Significance
Synaptic pruning in primate prefrontal cortical circuitry has been proposed to contribute to working memory maturation. However, pruning of excitatory synapses has only been shown on pyramidal neurons despite the well-recognized role of parvalbumin (PV) interneurons in working memory. Moreover, in schizophrenia, working memory deficits are thought to result from disturbances in the maturation of PV interneurons. Here we demonstrate in the monkey prefrontal cortex that excitatory synapses on PV interneurons are pruned across adolescence, the remaining synapses are strengthened, and splicing of erb-b2 receptor tyrosine kinase 4 (ErbB4) may mediate these effects. These findings provide a developmental context for deficient excitatory synaptic inputs to PV interneurons in schizophrenia and implicate dysregulated ErbB4 splicing as a potential molecular mechanism underlying this process.
Keywords: synaptic pruning, parvalbumin interneuron, ErbB4, alternative splicing, schizophrenia
Abstract
Working memory requires efficient excitatory drive to parvalbumin-positive (PV) interneurons in the primate dorsolateral prefrontal cortex (DLPFC). Developmental pruning eliminates superfluous excitatory inputs, suggesting that working memory maturation during adolescence requires pruning of excitatory inputs to PV interneurons. Therefore, we tested the hypothesis that excitatory synapses on PV interneurons are pruned during adolescence. The density of excitatory synapses, defined by overlapping vesicular glutamate transporter 1-positive (VGlut1+) and postsynaptic density 95-positive (PSD95+) puncta, on PV interneurons was lower in postpubertal relative to prepubertal monkeys. In contrast, puncta levels of VGlut1 and PSD95 proteins were higher in postpubertal monkeys and positively predicted activity-dependent PV levels, suggesting a greater strength of the remaining synapses after pruning. Because excitatory synapse number on PV interneurons is regulated by erb-b2 receptor tyrosine kinase 4 (ErbB4), whose function is influenced by alternative splicing, we tested the hypothesis that pruning of excitatory synapses on PV interneurons is associated with developmental shifts in ErbB4 expression and/or splicing. Pan-ErbB4 expression did not change, whereas the minor-to-major splice variant ratios increased with age. In cell culture, the major, but not the minor, variant increased excitatory synapse number on PV interneurons and displayed greater kinase activity than the minor variant, suggesting that the effect of ErbB4 signaling in PV interneurons is mediated by alternative splicing. Supporting this interpretation, in monkey DLPFC, higher minor-to-major variant ratios predicted lower PSD95+ puncta density on PV interneurons. Together, our findings suggest that ErbB4 splicing may regulate the pruning of excitatory synapses on PV interneurons during adolescence.
In primates, certain complex cognitive processes, such as working memory, depend in part on the proper activation of specific neural circuits in the dorsolateral prefrontal cortex (DLPFC) (1). In both monkeys and humans, recruitment of DLPFC activity and performance during working memory tasks continue to improve through adolescence (2–4). During this period, excitatory synapses and their principal targets, pyramidal neuron dendritic spines, massively decline in number in the primate DLPFC (5–8). Synaptic pruning is thought to eliminate unwanted or imprecise connections and to strengthen the remaining connections (9, 10), suggesting that this process could contribute to the maturation of working memory function during adolescence.
Working memory is thought to emerge from oscillatory activity of DLPFC neurons at gamma frequency (30–80 Hz) (11), which requires the activity of local GABAergic interneurons that express the calcium binding protein parvalbumin (PV) (12, 13). During working memory tasks, excitation from pyramidal neurons recruits PV interneurons, which in turn provide phasic inhibition that synchronizes the firing of pyramidal neurons at gamma frequency (14). Due to the narrow time window of PV interneuron recruitment during gamma oscillations, PV interneurons require efficient detection of glutamatergic inputs with high precision (15, 16). Therefore, pruning of unnecessary synaptic connections and strengthening of the remaining excitatory inputs to PV interneurons during development could play a crucial role in improving PV interneuron recruitment during gamma oscillations and consequently in the maturation of working memory function. However, evidence for pruning of excitatory synapses on PV interneurons in the DLPFC, and the molecular mechanisms that could regulate cell type-specific pruning, have not been reported.
Excitatory synapses on PV interneurons are modulated in part by erb-b2 receptor tyrosine kinase 4 (ErbB4) (17, 18). Activation of ErbB4 increases the number of excitatory synapses in a kinase-dependent manner (19) and the loss of ErbB4 results in fewer excitatory synapses on PV interneurons (20). Moreover, ErbB4 transcripts are alternatively spliced at two loci, generating four different splice variants with distinct downstream signaling pathways (21). Although their functional consequences are not known in PV interneurons, ErbB4 splice variants display different levels of tyrosine kinase activity and exert different physiological effects in nonneuronal cells (22–24), suggesting that the effect of ErbB4 signaling could be modulated by alternative splicing. Thus, shifts in the expression level and/or splicing of ErbB4 in PV interneurons during adolescence might function as a developmental switch regulating the pruning of excitatory synapses on these neurons.
In this study, we tested the hypotheses that (i) excitatory synapses on PV interneurons are pruned during postnatal development in monkey DLPFC and (ii) this pruning process is attributable to shifts in the expression levels and/or splicing of ErbB4 transcripts. We found that excitatory synapses on PV interneurons undergo pruning during adolescence in monkey DLPFC. Moreover, total ErbB4 expression did not change but the alternative splicing of ErbB4 transcripts shifted from the major to minor variants with age. Overexpression of ErbB4 major variant increased the number of excitatory synapses on PV neurons, whereas the minor variant had no effect. Furthermore, the major ErbB4 variant displayed greater kinase activity than the minor variant in response to neuregulin 1 stimulation. Finally, higher ratio of minor-to-major splice variants predicted lower density of excitatory synapses on PV interneurons across all monkeys. Thus, our findings indicate that shifts in ErbB4 splicing in PV interneurons modulate the pruning of excitatory synapses on these neurons during postnatal development.
Methods
Animals and Tissue Preparation.
We studied 13 female rhesus monkeys (Macaca mulatta) of two age groups: postnatal 3–9 mo (prepubertal group; n = 7) and 42–46 mo (postpubertal group; n = 6); these age groups capture the plateau phase and the declining phase, respectively, of excitatory synapse density in the primate DLPFC (5). Housing and experimental procedures were conducted in accordance with guidelines set by the US Department of Agriculture and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee. Animals were perfused transcardially with ice-cold artificial cerebrospinal fluid following ketamine- and pentobarbital-induced anesthesia. The brain was removed and the right hemisphere was blocked coronally, frozen in isopentane on dry ice, and stored at −80 °C. The left hemisphere was blocked coronally, immersed in phosphate-buffered 4% (wt/vol) paraformaldehyde at 4 °C for 48 h, washed in sucrose solutions at 4 °C, and stored at −30 °C in a cryoprotectant solution containing glycerin and ethylene glycol (25).
Layer-Specific RNA Extraction and Quantitative PCR.
Cryostat sections (12 µm) containing right DLPFC area 46 were mounted on glass polyethylene naphthalate membrane slides (Leica Microsystems), fixed in ethanol/sodium acetate, stained in 0.5% thionin, and dehydrated with ethanol. The boundaries of layer 4 were determined on the basis of the size and packing density of stained neurons (26). A Leica microdissection system (LMD 6500, Leica Microsystems) was used to collect ∼3 × 106 µm2 of tissue from layer 4 of each monkey. The collected tissue samples were lysed by vortexing for 30 s in 200 μL of RLTplus buffer (Qiagen). RNA was extracted using the RNeasy Plus Micro Kit (Qiagen), and cDNA was synthesized using the qScript cDNA SuperMix (Quanta Bioscience). The quantitative PCR (qPCR) reactions were performed in quadruplicate using an ABI StepOnePlus Real-Time PCR System (Applied Biosystems) with previously described primer sets for PV, four ErbB4 splicing variants (JM-a, JM-b, CYT-1, and CYT-2), and pan-ErbB4 (26). Beta-actin and cyclophilin A were used as reference genes to normalize the expression levels of transcripts, as these housekeeping genes have stable levels of expression across postnatal development in monkey DLPFC (27). The delta cycle thresholds (dCTs) were calculated for each sample by using the geometric mean of the two endogenous reference genes as the normalizing factor. Then the expression level for each transcript was calculated as the expression ratio value (expression ratio = 2−dCTs).
Fluorescent Immunohistochemistry.
Cryostat sections (40 µm) containing left DLPFC area 46 were pretreated for antigen retrieval (0.01 M sodium citrate for 75 min at 80 °C) and then incubated for 72 h at 4 °C in the following primary antibodies: PV (mouse, 1:1,000; Swant), postsynaptic density 95 (PSD95; rabbit, 1:250; Cell Signaling), and vesicular glutamate transporter 1 (VGlut1; guinea pig, 1:250; Millipore). Tissue sections were washed three times in PBS and then incubated for 24 h at 4 °C with secondary antibodies (donkey) conjugated to Alexa 488 (anti-mouse, 1:500; Invitrogen), 568 (anti-rabbit, 1:500; Invitrogen), or 647 (anti-guinea pig, 1:500; Millipore). After washing three times in PBS, sections were mounted in Prolong Gold Antifade reagent (Life Technologies) and stored at 4 °C until imaging. The specificity of these antibodies has been described previously (28).
ErbB4 Plasmid Cloning.
Full-length cDNAs encoding ErbB4 JM-a/CYT-1 variant (29) (a gift from Lin Mei, Georgia Regents University, Augusta, GA) or JM-b/CYT-1 variant (30) (a gift from Gabriel Corfas, University of Michigan, Ann Arbor, MI) were amplified using a primer pair ErbB4_Exon1_Bgl II_F (ACGTAGATCTATGAAGCCGGCGACAGGACTTTGG) and ErbB4_Exon28_Sal I_R (ACGTGTCGACTTACACCACAGTATTCCGGTGTCTG). The amplified products were digested with BglII and SalI and ligated with T4 ligase (Invitrogen) into Pires2-DsRed-Express2 vector (Clontech). Pires2 vector contains an internal ribosome entry site (IRES) between cloning sites and fluorescent protein coding region, which allows both the gene of interest and the fluorescent protein to be translated from a single mRNA in mammalian cells (31). To generate cDNA encoding the JM-b/CYT-2 variant, the DNA sequence corresponding to exon 26 of JM-b/CYT-1 was deleted using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies) with a primer pair Del172-219_F (ATCTCGGTATACAAACTGGTTCCTATTCGAGTCAATTCTTGC) and Del172-219_R (GCAAGAATTGACTCGAATAGGAACCAGTTTGTATACCGAGAT). The DNA sequence of all constructs was verified by sequencing.
Dissociated Neuronal Culture and Immunocytochemistry.
Dissociated rat cortical neurons were prepared from postnatal day 1 Long–Evans rats (Charles River Laboratories). In 24-well plates, cortical neurons were placed at 1 × 105 cells per well on acid-washed coverslips coated with poly-d-lysine (20 µg/mL) and laminin (3.4 µg/mL). Cortical neurons were maintained in neurobasal medium supplemented with B27 (all from Invitrogen), penicillin/streptomycin (100 units/mL and 100 mg/mL, respectively), and 2 mM glutamine. One half of the media in each well was replaced every 4 d. At 7 d in vitro (DIV) cortical neurons were transfected with 1 µg of DNA plasmid per well using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Neurons at 21 DIV were fixed for 8 min at room temperature with 4% (wt/vol) paraformaldehyde/4% (wt/vol) sucrose in PBS. Fixed neurons were washed with PBS and then incubated for 24 h at 4 °C in 1× GDB [15 mM phosphate buffer (pH 7.4) containing 0.1% gelatin, 0.3% Triton X-100, and 0.25M NaCl] (32) with the following primary antibodies: PV (goat, 1:100; Swant), DsRed (rabbit, 1:500; Abcam), PSD95 (mouse, 1:250; Synaptic Systems), and VGlut1 (guinea pig, 1:2,000; Synaptic Systems). Neurons were washed three times in PBS and then incubated for 2 h with secondary antibodies (donkey) conjugated to Alexa 405 (anti-goat, 1:500; Abcam), 488 (anti-mouse, 1:500; Invitrogen), 568 (anti-rabbit, 1:500; Invitrogen), and 647 (anti-guinea pig, 1:500; Millipore). Coverslips were mounted using Fluoromount-G (Southern Biotech). All antibodies have been shown to specifically recognize the targeted protein as reported by the manufacturer.
Image Acquisition.
Images containing monkey sections were acquired on an Olympus IX81 microscope with a spinning disk confocal unit and a Hamamatsu EM-CCD digital camera at 60× magnification. Ten image stacks (512 × 512 pixel; 0.25 µm z step) in layer 4, defined as 50–60% of the pia-to-white matter distance, per each section were selected using a previously published method for systematic random sampling (33). The mean numbers of PV-immunoreactive neurons sampled in the same volume of tissue did not differ between age groups (t11 = 0.06, P = 0.952). Images containing primary neurons were acquired on a Nikon A1R laser scanning confocal microscope at 60× magnification. In each experiment, all image stacks (1,024 × 1,024 pixels; 0.5 µm z step) were acquired with identical settings for laser power, detector gain, and amplifier offset, with pinhole diameters set for 1.2 airy units. Images were rendered using a maximal intensity projection algorithm. Approximately 10% of cultured neurons were positive for PV staining (Fig. S1) and in each well one to two PV-positive (PV+) neurons were DsRed-positive (DsRed+), presumably reflecting low transfection efficiency. Ten to 12 DsRed+/PV+ neurons were obtained for each condition. The final data comprised four independent sets of experiment.
Fig. S1.
Cultured cortical primary neurons labeled with antibodies against PV (red) and NeuN (blue). Arrows indicate cells positive for PV and NeuN immunoreactivities. Approximately 10% of neurons were positive for PV labeling. (Scale bar, 20 µm.)
Postimage Processing.
For images containing monkey sections, each fluorescent channel was deconvolved using the Autoquant’s Blind Deconvolution algorithm to improve image contrast by reducing out-of-focus fluorescence. Pre- and postsynaptic elements of excitatory synapses were defined as VGlut1+ and PSD95+ puncta, respectively. Masking of VGlut1+ and PSD95+ puncta and PV cell body was performed using the previously described method (SI Methods) (28). Excitatory synapses on PV interneurons were defined by the overlap of VGlut1+ and PSD95+ (VGlut1+/PSD95+) puncta within PV cell bodies (Fig. 1). The validity of this approach was confirmed by comparing prior data obtained using the same approach (28) with the results from a previous electron microscopy (EM) study (34). For images containing primary neurons, the VGlut1+ and PSD95+ puncta were masked using the binary threshold function in NIS-Elements AR software (Nikon). The proximal dendrites were defined as neurites containing PSD95+ puncta within 30 µm from the PV+/DsRed+ cell body as previously characterized (19). The cell body and one randomly selected proximal dendrite from each sampled PV+/DsRed+ neuron were manually masked.
Fig. 1.
Sampling of excitatory synapses on PV interneurons in monkey DLPFC layer 4. (A) Representative deconvolved image of monkey DLPFC layer 4 labeled with antibodies against PV (gray), VGlut1 (green), and PSD95 (red). (Scale bar, 10 μm.) (B) Single (white) and combined (color) mask images of PV cell body, VGlut1+ puncta, and PSD95+ puncta from the boxed area in A. Combined mask image displays only overlapping VGlut1+ and PSD95+ puncta (yellow). Excitatory synapses on PV cell bodies were defined by the VGlut1+/PSD95+ puncta within a PV cell body, as indicated by yellow arrowheads. Overlapping VGlut1+/PSD95+, where only the PSD95+ puncta or the VGlut1+ puncta were located within a PV cell body, were not counted as excitatory synapses. (Scale bar, 5 μm.)
Neuregulin 1 Stimulation Assay and Western Blotting.
Human embryonic kidney 293 (HEK-293) cells were maintained in six-well plates in DMEM supplemented with 10% (vol/vol) FBS and 1% penicillin/streptomycin solution (all from Life Technologies) and transfected with 2 µg of DNA plasmid per well using the Lipofectamine 2000 kit (Invitrogen) according to the manufacturer’s instructions. Six hours after transfection, culture medium was replaced by DMEM without FBS. Cells were starved for 18 h, stimulated with 50 ng/mL neuregulin 1-beta 1 EGF domain protein (R&D Systems) for 10 min at 37 °C (24), and lysed in RIPA buffer supplemented with Halt Protease and Phosphatase Inhibitor Mixture (all from Thermo Scientific). Phosphorylation levels of ErbB4 splice variants were assessed by Western blotting with a phospho-specific ErbB4 antibody (rabbit, 1:1,000; Cell Signaling). A beta-tubulin antibody (mouse, 1:50,000; Millipore) was used as a loading control. The membranes were stripped with Restore Fluorescent Western Blot Stripping buffer (Thermo Scientific) and then reblotted with a ErbB4 antibody (rabbit, 1:1,000; Abcam). The specificity of phospho-specific and total ErbB4 antibodies has been validated for Western blotting assay by previous studies (35, 36).
Statistics.
Student’s t tests were performed to compare the dependent measures between age groups. Pearson’s correlation analyses were performed to assess the association between the ErbB4 splice variant ratios and the density of VGlut1+, PSD95+, or VGlut1+/PSD95+ puncta on PV cell bodies across all animals. One-way analysis of variance (ANOVA) was used to assess the main effect of Pires2 constructs on the dependent measures in primary neuronal culture or in HEK-293 cells.
SI Methods
In Situ Hybridization.
Cryostat sections (20 µm) containing right DLPFC area 46 were mounted onto glass slides from a subset of monkeys (five prepubertal and three postpubertal) due to limitations in tissue availability. Sections were fixed with 4% (wt/vol) paraformaldehyde in PBS and then acetylated with 0.25% acetic anhydrate in 0.1 M triethanolamine/0.9% NaCl for 10 min, dehydrated through a graded ethanol series, and defatted in chloroform for 10 min. The sections were then hybridized with 35S-labeled riboprobes (2 × 107 dpm/mL) targeting bases 59–403 of PV mRNA in hybridization buffer containing 50% (vol/vol) formamide, 0.75 M NaCl, 20 mM 1,4-piperazine diethane sulfonic acid, pH 6.8, 10 mM EDTA, 10% (wt/vol) dextran sulfate, 5× Denhardt's solution (0.2 mg/mL Ficoll, 0.2 mg/mL polyvinylpyrrolidone, 0.2 mg/mL BSA), 50 mM DTT, 0.2% SDS, and 100 μg/mL yeast tRNA at 56 °C for 16 h. The sections were then washed in a solution containing 0.3 M NaCl, 20 mM Tris⋅HCl, pH 8.0, 1 mM EDTA, pH 8.0, and 50% (vol/vol) formamide at 63 °C, treated with RNase A (20 μg/mL) at 37 °C, and washed in 0.1× SSC (150 mM NaCl, 15 mM sodium citrate) at 67 °C. Finally, sections were dehydrated through a graded ethanol series, air dried, and exposed to BioMax MR film (Kodak) for 72 h. Film optical density (OD) was quantified as nanocuries per gram of tissue by reference to radioactive C-14 standards. PV mRNA expression as a function of cortical layer was quantified in ∼1-mm-wide cortical traverses extending from the pial surface to the white matter. Each traverse was divided into 50 equal bins parallel to the pial surface and the OD was determined for each bin.
Masking of VGlut1+ and PSD95+ Puncta and PV Cell Body.
For the purpose of masking synaptic structures, a custom channel was made for each deconvolved channel of VGlut1 or PSD95 by calculating a difference of Gaussians using sigma values of 0.7 and 2. The Ridler–Calvard threshold value was applied to the fluorescence intensity histogram of either VGlut1+ or PSD95+ labeling and all pixels were reassigned to a binary value according to whether they were above or below the threshold value, and the resulting binary image was referred to as the object mask. These object masks were automatically defined as VGlut1+ or PSD95+ masks if they fell in the range of defined puncta size (0.06–0.7 µm3). Multiple iteration of binary masking process was then performed with the threshold level incrementally increasing for each iteration of masking until it reached the highest intensity value. The resulting object masks from each iteration were combined to represent the total population of VGlut1+ or PSD95+ puncta. Edges of PV cell bodies were segmented by the MATLAB edge function using the Canny edge detector operator. The edges of segmented objects were closed, filled, and size gated (>80 µm3) to limit the boundaries of PV cell bodies. All PV cell body masks were manually cleaned for final analyses.
Results
Developmental Pruning of Excitatory Synapses on PV Interneurons in Layer 4 of Monkey DLPFC.
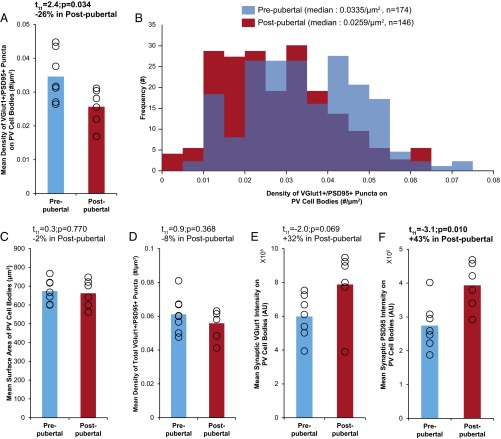
PV neurons were sampled in DLPFC layer 4 as this cortical layer has been shown to contain the highest density of PV neurons in monkey DLPFC (37). The mean (± SD) density of VGlut1+/PSD95+ puncta on PV cell bodies was significantly (t11 = 2.4, P = 0.034) 26% lower in the postpubertal (0.026 ± 0.005 per µm2) relative to the prepubertal group (0.035 ± 0.007 per µm2; Fig. 2A), indicating that excitatory synapses on PV interneurons are pruned during adolescence. Consistent with the mean group difference, the frequency distribution of VGlut1+/PSD95+ puncta density on individual PV cell bodies was shifted to the left in postpubertal relative to prepubertal animals (Fig. 2B). The mean surface area of PV cell bodies did not differ between age groups (prepubertal: 674 ± 64 µm2; postpubertal: 662 ± 73 µm2; t11 = 0.3, P = 0.770; Fig. 2C), demonstrating that the lower density of excitatory synapses on PV neurons in the postpubertal animals is not due to a larger surface area of PV cell bodies. Moreover, the density of total VGlut1+/PSD95+ puncta in DLPFC layer 4 did not differ between age groups (prepubertal: 0.061 ± 0.011 per µm3; postpubertal: 0.056 ± 0.008 per µm3; t11 = 0.9, P = 0.368; Fig. 2D), suggesting that the pruning of excitatory synapses during adolescence is more pronounced on PV interneurons than on other neural elements in DLPFC layer 4. Finally, the mean synaptic levels [i.e., relative fluorescence intensities, reported in arbitrary units (AU), within VGlut1+/PSD95+ puncta on PV cell bodies] of VGlut1 (prepubertal: 6.0 × 105 ± 1.3 × 105 AU, postpubertal: 7.9 × 105 ± 2.1 × 105 AU; t11 = −2.0, P = 0.069; Fig. 2E) and PSD95 (prepubertal: 2.7 × 105 ± 0.7 × 105 AU, postpubertal: 3.9 × 105 ± 0.7 × 105 AU; t11 = −3.1, P = 0.010; Fig. 2F) proteins were 32% and 43% higher, respectively, in the postpubertal group. These findings suggest that the maturation of excitatory synaptic inputs to cortical PV interneurons during adolescence involves the pruning of a subset of synapses and higher levels of VGlut1 and PSD95 proteins in the remaining synapses.
Fig. 2.
Developmental pruning of excitatory synapses on PV interneurons through adolescence in layer 4 of monkey DLPFC. (A and C–F) Group mean (bar) and individual monkey (open circles) levels of dependent measures, as indicated by the y axis labels, in DLPFC layer 4 of monkeys in the prepubertal group (blue) or postpubertal group (red). Statistics from Student’s t test for each dependent measure are shown above the corresponding graph. (A) Mean density of VGlut1+/PSD95+ puncta on PV cell bodies was significantly lower in the postpubertal group. (B) The frequency distributions of VGlut1+/PSD95+ puncta density on PV cell bodies sampled from prepubertal monkeys (blue) and postpubertal monkeys (red). Each bin represents 0.05 puncta per square micrometer. (C and D) The mean surface area of PV cell bodies (C) and the density of total VGlut1+/PSD95+ puncta in DLPFC layer 4 (D) did not differ between age groups. (E and F) Mean VGlut1 (E) and PSD95 (F) levels within VGlut1+/PSD95+ puncta on PV cell bodies were higher in the postpubertal group. AU, arbitrary units of relative fluorescence intensity.
Association Between Maturation of Excitatory Synapses on PV Interneurons and PV Levels.
As PV expression depends on neuronal activity (20, 38, 39), we next assessed changes in PV immunoreactivity between age groups. Mean PV protein levels per neuron were significantly (t11 = −3.5, P = 0.005) 73% higher in the postpubertal (4.2 × 108 ± 0.7 × 108 AU) relative to the prepubertal group (2.4 × 108 ± 1.0 × 108 AU; Fig. 3A). Moreover, mean PV protein levels per neuron were negatively correlated with the mean density of VGlut1+/PSD95+ puncta on PV cell bodies (R = −0.620, P = 0.024; Fig. 3B) and positively correlated with the mean synaptic VGlut1 (R = 0.603, P = 0.029; Fig. 3C) and PSD95 levels (R = 0.720, P = 0.006; Fig. 3D) across all animals. These data suggest that although a subset of excitatory synaptic inputs to PV interneurons is pruned during adolescence, the increase in VGlut1 and PSD95 levels in the remaining excitatory synapses contribute to the developmental up-regulation of activity-dependent PV expression.
Fig. 3.
Lower density of excitatory synapses on PV interneurons and higher synaptic levels of VGlut1 and PSD95 proteins predict higher PV levels across all animals. (A) Group mean (bar) and individual monkey (open circles) levels of PV immunoreactivity in PV cell bodies for each age group. Mean PV immunoreactivity levels in PV cell bodies were significantly higher in the postpubertal group relative to the prepubertal group. Statistic from Student’s t test is shown above the graph. (B–D) Correlation graphs plotting the mean PV intensity in PV cell bodies on the y axis and the mean density of VGlut1+/PSD95+ puncta on PV cell bodies (B), mean synaptic VGlut1 intensity (C), or mean synaptic PSD95 intensity (D) on the x axis. Trendlines represent significant regression line across all animals (n = 13). AU, arbitrary units of relative fluorescence intensity.
Developmental Shifts in Alternative Splicing of ErbB4 Transcripts in Layer 4 of Monkey DLPFC.
Next, we assessed differences in the expression level and alternative splicing of ErbB4 transcripts in PV interneurons between age groups. In the primate DLPFC, most ErbB4-positive neurons contain either PV or the calcium-binding protein calretinin (CR) (40). Microdissection of DLPFC layer 4 yields samples highly enriched in PV relative to CR mRNA, which allows an assessment of ErbB4 expression predominantly in PV interneurons (26). Moreover, an in situ hybridization assay (SI Methods) demonstrated that layer 4 had the highest PV mRNA levels of any layer in the DLPFC and the greatest laminar difference in PV expression between age groups (+46% in postpubertal; t6 = −2.6, P = 0.042; Fig. 4A). Thus, we microdissected DLPFC layer 4 (Fig. 4B) and quantified mRNA levels of PV and pan-ErbB4 by qPCR. Consistent with the immunohistochemistry (Fig. 3A) and the in situ hybridization data (Fig. 4A), PV mRNA levels in layer 4 were significantly (t11 = −3.4, P = 0.006) 57% higher in the postpubertal (0.071 ± 0.015) relative to the prepubertal group (0.045 ± 0.012; Fig. 4C). In contrast, pan-ErbB4 mRNA levels in layer 4 did not differ between age groups (prepubertal: 0.032 ± 0.011; postpubertal: 0.038 ± 0.014; t11 = −1.7, P = 0.362; Fig. 4D).
Fig. 4.
Developmental shifts in alternative splicing of ErbB4 transcripts through adolescence in layer 4 of monkey DLPFC. (A) Mean PV mRNA optical density as a function of cortical layer in monkey DLPFC for prepubertal (blue) and postpubertal (red) groups. (B) Representative image of a thionin-stained coronal section before and after microdissection of layer 4. Layer 4 was identified based on the size and packing density of stained cells. (Scale bar, 100 µm.) (C, D, F, and G) Group mean (bar) and individual monkey (open circles) levels of dependent measures, as indicated by the y axis labels, in DLPFC layer 4. Statistics from Student’s t test for each dependent measure are shown above the corresponding graph. (C) Mean PV mRNA levels were significantly higher in the postpubertal group, (D) whereas mean pan-ErbB4 levels did not differ. (E) Schematic diagram depicting the alternative splicing loci of ErbB4. (F and G) The JM-a:JM-b variant ratio (F) and the CYT-1:CYT-2 variant ratio (G) were significantly higher in the postpubertal group.
We then assessed shifts in ErbB4 splicing between age groups in layer 4. ErbB4 transcripts are alternatively spliced at two loci (Fig. 4E) (21). Splicing at the juxtamembrane (JM) locus produces the minor JM-a variant and the major JM-b variant based on the inclusion of exon 16 or 15b, respectively. Inclusion or exclusion of exon 26 at the cytoplasmic (CYT) locus yields the minor CYT-1 variant and the major CYT-2 variant, respectively. To investigate shifts in splicing, we assessed the ratio of minor-to-major splicing variant levels at each locus (i.e., JM-a:JM-b ratio and CYT-1:CYT-2 ratio). The JM-a:JM-b ratio (prepubertal: 0.073 ± 0.020; postpubertal: 0.117 ± 0.048; t11 = −3.0, P = 0.013; Fig. 4F) and the CYT-1:CYT-2 ratio (prepubertal: 0.253 ± 0.090; postpubertal: 0.446 ± 0.168; t11 = −3.5, P = 0.005; Fig. 4G) were significantly 62% and 76% higher, respectively, in the postpubertal group, demonstrating a developmental shift in ErbB4 splicing from the major JM-b/CYT-2 to minor JM-a/CYT-1 variants during adolescence.
Differential Regulation of Excitatory Synapse Number on PV Interneurons by ErbB4 Splice Variants.
To investigate whether ErbB4 major JM-b/CYT-2 and minor JM-a/CYT-1 splice variants differentially regulate the number of excitatory synapses on PV interneurons, we transfected constructs expressing either ErbB4 JM-a/CYT-1 or JM-b/CYT-2 in rat primary neurons (Fig. 5A). Each construct bicistronically translates an ErbB4 splice variant and DsRed from a single mRNA, so that the intensity of DsRed reflects the levels of the transfected ErbB4 variant. The DsRed levels did not differ in PV neurons transfected with each construct (cell body: F2,145 = 1.4, P = 0.261; proximal dendrites: F2,145 = 1.3, P = 0.267; Fig. 5B), suggesting that the expression levels of these inserts are comparable in PV neurons. The surface areas of cell bodies and proximal dendrites did not differ in PV+/DsRed+ neurons transfected with each construct (cell body: F2,145 = 2.9, P = 0.058; proximal dendrites: F2,145 = 0.3, P = 0.737; Fig. 5C). Overexpression of the JM-b/CYT-2 variant increased the density of VGlut+/PSD95+ puncta on the cell bodies and proximal dendrites of PV neurons relative to DsRed controls, whereas JM-a/CYT-1 overexpression had no discernible effect (cell body: F2,145 = 4.3, P = 0.016; proximal dendrites: F2,145 = 5.6, P = 0.004; Fig. 5D). These findings suggest that the JM-b/CYT-2 variant primarily drives the ErbB4 signaling effect on the number of excitatory inputs to PV interneurons, whereas the JM-a/CYT-1 variant does not contribute to this effect.
Fig. 5.
Differential regulation of excitatory synapse number on PV interneurons by ErbB4 splice variants in rat primary neuronal culture. (A) Representative images of cultured cortical neurons transfected with either JM-b/CYT-2 variant or JM-a/CYT-1 variant and labeled with antibodies against PV (blue), DsRed (gray), VGlut1 (green), and PSD95 (red). Transfected neurons are labeled with bicistronic DsRed expression. (Scale bar, 5 µm.) Below are the masked images of VGlut1+ (green) and PSD95+ (red) puncta within PV dendrites (outlined with dotted lines) from the boxed area. (B–D) Bar graphs showing the levels of dependent measures, as indicated by the y axis labels, in the cell bodies (Left) and the proximal dendrites (Right) of PV+/DsRed+ neurons overexpressing DsRed only (green bar), JM-b/CYT-2 (blue bar), or JM-a/CYT-1 (red bar). Data shown are mean percentage (+SE of mean) from four individual experiments normalized to DsRed only (DsRed only: n = 46, JM-b/CYT-2: n = 55, JM-a/CYT-1: n = 47). One-way ANOVA statistics for each dependent measure are shown above the corresponding graph. *P < 0.05 from Tukey’s post hoc test.
Differential Kinase Activity of ErbB4 Splice Variants in Response to Neuregulin 1 Stimulation.
ErbB4 kinase activity is necessary for excitatory synapse formation (19, 29). To investigate whether the differential effect of ErbB4 splice variants reflects differences in their kinase activity, we expressed ErbB4 JM-a/CYT-1 or JM-b/CYT-2 variant in HEK-293 cells and assessed phosphorylation levels of ErbB4 in response to neuregulin 1 stimulation. Expression of ErbB4 splice variants yielded bands corresponding to glycosylated mature ErbB4 protein (∼180 kDa), partially glycosylated intermediate ErbB4 protein (∼160 kDa), and the cleaved intracellular domain of ErbB4 (∼80 kDa), which is the product of the cleavage site localized selectively in the JM-a splice domain (41, 42) (Fig. 6A). The level of autophosphorylation did not differ between the JM-a/CYT-1 and JM-b/CYT-2 variants. However, neuregulin 1 stimulation produced a significantly greater increase in phosphorylation levels of the JM-b/CYT-2 variant than the JM-a/CYT-1 variant, selectively for the band corresponding to the mature ErbB4 protein (180 kDa: F3,16 = 17.2, P < 0.0001; 160 kDa: F3,16 = 2.8, P = 0.073; 80 kDa: t8 = 1.8, P = 0.106; Fig. 6 B–D). This differential kinase activity of the mature ErbB4 JM-a/CYT-1 and JM-b/CYT-2 variants suggests that shifts in alternative splicing could be a key determinant of ErbB4 signaling activity and consequently of the number of excitatory inputs to PV interneurons.
Fig. 6.
Differential kinase activity of ErbB4 splice variants in response to neuregulin 1 stimulation in HEK-293 cells. (A) Representative image of Western blot for phospho-ErbB4 and total ErbB4 from HEK-293 cells transfected with either the JM-a/CYT-1 or JM-b/CYT-2 variant and treated with neuregulin 1. (B–D) Bar graphs showing the ratio of phospho-ErbB4 to total ErbB4 band intensities for JM-a/CYT-1 and JM-b/CYT-2 variants with (green) or without (yellow) neuregulin 1 treatment. (B) The glycosylated mature protein (∼180 kDa) of the JM-b/CYT-2 variant displayed a significantly greater increase in phosphorylation levels than that of the JM-a/CYT-1 variant in response to neuregulin 1 stimulation. (C and D) Phosphorylation levels of the intermediate proteins or the cleaved intracellular domains did not change with neuregulin 1 stimulation. Data shown are mean ratio (+SD) from five individual experiments. One-way ANOVA statistics or Student’s t test statistics are shown above the corresponding graph. *P < 0.05 and **P < 0.001 from Tukey’s post hoc test.
Association Between ErbB4 Splicing Shifts and Pruning of Excitatory Synapses on PV Interneurons in Monkey DLPFC.
Finally, to investigate whether the effect of ErbB4 splicing shifts on the number of excitatory inputs to PV neurons is reflected during pruning in primate DLPFC, we assessed the relationships between ErbB4 splicing shifts and the density of excitatory synapses on PV interneurons across all monkeys. Previous studies have demonstrated that ErbB4 signaling regulates the formation of excitatory synapses on PV interneurons primarily by its effect on synaptic recruitment of PSD95 (19, 29, 43). Consistent with these findings, the JM-a:JM-b (R = −0.612, P = 0.026) and CYT-1:CYT-2 (R = −0.642, P = 0.018) ratios were both significantly negatively correlated with the mean density of PSD95+ puncta on PV cell bodies across all animals.
Discussion
In this study, we tested the hypotheses that excitatory synapses on cortical PV interneurons are pruned during adolescence and this pruning process is attributable to shifts in ErbB4 expression and/or splicing. In layer 4 of monkey DLPFC, the density of VGlut1+/PSD95+ puncta on PV neurons was lower in postpubertal relative to prepubertal monkeys, demonstrating that excitatory synapses on cortical PV interneurons undergo pruning through adolescence. Moreover, although pan-ErbB4 mRNA levels did not differ between age groups, the JM-a:JM-b and CYT-1:CYT-2 variant ratios were higher in postpubertal monkeys. Furthermore, these ratios were negatively correlated with the density of PSD95+ puncta on PV neurons across all animals; these findings suggest that pruning of excitatory synapses on cortical PV interneurons might be regulated by developmental shifts in ErbB4 splicing. This interpretation was supported by findings that overexpression of the JM-b/CYT-2 variant increased the density of VGlut1+/PSD95+ puncta on the proximal dendrites and the cell bodies of PV neurons, whereas overexpression of the JM-a/CYT-1 variant had no effects.
Our findings indicate that PV protein levels increase in association with the pruning of excitatory synapses on PV interneurons in primate DLPFC. These results might seem contradictory with prior findings that (i) PV expression is activity dependent (20, 38, 39), and (ii) the density of excitatory synapses on PV neurons positively predicts PV immunoreactivity levels in mouse hippocampus (44) and human DLPFC (28). However, we also found that the excitatory synapses remaining after pruning have higher apparent levels of VGlut1 and PSD95 proteins. Higher levels of VGlut1 and PSD95 have been shown to increase the amplitude of excitatory postsynaptic currents and to correlate with the maturation of excitatory synapses (45, 46). Consequently, the excitatory synapses on PV interneurons that remain after pruning likely represent mature synaptic connections with stronger excitatory neurotransmission. Therefore, our findings suggest that the maturation of excitatory synaptic inputs to PV interneurons during adolescence involves a reduction in the total number of synapses and a strengthening of the remaining synapses. More refined and mature glutamatergic inputs to PV interneurons could support more precise excitation of these neurons during gamma oscillations and consequently contribute to the improvement of working memory function during adolescence.
Our results demonstrate that the transcription of ErbB4 does not change with age, but that shifts in ErbB4 splicing from the JM-b/CYT-2 to JM-a/CYT-1 variants occur in PV interneurons during adolescence in primate DLPFC. Activation of the ErbB4 signaling pathway by its ligand neuregulin 1 has been associated with multiple aspects of neuronal development (47), including the positive modulation of excitatory synapse number on PV interneurons (17, 18). Similar to other genes that are involved in neurodevelopment (48), the functional consequence of ErbB4 signaling in PV neurons could be modulated by alternative splicing. Our study demonstrates that the JM-b/CYT-2 variant displays greater kinase activity than the JM-a/CYT-1 variant in response to neuregulin 1 stimulation, suggesting that the efficacy of ErbB4 signaling could be regulated by alternative splicing. Consistent with the differential kinase activity of ErbB4 splice variants, the JM-b/CYT-2 variant increased the number of excitatory synapses on PV interneurons, whereas the JM-a/CYT-1 variant had no effects. These results support the hypothesis that shifts in ErbB4 splicing from the JM-b/CYT-2 variant to the JM-a/CYT-1 variant result in lower ErbB4 signaling and consequently in a lower number of excitatory synapses on PV interneurons. This relationship is reflected in the development of excitatory synaptic inputs to PV neurons in primate DLPFC, which revealed a negative correlation between the density of PSD95+ puncta on PV interneurons and the ratios of JM-a:JM-b and CYT-1:CYT-2 variants. Thus, our study provides evidence that developmental shifts in ErbB4 splicing could function as a molecular switch triggering the pruning of excitatory synapses on PV interneurons in primate DLPFC during adolescence.
Other molecules that converge onto the ErbB4 signaling pathway could also participate in the pruning of excitatory synapses on PV interneurons (49). For example, disrupted-in-schizophrenia 1 (DISC1) can interfere with the interaction between ErbB4 and PSD95, resulting in an inhibition of ErbB4 signaling in GABAergic interneurons (43). Similar to ErbB4, alternative splicing of the DISC1 transcript is developmentally regulated and may modulate the functional consequences of the DISC1 signaling pathway (50). Thus, if shifts in DISC1 expression and/or splicing occur in PV interneurons during adolescence, DISC1 could trigger the pruning of excitatory synapses on these neurons synergistically with the developmental changes in ErbB4 splicing.
Several methodological issues are important to consider in interpreting the results of this study. First, our sampling of excitatory synapses excluded the VGlut2-containing thalamocortical excitatory synapses (51). However, previous EM studies demonstrated that thalamic excitatory inputs represent only ∼10% of total excitatory synapses in the cortex (52) and only a small percentage (∼2%) of thalamic inputs innervate PV interneurons (53). Second, we excluded sampling of synapses on the dendrites of PV interneurons in monkey DLPFC due to the lack of PV immunoreactivity within these structures. Although PV neurons receive excitatory inputs more frequently on their dendrites than cell bodies (54), somal excitatory inputs produce much stronger depolarization in PV neurons than dendritic excitatory inputs (55, 56). Moreover, the densities of excitatory inputs to the dendrites (44) and the cell bodies (28) of PV neurons both similarly predict activity-dependent PV levels and are similarly affected by ErbB4 expression in the current study. Thus, these findings suggest that our sampling approach sufficiently captures functionally important excitatory synapses on PV interneurons in primate DLPFC. Lastly, our findings from the primate DLPFC are from female monkeys and might not generalize to male monkeys. However, neither the density of excitatory inputs to PV neuron (28), the development of parvalbumin expression (57), nor the trajectory of synaptic pruning (8) differs between sexes in the DLPFC.
In conclusion, our study demonstrates a previously unrecognized role of synaptic pruning in the maturation of excitatory synapses on cortical PV interneurons during adolescence that is mediated by a shift in ErbB4 splicing. Deficits in cortical PV interneuron maturation have been linked to several neuropsychiatric disorders, including schizophrenia (58). In the DLFPC of individuals with schizophrenia, a pathogenic shift in ErbB4 splicing from the JM-b to JM-a variants has been associated with fewer excitatory synaptic inputs to PV interneurons (26, 28), which could reflect an exaggerated pruning process in these neurons. Therefore, findings from our study support the view that schizophrenia is a neurodevelopmental disorder with disturbances in the normal maturation process of prefrontal inhibitory circuits (59).
Acknowledgments
We thank Kelly Rogers, MS (University of Pittsburgh) and H. Holly Bazmi, MS (University of Pittsburgh) for their technical assistance. This work was supported by NIH grants MH043784 (to D.A.L.), MH103204 (to D.A.L.), and MH096985 (to K.N.F.).
Footnotes
Conflict of interest statement: D.A.L. currently receives investigator-initiated research support from Pfizer and in 2013–2015 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. All other authors report no biomedical financial interests or potential conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610077114/-/DCSupplemental.
References
- 1.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 2.Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–622. [PubMed] [Google Scholar]
- 4.Alexander GE. Functional development of frontal association cortex in monkeys: Behavioural and electrophysiological studies. Neurosci Res Program Bull. 1982;20(4):471–479. [PubMed] [Google Scholar]
- 5.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 7.Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 8.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 10.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 11.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 12.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77(12):1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: Fast in, fast out--temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27(1):30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science. 2014;345(6196):1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 17.Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21(3):262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ting AK, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31(1):15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79(6):1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Veikkolainen V, et al. Function of ERBB4 is determined by alternative splicing. Cell Cycle. 2011;10(16):2647–2657. doi: 10.4161/cc.10.16.17194. [DOI] [PubMed] [Google Scholar]
- 22.Muraoka-Cook RS, et al. ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Mol Cell Biol. 2009;29(18):4935–4948. doi: 10.1128/MCB.01705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundvall M, et al. Differential nuclear localization and kinase activity of alternative ErbB4 intracellular domains. Oncogene. 2007;26(48):6905–6914. doi: 10.1038/sj.onc.1210501. [DOI] [PubMed] [Google Scholar]
- 24.Sundvall M, et al. Cell death or survival promoted by alternative isoforms of ErbB4. Mol Biol Cell. 2010;21(23):4275–4286. doi: 10.1091/mbc.E10-04-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Burgos G, et al. Functional maturation of GABA synapses during postnatal development of the monkey dorsolateral prefrontal cortex. Cereb Cortex. 2015;25(11):4076–4093. doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung DW, et al. Dysregulated ErbB4 splicing in schizophrenia: Selective effects on parvalbumin expression. Am J Psychiatry. 2016;173(1):60–68. doi: 10.1176/appi.ajp.2015.15020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 2012;22(5):1215–1223. doi: 10.1093/cercor/bhr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung DW, Fish KN, Lewis DA. Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am J Psychiatry. 2016;173(11):1131–1139. doi: 10.1176/appi.ajp.2016.16010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26(2):443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 30.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127(1):185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Chung DW, Rudnicki DD, Yu L, Margolis RL. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20(17):3467–3477. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills ZP, et al. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 2012;73(3):466–481. doi: 10.1016/j.neuron.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: Differential targeting of subclasses of GABA neurons. Cereb Cortex. 2003;13(5):452–460. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- 35.Kurppa KJ, Denessiouk K, Johnson MS, Elenius K. Activating ERBB4 mutations in non-small cell lung cancer. Oncogene. 2016;35(10):1283–1291. doi: 10.1038/onc.2015.185. [DOI] [PubMed] [Google Scholar]
- 36.Veikkolainen V, et al. ErbB4 modulates tubular cell polarity and lumen diameter during kidney development. J Am Soc Nephrol. 2012;23(1):112–122. doi: 10.1681/ASN.2011020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: Distribution and morphology. J Comp Neurol. 1994;341(1):95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 38.Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 40.Neddens J, et al. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: Implications for schizophrenia. Biol Psychiatry. 2011;70(7):636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Määttä JA, et al. Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol Biol Cell. 2006;17(1):67–79. doi: 10.1091/mbc.E05-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HJ, et al. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. J Biol Chem. 2002;277(8):6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 43.Seshadri S, et al. Interneuronal DISC1 regulates NRG1-ErbB4 signalling and excitatory-inhibitory synapse formation in the mature cortex. Nat Commun. 2015;6:10118. doi: 10.1038/ncomms10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504(7479):272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 45.Wilson NR, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25(26):6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 47.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norris AD, Calarco JA. Emerging roles of alternative pre-mRNA splicing regulation in neuronal development and function. Front Neurosci. 2012;6:122. doi: 10.3389/fnins.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaaro-Peled H, et al. Neurodevelopmental mechanisms of schizophrenia: Understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32(9):485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakata K, et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci USA. 2009;106(37):15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fremeau RT, Jr, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 52.Latawiec D, Martin KA, Meskenaite V. Termination of the geniculocortical projection in the striate cortex of macaque monkey: A quantitative immunoelectron microscopic study. J Comp Neurol. 2000;419(3):306–319. doi: 10.1002/(sici)1096-9861(20000410)419:3<306::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: Synaptic relationships to subclasses of interneurons. J Comp Neurol. 2005;490(3):220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- 54.Hioki H. Compartmental organization of synaptic inputs to parvalbumin-expressing GABAergic neurons in mouse primary somatosensory cortex. Anat Sci Int. 2015;90(1):7–21. doi: 10.1007/s12565-014-0264-8. [DOI] [PubMed] [Google Scholar]
- 55.Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327(5961):52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- 56.Nörenberg A, Hu H, Vida I, Bartos M, Jonas P. Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proc Natl Acad Sci USA. 2010;107(2):894–899. doi: 10.1073/pnas.0910716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fung SJ, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167(12):1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 58.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]