Significance
Fishing marine ecosystems indiscriminately and intensely can have negative impacts on biodiversity, but it may increase the biomass of fish available for capture in the system. We explore the possibility that China’s high fishery catches are a result of predator removal using an ecosystem model of the East China Sea (ECS). We show that China’s high fishery catches can be explained by the removal of larger predatory fish and consequent increases in the production of smaller fish. We project that single-species management would decrease catches in the ECS by reversing these ecosystem effects. Fisheries similar to those in China produce a large fraction of global catch; management reform in these areas must consider the entire ecosystem, rather than individual species.
Keywords: ecosystem management, China, fisheries, trophic cascades, food security
Abstract
Indiscriminate and intense fishing has occurred in many marine ecosystems around the world. Although this practice may have negative effects on biodiversity and populations of individual species, it may also increase total fishery productivity by removing predatory fish. We examine the potential for this phenomenon to explain the high reported wild catches in the East China Sea—one of the most productive ecosystems in the world that has also had its catch reporting accuracy and fishery management questioned. We show that reported catches can be approximated using an ecosystem model that allows for trophic cascades (i.e., the depletion of predators and consequent increases in production of their prey). This would be the world’s largest known example of marine ecosystem “engineering” and suggests that trade-offs between conservation and food production exist. We project that fishing practices could be modified to increase total catches, revenue, and biomass in the East China Sea, but single-species management would decrease both catches and revenue by reversing the trophic cascades. Our results suggest that implementing single-species management in currently lightly managed and highly exploited multispecies fisheries (which account for a large fraction of global fish catch) may result in decreases in global catch. Efforts to reform management in these fisheries will need to consider system wide impacts of changes in management, rather than focusing only on individual species.
Globally, marine ecosystems produced 81.5 million tons of wild-capture seafood during the year 2014, which provided 17% of the animal protein in the diet of the average global citizen (1). Societies around the globe use a range of methods to manage fisheries to ensure they continue to produce seafood. In closely managed systems like the United States and Europe, management is often based on identifying maximum sustainable yield for an individual species or population, which attempts to ensure that all species are protected (2). Single-species management was adopted at least partially in response to the observation that overfishing appeared to be occurring for some populations; single-species management has been successful in curbing overfishing in many cases (2). However, other fisheries around the world are managed using measures (e.g., seasonal or spatial closures) that do not afford focused protection for individual species. Areas of the world with minimal management measures in place and intense fishing (e.g., much of the Asia-Pacific region) continue to produce a large fraction of the world’s seafood without the protection of single-species management (1). A potential (but sometimes undiscussed) reason for high productivity in minimally managed marine ecosystems is the existence of trophic cascades. Trophic cascades can occur when larger predatory fish are removed from the system by fishing and the productivity of their prey consequently increases, which then allows for more exploitable biomass by fisheries (3).
The concept of increased total catches after the elimination of larger, predatory fish (4) should be intuitive when considering a trophic transfer efficiency of ∼10% (5)—eating lower on the food chain is more “efficient” because less energy is lost to transfer among trophic levels. Increases in ecosystem-wide harvests by shortening food chains have been demonstrated both theoretically and empirically in a variety of contexts. For example, high-yield agriculture occurs in simplified food chains, rather than in wild pastures or forests (6). Diverse models of marine ecosystems have predicted that obtaining maximum total yield involves removing predators from the system (3, 7), and this phenomenon has been observed widely in freshwater environments (8). Reported marine cases are fewer but also exist. Marine coral reef fisheries in Kenya have been shown to produce more catch with increasing effort, but with the result of decreasing functional diversity and trophic complexity (9). Indiscriminate marine fisheries in Cambodia continue to produce high catches despite high fishing mortality, but at the cost of reduced species diversity (10). Because trophic cascades can increase the total harvestable biomass in a system, reversing them in systems where predator removal has already occurred by implementing management that protects predators could result in decreases in catch. Here, we explore the potential for trophic cascades to explain the sustained, high catches (and the impacts of potentially reversing them) from one of the most productive (yet minimally managed) marine ecosystems in the world: the East China Sea.
China’s Fisheries
China produces 16% of global wild-capture catch from its fisheries (1), and the East China Sea is the most productive of China’s waters, producing ∼40% of that total (11–39). Current Chinese fisheries management consists of gear restrictions, seasonal closures, and catch and effort caps (40). Fishing practices are largely indiscriminate (∼50% trawl fisheries) and there is little discard because a market exists for a wider range of species and sizes than are typically salable in other locales. Despite relatively simple management, reported catches for many species plateaued during the late 1990s and have since been maintained at high levels (Fig. 1).
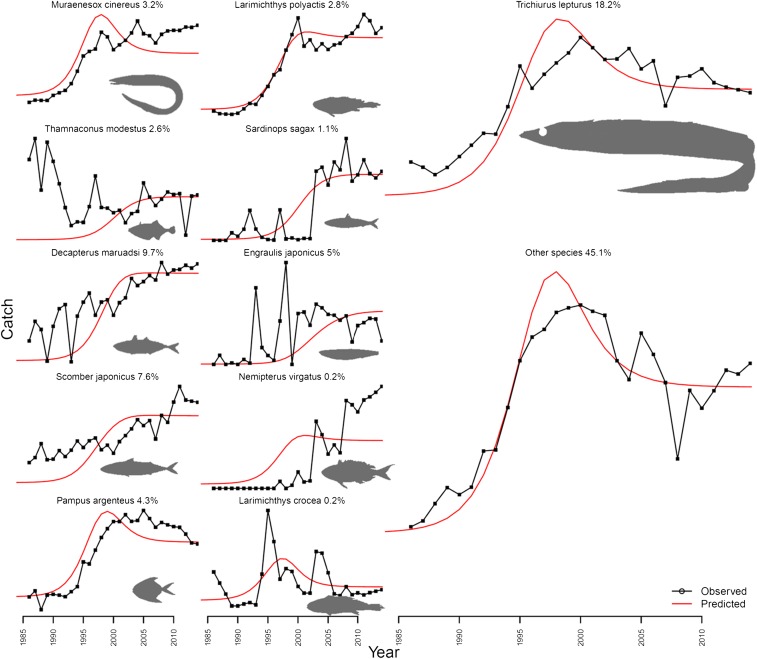
Fig. 1.
East China Sea model fitted to observed data for 11 species and 1 “other species” group. Size of plot roughly represents the proportion of the total catch a species represents (true proportions of the total catch that each species/group represented in 2014 are indicated after the group name).
The persistent magnitude of China’s catches led to suggestions that they were overreporting, because their catches exceeded other countries’ catches after controlling for environmental conditions [e.g., primary productivity, ice cover, distance from shore (41)]. However, this analysis excluded fishing effort, and China’s fleet is the largest and most powerful in the world (1). The Chinese modified their catch reports (∼10%) in response (42), but, even with these adjustments, catch from China often exceeds the combined catches of the next three largest fishing countries. It has also been suggested that China reports catches taken in other countries’ waters as their own, artificially buoying Chinese catches (43, 44), but it is difficult to explain why catch for some (often smaller, lower-value) species have increased while other (often larger, higher-value) species decreased if the catches are coming from other waters. Fishers generally target larger, high-value species when developing new fisheries (45).
In addition to the validity of the reported catches, the sustainability of Chinese fisheries management has also been questioned. For example, it has recently been suggested that the catch extracted from the East China Sea exceeds the potential of the input primary productivity (46), which implies unsustainable fishing at the ecosystem level. The age structure for most fished populations in the East China Sea consists largely of 1-y-olds and exploitation rates in the fisheries appear to be very high (47), which suggests decreasing harvest rates could result in large benefits [from the perspective of single-species assessment methods (48)]. However, the current levels of total catch have been sustained over at least 10 generations of fish. If these records are even roughly accurate, they suggest Chinese fishing practices may be “sustainable” in terms of total fishery production. It is unclear whether these practices are desirable because some species have been exploited to low levels (49) and catch of “high”-quality species has been replaced by “low”-quality species over time (50, 51).
Given the sustained production from Chinese waters, the concern about the validity of reported catches from Chinese waters, and questions of the sustainability of Chinese fisheries management, here, we ask the following: (i) Can the reported catch from the East China Sea be explained as a consequence of intense fishing that removed large fish and subsequently induced an increase in the productivity of smaller fish? If this “ecosystem engineering” explanation is feasible, we would then like to assess its relevance for fishery management, by asking the following: (ii) What would be the effects of single-species management (that ignores ecosystem interactions) in an engineered ecosystem? Specifically, under a range of management alternatives, what would be the effects on biomass in the water, catch in the nets, and revenue at market?
We developed a size-spectrum ecosystem model for the East China Sea in the R programming language [package: mizer (52)] and fit it to catch data for the 11 species with the largest catches reported by biomass, plus one “other fish” category (Materials and Methods). Collectively, these taxa represent >95% of reported finfish catch from the East China Sea (11–39) (see SI Appendix for discussion of why we used a size-spectrum model, its specifics, and the data used). Size-spectrum models are based on known relationships between body size, physiology, and life history, and the idea that big fish eat smaller fish (53). Size-spectrum models allow prey switching, interspecific and intraspecific predator–prey interactions, and size-selective fishing practices, all while using relatively few parameters compared with other ecosystem models (an advantage in data-poor contexts).
We used our fitted model to evaluate the performance of several types of management strategies, including the following: (i) the status quo (an indiscriminate fishery with high effort), (ii) single-species management in which each species was fished with a selectivity [defined here as the sizes of fish captured by the fishery, and measured by the length at 50% capture (L50%)—the higher the selectivity, the more catch is restricted to larger individuals] and fishing effort tailored to its mortality and maturity schedules, and (iii) strategies in which effort and selectivity were modified within an indiscriminate fishery to maximize catch, value, or biomass. For each scenario, the biomass and catch in year 2050 of the projection were recorded for all species and the value of the catch by species was calculated based on observed average price per kilogram over the last 6 y (www.zjscxh.com/newslist/1068.html) (SI Appendix).
Results and Discussion
Our model approximately reproduced the reported catch trends for most species despite the relatively limited information available (catch-weighted r2 = 0.90; Fig. 1). In addition, the average size of fish decreased within the model over time in a manner consistent with observed changes in the size spectrum of the East China Sea (54) (SI Appendix). Year-to-year variability in catch was not well captured because no data were available to determine recruitment variability. Our model was able to approximate China’s high, sustained catches by explicitly incorporating trophic relationships between large and small fish (Fig. 2). Good fits to the data do not necessarily mean that the Chinese catch data are perfectly accurate; however, it does mean that explanations for high catches other than inaccurate reporting are plausible.
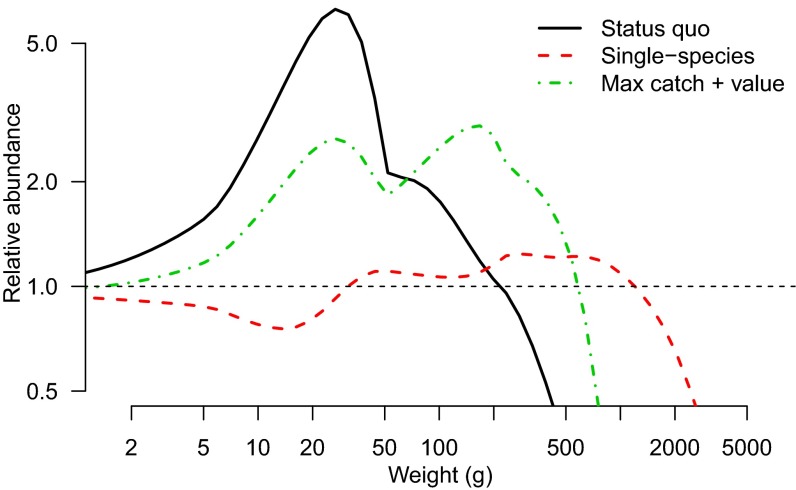
Fig. 2.
Trophic cascades resulting from different management strategies implemented in the size-spectrum model of the East China Sea. Changes in abundance are relative to unfished levels, indicated by the dotted black line at a relative abundance of “1”; x axis is the weight in grams of fish within the model.
Unaccounted-for changes in trophic level could explain the apparent outpacing of primary productivity of the East China Sea by the fisheries yield. For example, largehead hairtail (Trichiurus lepturus) [reported trophic level, 4.4 (FishBase; www.fishbase.org/)] accounted for 15% of catch in the East China Sea on average, but today’s hairtail are smaller than in the past, and therefore occupy a lower trophic level. Reducing trophic levels by only 14% for secondary consumers and above can bring fisheries in balance with primary productivity (reproducing ref. 46; SI Appendix); our model predicted changes in trophic level of up to 27% for species like largehead hairtail from those used in Watson et al. (46). If China’s fishery catch data are roughly correct, they appear to have, perhaps unwittingly, performed the world’s largest experiment in marine predator removal with the result of increases in harvestable biomass.
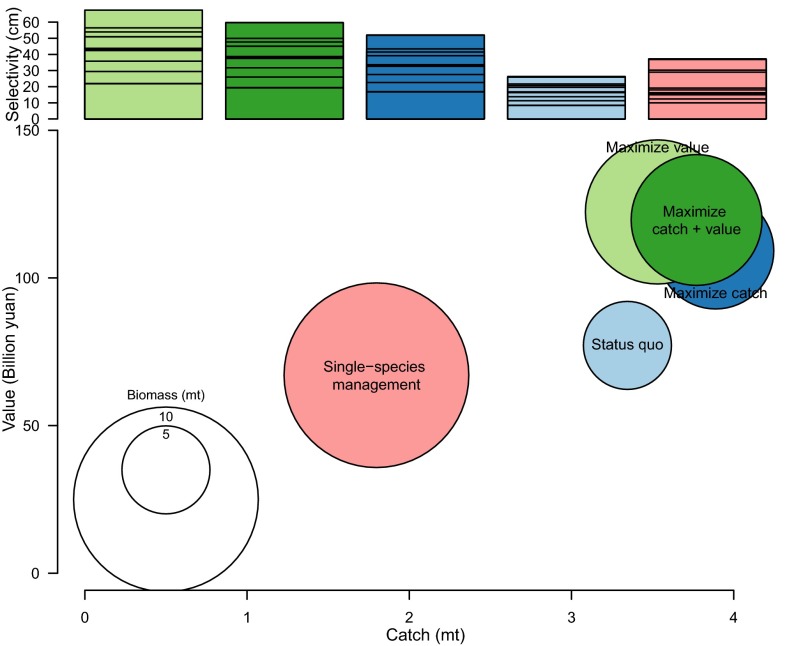
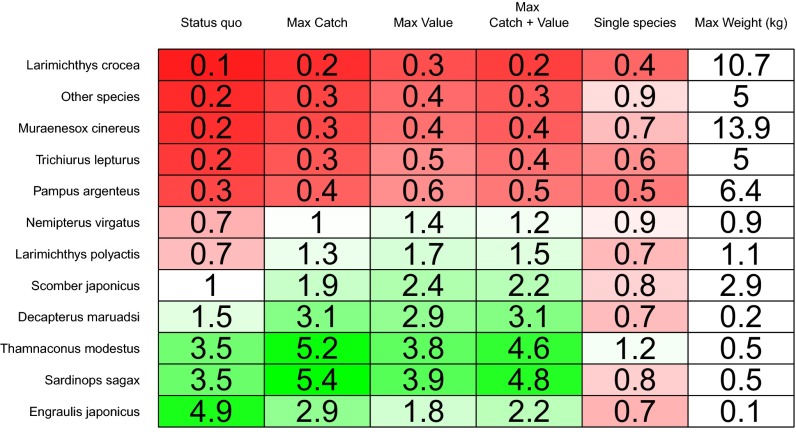
Our projections suggest that single-species management would increase fish biomass by 109% compared with the status quo, but at a cost of decreased catches (−46%) and value (−13%) (Fig. 3). Optimizing selectivity and effort to maximize a given objective produced trade-offs, so we used weighted objective functions to determine the optimal selectivity and effort for a desired “portfolio” of benefits. For example, weighting catch and value equally (but ignoring biomass) resulted in increases in all three objectives compared with the status quo (biomass, 59%; catch, 8%; value, 58%). These increases could be accomplished by increasing the selectivity of Chinese fleets by a factor of 2 through increasing the mesh size of their nets. Scenarios in which catch or value were maximized resulted in some species (late maturing and/or slow growing) being reduced to small fractions of unexploited levels (Fig. 4), but even then only Larimichthys crocea was reduced close to 20% of virgin biomass, which is a threshold biomass sometimes used in fisheries management to identify collapsed stocks.
Fig. 3.
Ecosystem-wide catch (x axis), biomass (circle size), and value (y axis) by management strategies. Bar plot at the Top displays the selectivity by strategy, color-coded to match the circles representing scenarios within the main figure. The lines in the selectivity bars represent the individual selectivities for each species and can be seen in tabular format in SI Appendix, Fig. S9.
Fig. 4.
Proportion of virgin biomass levels predicted by the size-spectrum model for species under different management strategies. Values greater than 1 indicate the biomass of the species is greater than the virgin levels as a result of predatory release. “Max weight” is the maximum weight in kilograms of an individual from a given species or group.
Our results provide a cautionary tale for single-species fisheries management reform in areas of the world in which fishing is intense and indiscriminate, species and sizes are broadly salable, and the food webs have been compressed. In these fisheries, “recovering” all species using single-species management could reverse trophic cascades and incur devastating social effects. Beyond China, this may include other large Asia-Pacific seafood producers (e.g., Indonesia, Thailand, Philippines; ref. 1). Our results also underscore the need for accurate and longitudinal data collection to characterize ecosystem dynamics and identify appropriate management reform. Management reform is needed in many areas of the globe, and the East China Sea is not in a state to which many managers would aspire, given the numbers of species depleted to low levels. Enforcing appropriate restrictions on fishing gear (as shown here and suggested in ref. 55) and reducing capacity (56) could provide benefits for China and likely many of the world’s undermanaged fisheries. However, we show that trade-offs (for example, between catch and the proportion of virgin biomass remaining in the ocean for larger species) exist among management strategies. When trade-offs exist, there is no simple answer to the “best” management strategy. Efficient strategies should be identified, and potential win–wins exhausted (57), but then each society must make a value-based judgment on where on the efficiency frontier it wishes to be. Given the diversity in values and priorities among societies, societies may differ in their judgments even when facing similar trade-offs.
A key problem with forecasting the impacts of management reform based on the analysis of single species is that single-species models assume consistent ecosystem dynamics to project benefits (58, 59). Implementing single-species reform in an ecosystem context will violate this assumption as the strength and direction of interactions between species changes. For example, fishing the predator of a prey species more or less heavily influences the productivity of the prey species and consequently its expected maximum sustainable yield. We do not advocate for the “engineering” of ecosystems through predator removal given the potential consequences on biodiversity, the difficulty of prediction of ecological systems, and uncertainty of compressed systems’ resilience to a changing climate. Rather, we stress that management reforms should be designed, acknowledging that the benefits of reform may be overstated by single-species models in systems in which predator removal has occurred (a point made in ref. 48). Reform should be designed from an ecosystem perspective to avoid short-term pain (i.e., decreased catches and income) while the promised future gain [i.e., increased catches and income (48, 60)] is eaten by recovered populations of predators.
Materials and Methods
Below, we briefly describe the data and ecosystem model used, and the management strategies evaluated. In SI Appendix, we provide a more in-depth discussion of the data and model, provide the specified and estimated parameter values, discuss the fits to the data and the rationale for using a size-spectrum model, describe the tested management strategies in more detail, provide additional results, and examine some of the concerns with Chinese fishing practices more closely.
Data.
Catch data were collated from the China Fishery Statistical Yearbook [中国渔业统计年鉴 (11–39)]. Annual catches (by species or species group) during the years 1986–2014 from the East China Sea were calculated by adding the catch landed in Shanghai, Zhejiang, and Fujian provinces. Catch time series were used for groups that were reported to the species level (SI Appendix, Table S1) and had been consistently reported since 1986. An “other fish” category was included in the analysis and was composed of species with relatively low catches, fish that were not identified to any group, and species for which reporting to species level only began in the last decade (SI Appendix, Fig. S1). The reported catches (rather than “corrected” time series) were used in this analysis (see SI Appendix for discussion). A price database was collated from the monthly price reports from 2009 to 2015 from www.zjscxh.com/newslist/1068.html for use in calculating the value of catches resulting from different management strategies. Species specific prices were available for the six most valuable species; prices for the other species were based on the conger eel (SI Appendix).
Ecosystem Model.
We fit a size-spectrum model [mizer (52)] to the reported catch data via sum of squares by specifying eight species-specific life history parameters (e.g., maximum weight, growth, length at weight, and weight at maturity; SI Appendix) and estimating parameters associated with fishing mortality, selectivity, and recruitment. Life history data were gathered from FishBase (www.fishbase.org/) when available and from published literature when absent from FishBase (SI Appendix, Tables S1 and S2). Fishing mortality was assumed to follow a logistic curve based on the effort data reported in refs. 11–39; the inflection point and the slope of the curve were estimated parameters. Maximum fishing mortality was based on an average rate collected from the literature (1.36) (SI Appendix) and ramped from a value of 5% of the maximum in 1950. A “maximum recruitment” parameter was estimated for each species (which primarily scales catches derived from a species) in a density-dependent stock–recruit relationship (52). Selectivity for each species within the fishery was extrapolated from the length at 25% and 50% probability of selection in the minimum mesh size trawl nets reported in Huang et al. (55) for small yellow croaker and largehead hairtail. Size-spectrum models represent a modeling framework sufficiently complex to capture trophic dynamics of complex ecosystems while still being applicable in relatively data-limited scenarios.
Evaluating Management Strategies.
After fitting the model to the data, the estimated parameters were used to project the model under a variety of management strategies in which the specified selectivities and fishing mortalities changed. A range of potential management strategies exist, but we considered only the following because they represent a shift to the “international standard” of single-species management (which is the focus of much of the Chinese literature on fisheries management) or they represent a management strategy that could be easily implemented within the status quo system (i.e., effort could be regulated by the length of seasons and selectivity could be modified by restricting the mesh size of the gear used). The “status quo” management strategy was represented by a continuation of current fishing mortality and selectivity and was the baseline for comparison of other management strategies. Single-species management was implemented by first setting the fishing mortality for a species equal to its natural mortality, which is often used as a proxy for the fishing mortality at which maximum sustainable yield would occur in data-poor fisheries (species-specific values were derived from the available literature). Next, the length at 50% capture (L50%) was set to the length at maturity for each species, and the relationship between L50% and L25% was preserved to mimic the selectivity of trawl fleets. Surfaces of biomass, catch, and value were created for each indiscriminate fishing management strategy (i.e., a strategy in which a multispecies trawl is still the dominant fishing practice and selectivity for all species is linked and determined by their weight at length) by performing grid searches over possible selectivity and effort combinations. Equilibrium biomasses for each strategy were recorded and compared with the virgin biomasses (i.e., the biomass under a scenario in which no fishing occurs) to determine the “depletion” resulting from a management strategy for each species. Ecosystem-wide trophic cascades were quantified for given management strategies by comparing the biomass in a given weight class under no fishing to the equilibrium biomass resulting from a given management strategy.
Supplementary Material
Acknowledgments
We are grateful to researchers at the East China Sea Fisheries Research Institute for their insights, and The Nature Conservancy and China Blue Sustainability Institute for their support in China. We also thank two anonymous reviewers for comments that improved this manuscript. We are grateful to the Packard Foundation for their funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 634.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612722114/-/DCSupplemental.
References
- 1.Food and Agriculture Organization of the United Nations 2016 The State of World Fisheries and Aquaculture (Food and Agriculture Organization of the United Nations, Rome). Available at www.fao.org/fishery/sofia/en. Accessed June 22, 2016.
- 2.Hilborn R, Ovando D. Reflections on the success of traditional fisheries management. ICES J Mar Sci. 2014;71(5):1040–1046. [Google Scholar]
- 3.Matsuda H, Abrams PA. Maximal yields from multispecies fisheries systems: Rules for systems with multiple trophic levels. Ecol Appl. 2006;16(1):225–237. doi: 10.1890/05-0346. [DOI] [PubMed] [Google Scholar]
- 4.Bax NJ. A comparison of fish biomass flow to fish, fisheries, and mammals in six marine ecosystems. ICES Mar Sci Symp. 1991;193:217–224. [Google Scholar]
- 5.Trebilco R, Baum JK, Salomon AK, Dulvy NK. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol Evol. 2013;28(7):423–431. doi: 10.1016/j.tree.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 7.Andersen KH, Brander K, Ravn-Jonsen L. Trade-offs between objectives for ecosystem management of fisheries. Ecol Appl. 2015;25(5):1390–1396. doi: 10.1890/14-1209.1. [DOI] [PubMed] [Google Scholar]
- 8.Brett MT, Goldman CR. A meta-analysis of the freshwater trophic cascade. Proc Natl Acad Sci USA. 1996;93(15):7723–7726. doi: 10.1073/pnas.93.15.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClanahan TR, Hicks CC, Darling ES. Malthusian overfishing and efforts to overcome it on Kenyan coral reefs. Ecol Appl. 2008;18(6):1516–1529. doi: 10.1890/07-0876.1. [DOI] [PubMed] [Google Scholar]
- 10.McCann KS, et al. Food webs and the sustainability of indiscriminate fisheries. Can J Fish Aquat Sci. 2015;73(4):656–665. [Google Scholar]
- 11. China Agriculture Press (1986) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 12. China Agriculture Press (1987) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 13. China Agriculture Press (1988) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 14. China Agriculture Press (1989) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 15. China Agriculture Press (1990) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 16. China Agriculture Press (1991) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 17. China Agriculture Press (1992) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 18. China Agriculture Press (1993) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 19. China Agriculture Press (1994) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 20. China Agriculture Press (1995) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 21. China Agriculture Press (1996) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 22. China Agriculture Press (1997) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 23. China Agriculture Press (1998) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 24. China Agriculture Press (1999) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 25. China Agriculture Press (2000) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 26. China Agriculture Press (2001) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 27. China Agriculture Press (2002) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 28. China Agriculture Press (2003) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 29. China Agriculture Press (2004) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 30. China Agriculture Press (2005) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 31. China Agriculture Press (2006) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 32. China Agriculture Press (2007) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 33. China Agriculture Press (2008) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 34. China Agriculture Press (2009) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 35. China Agriculture Press (2010) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 36. China Agriculture Press (2011) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 37. China Agriculture Press (2012) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 38. China Agriculture Press (2013) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 39. China Agriculture Press (2014) China Fishery Statistical Yearbook (China Agriculture Press, Beijing). Chinese.
- 40.Shen G, Heino M. An overview of marine fisheries management in China. Mar Policy. 2013;44:265–272. [Google Scholar]
- 41.Watson R, Pauly D. Systematic distortions in world fisheries catch trends. Nature. 2001;414(6863):534–536. doi: 10.1038/35107050. [DOI] [PubMed] [Google Scholar]
- 42.Food and Agriculture Organization of the United Nations . The State of World Fisheries and Aquaculture. Food and Agriculture Organization of the United Nations; Rome: 2012. [Google Scholar]
- 43.Pauly D, et al. China’s distant water fisheries in the 21st century. Fish Fish. 2014;15:474–488. [Google Scholar]
- 44.Pauly D, Zeller D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat Commun. 2016;7:10244. doi: 10.1038/ncomms10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi SA, Branch TA, Watson R. Global fishery development patterns are driven by profit but not trophic level. Proc Natl Acad Sci USA. 2010;107(27):12163–12167. doi: 10.1073/pnas.1003236107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson R, Zeller D, Pauly D. Primary productivity demands of global fishing fleets. Fish Fish. 2014;15:231–241. [Google Scholar]
- 47.Lin L-S, Zheng Y-J, Cheng J-H, Liu Y, Ling J-Z. A preliminary study on fishery biology of main commercial fishes surveyed from the bottom trawl fisheries in the East China Sea. Mark Sci. 2006;30(2):21–25. [Google Scholar]
- 48.Costello C, et al. Global fishery futures under contrasting management regimes. Proc Natl Acad Sci USA. 2016;113(18):5125–5129. doi: 10.1073/pnas.1520420113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu M, De Mitcheson YS. Profile of a fishery collapse: Why mariculture failed to save the large yellow croaker. Fish Fish. 2008;9(3):219–242. [Google Scholar]
- 50.Chen W-Z, Zheng Y-Z, Chen Y-Q, Mathews C-P. An assessment of fishery yields from the East China Sea ecosystem. Mar Fish Rev. 1997;59(4):1–7. [Google Scholar]
- 51.Cao L, et al. Global food supply. China’s aquaculture and the world’s wild fisheries. Science. 2015;347(6218):133–135. doi: 10.1126/science.1260149. [DOI] [PubMed] [Google Scholar]
- 52.Scott F, Blanchard JL, Andersen KH. mizer: An R package for multispecies, trait-based and community size spectrum ecological modelling. Methods Ecol Evol. 2014;5(10):1121–1125. doi: 10.1111/2041-210X.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen KH, Jacobsen NS, Farnsworth KD. The theoretical foundations for size spectrum models of fish communities. Can J Fish Aquat Sci. 2016;73:1–14. [Google Scholar]
- 54.Cheng J-H, et al. Changes of fish community structure in the coast zone of the northern part of the East China Sea in summer. J Nat Res. 2009;21(5):775–780. [Google Scholar]
- 55.Huang H-L, Wang M-Y, Xu B-S, Zhang X, Tang Z-M. Study on selectivity of mesh size of cod-end trawl in the East China Sea region. J Fish China. 2005;29(2):232–237. [Google Scholar]
- 56.Cheung WWL, Sumalia UR. Trade-offs between conservation and socio-economic objectives in managing a tropical marine ecosystem. Ecol Econ. 2007;6(1):193–210. [Google Scholar]
- 57.Jacobsen NS, Burgess MG, Andersen KH. Efficiency of fisheries is increasing at the ecosystem level. Fish Fish. July 9, 2016 doi: 10.1111/faf.12171. [DOI] [Google Scholar]
- 58.Szuwalski CS, Hollowed AB. Climate change and non-stationary population processes in fisheries management. ICES J Mar Sci. 2016;73(5):1297–1305. [Google Scholar]
- 59.Burgess MG, Giacomini HC, Szuwalski CS, Costello C, Gaines SD. Describing ecosystem contexts with single-species models: A theoretical synthesis for fisheries. Fish Fish. September 20, 2016 doi: 10.1111/faf.12179. [DOI] [Google Scholar]
- 60.Sumaila UR, et al. Benefits of rebuilding global marine fisheries outweigh costs. PLoS One. 2012;7(7):e40542. doi: 10.1371/journal.pone.0040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.