Abstract
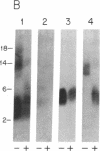
The complete primary structure of Fel dI (International Union of Immunological Societies nomenclature), the major allergen produced by the domestic cat, Felis domesticus, was determined by protein sequence analysis and cDNA cloning. Protein sequencing of Fel dI from an immunoaffinity-purified extract of house dust revealed that the allergen is composed of two polypeptide chains. Degenerate oligonucleotides derived from the protein sequence were used in polymerase chain reaction amplification of cat salivary gland cDNA to demonstrate that the two chains are encoded by different genes. Chain 1 of Fel dI shares amino acid homology with rabbit uteroglobin, while chain 2 is a glycoprotein with N-linked oligosaccharides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. C., Baer H. Allergenically active components of cat allergen extracts. J Immunol. 1981 Sep;127(3):972–975. [PubMed] [Google Scholar]
- Anderson M. C., Baer H., Ohman J. L., Jr A comparative study of the allergens of cat urine, serum, saliva, and pelt. J Allergy Clin Immunol. 1985 Oct;76(4):563–569. doi: 10.1016/0091-6749(85)90776-6. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Dixon J. E. A procedure for in situ alkylation of cystine residues on glass fiber prior to protein microsequence analysis. Anal Biochem. 1987 Mar;161(2):524–528. doi: 10.1016/0003-2697(87)90484-2. [DOI] [PubMed] [Google Scholar]
- Beato M., Baier R. Binding of progesterone to the proteins of the uterine luminal fluid. Identification of uteroglobin as the binding protein. Biochim Biophys Acta. 1975 Jun 12;392(2):346–356. doi: 10.1016/0304-4165(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Oman C. L., Margolies M. N. Use of o-phthalaldehyde to reduce background during automated Edman degradation. Anal Biochem. 1984 Feb;137(1):134–142. doi: 10.1016/0003-2697(84)90359-2. [DOI] [PubMed] [Google Scholar]
- Brown P. R., Leitermann K., Ohman J. L., Jr Distribution of cat allergen 1 in cat tissues and fluids. Int Arch Allergy Appl Immunol. 1984;74(1):67–70. doi: 10.1159/000233518. [DOI] [PubMed] [Google Scholar]
- Bryant D. H., Burns M. W. Skin-prick test reactions to inhalant allergens in asthmatic patients. Med J Aust. 1976 Jun 12;1(24):918–924. [PubMed] [Google Scholar]
- Chapman M. D., Aalberse R. C., Brown M. J., Platts-Mills T. A. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988 Feb 1;140(3):812–818. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Coulson A. F., Lyall A. The significance of protein sequence similarities. Comput Appl Biosci. 1988 Mar;4(1):67–71. doi: 10.1093/bioinformatics/4.1.67. [DOI] [PubMed] [Google Scholar]
- Dabrowski A. J., Van der Brempt X., Soler M., Seguret N., Lucciani P., Charpin D., Vervloet D. Cat skin as an important source of Fel d I allergen. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 1):462–465. doi: 10.1016/s0091-6749(05)80200-3. [DOI] [PubMed] [Google Scholar]
- Duffort O. A., Carreira J., Nitti G., Polo F., Lombardero M. Studies on the biochemical structure of the major cat allergen Felis domesticus I. Mol Immunol. 1991 Apr-May;28(4-5):301–309. doi: 10.1016/0161-5890(91)90141-6. [DOI] [PubMed] [Google Scholar]
- Duffort O., Carreira J., Lombardero M. Characterization of the main IgE-binding components of cat dander. Int Arch Allergy Appl Immunol. 1987;84(4):339–344. doi: 10.1159/000234447. [DOI] [PubMed] [Google Scholar]
- Freidhoff L. R., Meyers D. A., Marsh D. G. A genetic-epidemiologic study of human immune responsiveness to allergens in an industrial population. II. The associations among skin sensitivity, total serum IgE, age, sex, and the reporting of allergies in a stratified random sample. J Allergy Clin Immunol. 1984 Apr;73(4):490–499. doi: 10.1016/0091-6749(84)90360-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Leitermann K., Ohman J. L., Jr Cat allergen 1: Biochemical, antigenic, and allergenic properties. J Allergy Clin Immunol. 1984 Aug;74(2):147–153. doi: 10.1016/0091-6749(84)90278-1. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Luczynska C. M., Li Y., Chapman M. D., Platts-Mills T. A. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990 Feb;141(2):361–367. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- Løwenstein H., Lind P., Weeke B. Identification and clinical significance of allergenic molecules of cat origin. Part of the DAS 76 Study. Allergy. 1985 Aug;40(6):430–441. doi: 10.1111/j.1398-9995.1985.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Facchiano A., Mukherjee A. B. Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature. 1988 Oct 20;335(6192):726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Mukherjee A. B. Uteroglobin: structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr Rev. 1987 Nov;8(4):474–490. doi: 10.1210/edrv-8-4-474. [DOI] [PubMed] [Google Scholar]
- Morel A. M., Delaage M. A. Immunoanalysis of histamine through a novel chemical derivatization. J Allergy Clin Immunol. 1988 Oct;82(4):646–654. doi: 10.1016/0091-6749(88)90978-5. [DOI] [PubMed] [Google Scholar]
- Mornon J. P., Fridlansky F., Bally R., Milgrom E. X-ray crystallographic analysis of a progesterone-binding protein. The C2221 crystal form of oxidized uteroglobin at 2.2 A resolution. J Mol Biol. 1980 Mar 15;137(4):415–429. doi: 10.1016/0022-2836(80)90166-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. C., Agrawal A. K., Manjunath R., Mukherjee A. B. Suppression of epididymal sperm antigenicity in the rabbit by uteroglobin and transglutaminase in vitro. Science. 1983 Feb 25;219(4587):989–991. doi: 10.1126/science.6130601. [DOI] [PubMed] [Google Scholar]
- O'Hehir R. E., Garman R. D., Greenstein J. L., Lamb J. R. The specificity and regulation of T-cell responsiveness to allergens. Annu Rev Immunol. 1991;9:67–95. doi: 10.1146/annurev.iy.09.040191.000435. [DOI] [PubMed] [Google Scholar]
- Ohman J. L., Jr, Findlay S. R., Leitermann K. M. Immunotherapy in cat-induced asthma. Double-blind trial with evaluation of in vivo and in vitro responses. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 1):230–239. doi: 10.1016/0091-6749(84)90251-3. [DOI] [PubMed] [Google Scholar]
- Ohman J. L., Jr, Lowell F. C., Bloch K. J. Allergens of mammalian origin. III. Properties of a major feline allergen. J Immunol. 1974 Dec;113(6):1668–1677. [PubMed] [Google Scholar]
- Ohman J. L., Jr, Lowell F. C. IgE antibody to cat allergens in an allergic population. J Allergy Clin Immunol. 1977 Nov;60(5):317–323. doi: 10.1016/0091-6749(77)90112-9. [DOI] [PubMed] [Google Scholar]
- Pollart S. M., Chapman M. D., Fiocco G. P., Rose G., Platts-Mills T. A. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol. 1989 May;83(5):875–882. doi: 10.1016/0091-6749(89)90100-0. [DOI] [PubMed] [Google Scholar]
- Rafnar T., Griffith I. J., Kuo M. C., Bond J. F., Rogers B. L., Klapper D. G. Cloning of Amb a I (antigen E), the major allergen family of short ragweed pollen. J Biol Chem. 1991 Jan 15;266(2):1229–1236. [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Roux K. H., Dhanarajan P. A strategy for single site PCR amplification of dsDNA: priming digested cloned or genomic DNA from an anchor-modified restriction site and a short internal sequence. Biotechniques. 1990 Jan;8(1):48–57. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsfield J. K., Boyle A. G., Rowell E. M., Moriarty S. C. Pet sensitivities in asthmatic children. Arch Dis Child. 1976 Mar;51(3):186–189. doi: 10.1136/adc.51.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears M. R., Herbison G. P., Holdaway M. D., Hewitt C. J., Flannery E. M., Silva P. A. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989 Jul;19(4):419–424. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Nice E. C. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem Int. 1984 Jun;8(6):787–791. [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Brown W. E., Phillips S., Kennedy A. L., Anthony J., Squeglia N. Amino-acid and cDNA nucleotide sequences of human Clara cell 10 kDa protein. Biochim Biophys Acta. 1988 Sep 7;950(3):329–337. doi: 10.1016/0167-4781(88)90129-7. [DOI] [PubMed] [Google Scholar]
- Sundin B., Lilja G., Graff-Lonnevig V., Hedlin G., Heilborn H., Norrlind K., Pegelow K. O., Løwenstein H. Immunotherapy with partially purified and standardized animal dander extracts. I. Clinical results from a double-blind study on patients with animal dander asthma. J Allergy Clin Immunol. 1986 Mar;77(3):478–487. doi: 10.1016/0091-6749(86)90183-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre T. E., Jr, Marsh D. G., Adkinson N. F., Jr, Fish J. E., Kagey-Sobotka A., Norman P. S., Radden E. B., Jr, Rosenberg G. L. Dose of cat (Felis domesticus) allergen 1 (Fel d 1) that induces asthma. J Allergy Clin Immunol. 1986 Jul;78(1 Pt 1):62–75. doi: 10.1016/0091-6749(86)90116-8. [DOI] [PubMed] [Google Scholar]
- de Groot H., van Swieten P., van Leeuwen J., Lind P., Aalberse R. C. Monoclonal antibodies to the major feline allergen Fel d I. I. Serologic and biologic activity of affinity-purified Fel d I and of Fel d I-depleted extract. J Allergy Clin Immunol. 1988 Nov;82(5 Pt 1):778–786. doi: 10.1016/0091-6749(88)90079-6. [DOI] [PubMed] [Google Scholar]