Supplementary 1.
Statistical analysis
In our meta-analysis, we used the pooled odds ratios (ORs) and 95%CIs to analyze the relationship between bilirubin concentration and diabetic complications, which were calculated by using the inverse variance-weighting method. The Chi-square-based Q-test was used to evaluate the heterogeneity among the individual studies. Heterogeneity was quantified based on I2, which ranged from 0% to 100% (I2 = 0% to 25%, no heterogeneity; I2 = 25% to 50%, moderate heterogeneity; I2 = 50% to 75%, high heterogeneity; I2 = 75% to 100%, extreme heterogeneity). When I2 was larger than 50%, a DerSimonian and Laird random-effects model was used; otherwise, the fixed-effects model was used. If there was highly heterogeneity between studies, we used subgroup analysis and meta-regression to find the source of heterogeneity. We performed subgroup analysis by country, number of subjects, design, source of subjects, FBG, HbA1C, the study quality. Restricted maximum likelihood (REML) based random effects meta-regression was carried out to investigate the source of heterogeneity among studies according to the relevant factors. We also used the sensitivity analysis to evaluate the robustness of the results in our meta-analysis. In the sensitivity analysis, we excluded each study in turn and obtained the pooled estimates from the remaining studies. The purpose of sensitivity analysis was to evaluate the effect of a single study on the overall pooled estimates.
We conducted the dose–response meta-analysis to calculate study-specific slopes (i.e., linear trends) and 95%CIs, which proposed by Greenland S. et al.59 and Orsini N. et al.60. If the study reported exposure category by a range, the midpoint was calculated by averaging the lower and upper bound; if the lowest category was open ended, the lowest boundary was considered to be zero; if the highest exposure category was open-ended, the width of the open-ended interval was taken to be the same as the adjacent interval.
The possibility of publication bias was assessed using the Begg and Egger’s test. We also performed the Duval and Tweedie nonparametric “trim and fill” procedure to further assess the possible effect of publication bias in our meta-analysis. A two-sided P value < 0.05 in statistical process was considered significantly different. All statistical analysis was performed with the Stata software package (Version 12.0; Stata Corp., College Station, TX).
Additional Information
How to cite this article: Zhu, B. et al. Effect of bilirubin concentration on the risk of diabetic complications: A meta-analysis of epidemiologic studies. Sci. Rep. 7, 41681; doi: 10.1038/srep41681 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by General Foundation of scientific research in the Department of Education in Liaoning (L2015592).
Footnotes
The authors declare no competing financial interests.
Author Contributions B. Z., Y.Y. and X.M.W. performed articles collection and extracted data from the included studies. Y.Y. and X.M.W. performed the statistical analysis. B. Z., Y.F.B. and X.M.W. wrote the manuscript. All of the authors read and approved the manuscript.
References
- Chen L., Magliano D. J. & Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nature reviews. Endocrinology 8, 228–236 (2012). [DOI] [PubMed] [Google Scholar]
- Grubesic T. H., Miller J. A. & Murray A. T. Geospatial and geodemographic insights for diabetes in the United States. Applied Geography 55, 117–126 (2014). [Google Scholar]
- Boyle J. P., Thompson T. J., Gregg E. W., Barker L. E. & Williamson D. F. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics 8, 29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting D. R., Guariguata L., Weil C. & Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice 94, 311–321 (2011). [DOI] [PubMed] [Google Scholar]
- Ayodele O. E., Alebiosu C. O. & Salako B. L. Diabetic nephropathy–a review of the natural history, burden, risk factors and treatment. Journal of the National Medical Association 96, 1445–1454 (2004). [PMC free article] [PubMed] [Google Scholar]
- Thompson J. L. et al. Environmental, policy, and cultural factors related to physical activity in sedentary American Indian women. Women & health 36, 59–74 (2002). [DOI] [PubMed] [Google Scholar]
- Chan K. H. et al. Plasma total bilirubin levels predict amputation events in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 56, 724–736 (2013). [DOI] [PubMed] [Google Scholar]
- Breimer L. H. & Mikhailidis D. P. Is bilirubin a marker of vascular disease and/or cancer and is it a potential therapeutic target? Current pharmaceutical design 17, 3644–3655 (2011). [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Chau L. Y., Chen J. W. & Lin S. J. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes care 31, 1615–1620 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S. et al. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 21, 3450–3457 (2007). [DOI] [PubMed] [Google Scholar]
- Sedlak T. W. et al. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proceedings of the National Academy of Sciences of the United States of America 106, 5171–5176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Frontiers in pharmacology 3, 55 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S. et al. Relationship between bilirubin concentration, coronary endothelial function, and inflammatory stress in overweight patients. Journal of atherosclerosis and thrombosis 18, 403–412 (2011). [DOI] [PubMed] [Google Scholar]
- Mangalat N., Bell C., Graves A. & Imseis E. M. Natural history of conjugated bilirubin trajectory in neonates following parenteral nutrition cessation. BMC pediatrics 14, 298 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm H., Yun J. E., Jo J. & Jee S. H. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke; a journal of cerebral circulation 40, 3422–3427 (2009). [DOI] [PubMed] [Google Scholar]
- Inoguchi T., Sasaki S., Kobayashi K., Takayanagi R. & Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. Jama 298, 1398–1400 (2007). [DOI] [PubMed] [Google Scholar]
- Gazzin S., Vitek L., Watchko J., Shapiro S. M. & Tiribelli C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends in molecular medicine 22, 758–768 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang D., Liu X. & Liu B. Role of total bilirubin level in early diagnosis of diabetic retinopathy. Chin J Diabetes 23, 995–997 (2015). [Google Scholar]
- Wang J. et al. Association between serum bilirubin levels and decline in estimated glomerular filtration rate among patients with type 2 diabetes. J Diabetes Complications 30, 1255–1260 (2016). [DOI] [PubMed] [Google Scholar]
- Sekioka R., Tanaka M., Nishimura T. & Itoh H. Serum total bilirubin concentration is negatively associated with increasing severity of retinopathy in patients with type 2 diabetes mellitus. Journal of Diabetes and Its Complications 29, 218–221 (2015). [DOI] [PubMed] [Google Scholar]
- Kim E. S., Mo E. Y., Moon S. D. & Han J. H. Inverse association between serum bilirubin levels and arterial stiffness in Korean women with type 2 diabetes. PloS one 9, e109251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. S. et al. High Serum Bilirubin Is Associated with the Reduced Risk of Diabetes Mellitus and Diabetic Nephropathy. Tohoku Journal of Experimental Medicine 221, 133–140 (2010). [DOI] [PubMed] [Google Scholar]
- Toya K., Babazono T., Hanai K. & Uchigata Y. Association of serum bilirubin levels with development and progression of albuminuria, and decline in estimated glomerular filtration rate in patients with type 2 diabetes mellitus. Journal of Diabetes Investigation 5, 228–235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P. et al. Correlation between serum bilirubin level and cystatin-c in patients with type 2 diabetic nephropathy. Journal of Xi’an Jiaotong University (Medical Sciences) 92–97 (2016). [Google Scholar]
- Hamamoto S. et al. Low bilirubin levels are an independent risk factor for diabetic retinopathy and nephropathy in Japanese patients with type 2 diabetes. Diabetes & metabolism 41, 429–431 (2015). [DOI] [PubMed] [Google Scholar]
- Chen F., Huang W., Yan H., Ou X. & Li M. Relationship between bilirubin and diabetic retinopathy. Chin J Clinicians (Electronic Edition) 2665–2668 (2015). [Google Scholar]
- Wei W. & Lu Q. The relation of Bilirubin and malondialdehyde with diabetic retinopathy in Type 2 Diabetes Mellitus. Journal Of Inner Mongolia Medical University 448–452 (2015). [Google Scholar]
- Dave A., Kalra P., Gowda B. H. R. & Krishnaswamy M. Association of bilirubin and malondialdehyde levels with retinopathy in type 2 diabetes mellitus. Indian journal of endocrinology and metabolism 19, 373–377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Li X., Zeng Y. & Hu Q. A Correlation Study Serum Bilirubin Level and Early Stage Diabetic Nephropathy. Clinical Misdiagnosis & Mistherapy 28, 78–80 (2015). [Google Scholar]
- Najam S. S. et al. Serum total bilirubin levels and prevalence of diabetic retinopathy in a Chinese population. Journal of diabetes 6, 221–227 (2014). [DOI] [PubMed] [Google Scholar]
- Mashitani T., Hayashino Y., Okamura S., Tsujii S. & Ishii H. Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT 5]). Diabetes care 37, 252–258 (2014). [DOI] [PubMed] [Google Scholar]
- Luo Y. Relationship between serum bilirnbin in patients with type 2 diabetes and diabetes-related chronic complications, Dalian Medical University, (2013). [Google Scholar]
- Lai J. Study On the relationship between urUmry albumin and serum bilirubin in pafienb with type 2 diabetes memtus. Chinese Journal of Postgraduates of Medicine 16–19 (2013). [Google Scholar]
- Jia Y., Xu Y., Yao Z. & Zhao Y. The study on the relationship between the severity of diabetic retinopathy and serum bilirubin. Chinese Journal of Postgraduates of Medicine 33, 9–12 (2010). [Google Scholar]
- Su Z., Yang J. & Zhang L. The relationship between diabetic retinopathy and serum bilirubin level. Journal of Capital Medical University 117–119 (2010). [Google Scholar]
- Yasuda M. et al. High Serum Bilirubin Levels and Diabetic Retinopathy The Hisayama Study. Ophthalmology 118, 1423–1428 (2011). [DOI] [PubMed] [Google Scholar]
- Chung J. O., Cho D. H., Chung D. J. & Chung M. Y. Physiological serum bilirubin concentrations are inversely associated with the prevalence of cardiovascular autonomic neuropathy in patients with Type 2 diabetes. Diabetic medicine: a journal of the British Diabetic Association 31, 185–191 (2014). [DOI] [PubMed] [Google Scholar]
- Leem J. et al. Serum Total Bilirubin Levels Provide Additive Risk Information over the Framingham Risk Score for Identifying Asymptomatic Diabetic Patients at Higher Risk for Coronary Artery Stenosis. Diabetes & metabolism journal 39, 414–423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S., Lee S. W., Mo E. Y., Moon S. D. & Han J. H. Inverse association between serum total bilirubin levels and diabetic peripheral neuropathy in patients with type 2 diabetes. Endocrine 50, 405–412 (2015). [DOI] [PubMed] [Google Scholar]
- Wang R. Association of serum bilirubin with glucose, lipid and chronic complications in type 2 diabetic patients, Medical School of Chinese PLA, (2015). [Google Scholar]
- Choi S. W. et al. Association between total bilirubin and hemoglobin A1c in Korean type 2 diabetic patients. Journal of Korean medical science 27, 1196–1201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto R. et al. Mildly elevated serum total bilirubin levels are negatively associated with carotid atherosclerosis among elderly persons with type 2 diabetes. Clinical and experimental hypertension (New York, N. Y.: 1993) 38, 107–112 (2016). [DOI] [PubMed] [Google Scholar]
- Liu M., Li Y., Li J., Lv X. & He Y. Elevated serum total bilirubin levels are negatively associated with major diabetic complications among Chinese senile diabetic patients. J Diabetes Complications, doi: 10.1016/j.jdiacomp.2016.08.023 (2016). [DOI] [PubMed] [Google Scholar]
- Rodriguez A. J., Nunes Vdos S., Mastronardi C. A., Neeman T. & Paz-Filho G. J. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of controlled cross-sectional studies. J Diabetes Complications 30, 357–367 (2016). [DOI] [PubMed] [Google Scholar]
- Ndisang J. F., Lane N. & Jadhav A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. American journal of physiology. Endocrinology and metabolism 296, E1029–1041 (2009). [DOI] [PubMed] [Google Scholar]
- Tapan S. et al. Soluble CD40 ligand and soluble P-selectin levels in Gilbert’s syndrome: a link to protection against atherosclerosis? Clinical biochemistry 42, 791–795 (2009). [DOI] [PubMed] [Google Scholar]
- Hwang H. J., Lee S. W. & Kim S. H. Relationship between bilirubin and C-reactive protein. Clinical chemistry and laboratory medicine 49, 1823–1828 (2011). [DOI] [PubMed] [Google Scholar]
- Akboga M. K. et al. Association of serum total bilirubin level with severity of coronary atherosclerosis is linked to systemic inflammation. Atherosclerosis 240, 110–114 (2015). [DOI] [PubMed] [Google Scholar]
- Lin J. P., Vitek L. & Schwertner H. A. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clinical chemistry 56, 1535–1543 (2010). [DOI] [PubMed] [Google Scholar]
- Vitek L. & Ostrow J. D. Bilirubin chemistry and metabolism; harmful and protective aspects. Current pharmaceutical design 15, 2869–2883 (2009). [DOI] [PubMed] [Google Scholar]
- Vitek L. & Schwertner H. A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Advances in clinical chemistry 43, 1–57 (2007). [DOI] [PubMed] [Google Scholar]
- Novotny L. & Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Experimental biology and medicine (Maywood, N. J.) 228, 568–571 (2003). [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005). [DOI] [PubMed] [Google Scholar]
- Kumar A., Pant P., Basu S., Rao G. R. & Khanna H. D. Oxidative stress in neonatal hyperbilirubinemia. Journal of tropical pediatrics 53, 69–71 (2007). [DOI] [PubMed] [Google Scholar]
- Alberti K. G. & Zimmet P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine: a journal of the British Diabetic Association 15, 539–553 (1998). [DOI] [PubMed] [Google Scholar]
- Standards of medical care in diabetes–2010. Diabetes care 33 Suppl 1, S11–61 (2010). [DOI] [PMC free article] [PubMed]
- Eknoyan G. et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3, 5–14 (2013). [DOI] [PubMed] [Google Scholar]
- Kuehl M. & Stevens M. J. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nature reviews. Endocrinology 8, 405–416 (2012). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American journal of epidemiology 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American journal of epidemiology 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.