Significance
Immunosuppressive tumor microenvironment, insufficient migration, and reduced effector function of tumor-specific T cells are the main hurdles that hamper the efficacy of immunotherapy in treating solid tumors. In this study, we combined the strength of adoptive cell transfer (ACT) and pathogen-based cancer vaccine and developed an innovative strategy, Reenergized ACT (ReACT), to treat solid tumors. ReACT uses a pathogen not only to break the immunosuppression, but also to drive the expansion and migration of tumor-specific T cells to the site of the tumor. With this combinatorial approach, we have demonstrated that ReACT enhances antitumor efficacy in comparison with either ACT or pathogen-based cancer vaccine alone in primary tumor eradication and offers long-term protection against reoccurrence in preclinical cancer models.
Keywords: adoptive cell transfer, immunotherapy, CD8 T cells, Listeria, melanoma
Abstract
Because of insufficient migration and antitumor function of transferred T cells, especially inside the immunosuppressive tumor microenvironment (TME), the efficacy of adoptive cell transfer (ACT) is much curtailed in treating solid tumors. To overcome these challenges, we sought to reenergize ACT (ReACT) with a pathogen-based cancer vaccine. To bridge ACT with a pathogen, we genetically engineered tumor-specific CD8 T cells in vitro with a second T-cell receptor (TCR) that recognizes a bacterial antigen. We then transferred these dual-specific T cells in combination with intratumoral bacteria injection to treat solid tumors in mice. The dual-specific CD8 T cells expanded vigorously, migrated to tumor sites, and robustly eradicated primary tumors. The mice cured from ReACT also developed immunological memory against tumor rechallenge. Mechanistically, we have found that this combined approach reverts the immunosuppressive TME and recruits CD8 T cells with an increased number and killing ability to the tumors.
Adoptive cell transfer (ACT) of genetically engineered T cells has become a promising cancer immunotherapy for hematologic malignancies (1–4). However, the efficacy of such an approach is curtailed when treating solid tumors (2, 5, 6). The primary hurdles that must be overcome for ACT to be effective against solid tumors include inadequate responses of adoptively transferred T cells, especially in dealing with heterogeneous cancerous cells that bear a wide range of tumor-associated antigens (TAAs) (2, 6); reduced migration of adoptively transferred T cells into the tumor (7); and the immunosuppressive microenvironment within tumors that often induces a rapid loss of T-cell effector function (8).
Using infectious pathogens that stimulate a patient’s immune system and break immunosuppression in the tumor microenvironment is a century-old strategy that is now being rejuvenated to enhance cancer immunotherapy (9). Bacillus Calmette-Guérin (bacillus Calmette–Guérin), a live attenuated strain of Mycobacterium bovis, has been widely used in treating bladder cancer and melanoma for decades (10, 11). Although effective, bacillus Calmette–Guérin only induces transient and nonspecific antitumor immune responses. To generate a tumor-specific T-cell response, recombinant Listeria monocytogenes (LM) expressing TAAs has recently been developed and shown promising results in treating multiple cancers including breast and pancreatic cancer (9). Owing to the heterogeneity of tumor cells, it remains challenging for recombinant LM-based immunotherapies targeting a single TAA to provide durable and complete regression of cancer because cancer cells that do not express the targeted TAA are able to evade immunosurveillance (2, 6, 7, 9). Thus, there is a critical need for new strategies that generate robust T-cell responses with broad coverage of tumor antigens to improve pathogen-based cancer vaccines.
To overcome these hurdles and induce a vigorous antitumor T-cell response, we sought to combine the strength of ACT and pathogen-based cancer vaccines with a strategy named Reenergized ACT (ReACT). To bridge ACT with a pathogen, we genetically engineered tumor-reactive CD8 T cells with a second T-cell receptor (TCR) specific to a bacterial antigen to create dual-specific CD8 T cells (i.e., a single T-cell capable of recognizing two antigens). This technology was first developed by Kershaw and coworkers (12, 13). We then used a pathogen-based vaccine to drive the robust expansion of adoptively transferred bacteria- and tumor- (dual) specific T cells, recruit them to the tumor site, and concomitantly reverse immunosuppression in the tumor microenvironment. This combined approach has demonstrated robust efficacy in primary tumor eradication and long-term protection against recurrence in preclinical cancer models.
Results
ReACT Enhances Antitumor Efficacy.
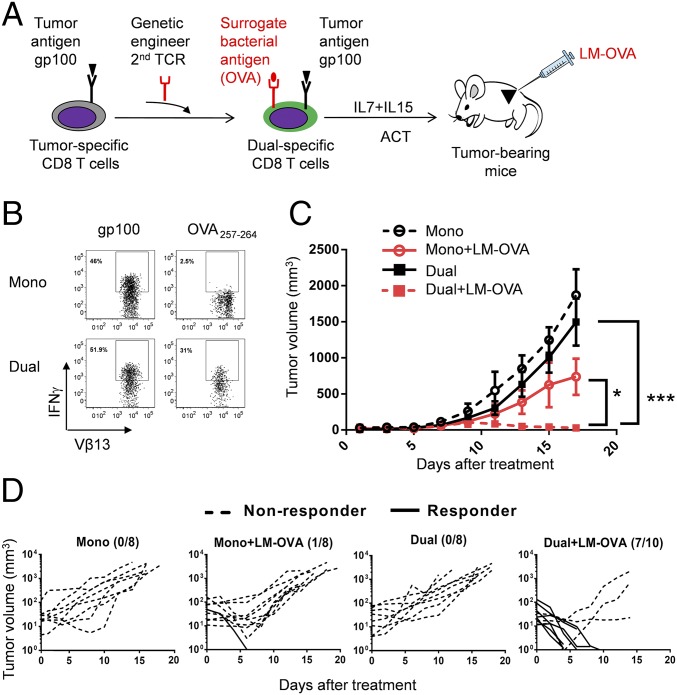
First, we used a well-established mouse B16-F10 melanoma model (14) to test the antitumor efficacy of ReACT. To generate dual-specific CD8 T cells, we started with Pmel-1 CD8 T cells, which express a TCR (Vα1 and Vβ13) that recognizes the glycoprotein 100 (gp100) epitope of murine melanoma (14). These cells were then genetically engineered to express OT-I TCR (Vα2 and Vβ5) by retroviral transduction in vitro (Fig. 1A). OT-I recognizes ovalbumin (OVA) residues 257–264, which served as a surrogate bacterial antigen expressed in a recombinant LM-OVA. We chose Listeria as a model organism because it is amenable to clinical use, and attenuated Listeria, like many other pathogen-based cancer vaccines, has shown promising antitumor effects in multiple cancer models in humans (https://clinicaltrials.gov/ct2/home) and mice (9). To validate dual specificity, we first examined the expression of the heterodimeric TCRs (Vα2 and Vβ5) on control (empty vector transduced; referred to as monospecific CD8 T cells henceforth) and OT-I TCR transduced (referred to as dual-specific CD8 T cells henceforth) Pmel-1 cells and confirmed the expression of heterodimeric TCRs (Vα2 and Vβ5) on dual-specific cells (Fig. S1A). Next, we stimulated both types of T cells in vitro by antigenic peptides. Monospecific CD8 T cells produced IFN-γ after stimulation with gp100 but not OVA257–264 peptide (Fig. 1B). In contrast, dual-specific CD8 T cells responded to both gp100 and OVA257–264 peptides (Fig. 1B).
Fig. 1.
ReACT shows significantly enhanced antitumor efficacy. (A) The experimental scheme of ReACT. Pmel-1 CD8 T cells are transduced in vitro to express a second TCR (OT-I) to generate T cells that could recognize both a TAA gp100 and a surrogate bacterial antigen OVA257–264. These dual-specific CD8 T cells were expanded in vitro and transferred to tumor-bearing mice followed by i.t. LM-OVA infection. (B) Dot plots show the intracellular IFN-γ staining in Pmel-1+ or OT-I+ Pmel-1+ CD8 T cells after 6 h of stimulation with gp100 or OVA peptide, respectively. (C) The B16-F10 tumor-bearing mice received the following combinations of treatments: monospecific CD8 T-cell transfer, monospecific CD8 T-cell transfer accompanied by i.t. injection of LM-OVA, dual-specific CD8 T-cell transfer or dual-specific CD8 T-cell transfer accompanied by i.t. injection of LM-OVA. In each group, 5 × 105 CD8 T cells were transferred into each mouse. The overall tumor growth is shown as mean volume ± SEM. *P < 0.05, ***P < 0.001. (D) The individual tumor growth curves following each treatment as stated in C were analyzed by Kruskai–Wallis with Dunn’s multiple comparison tests. The number on top right represents the responder/total mice ratio. Data shown are pooled from two to three independent experiments.
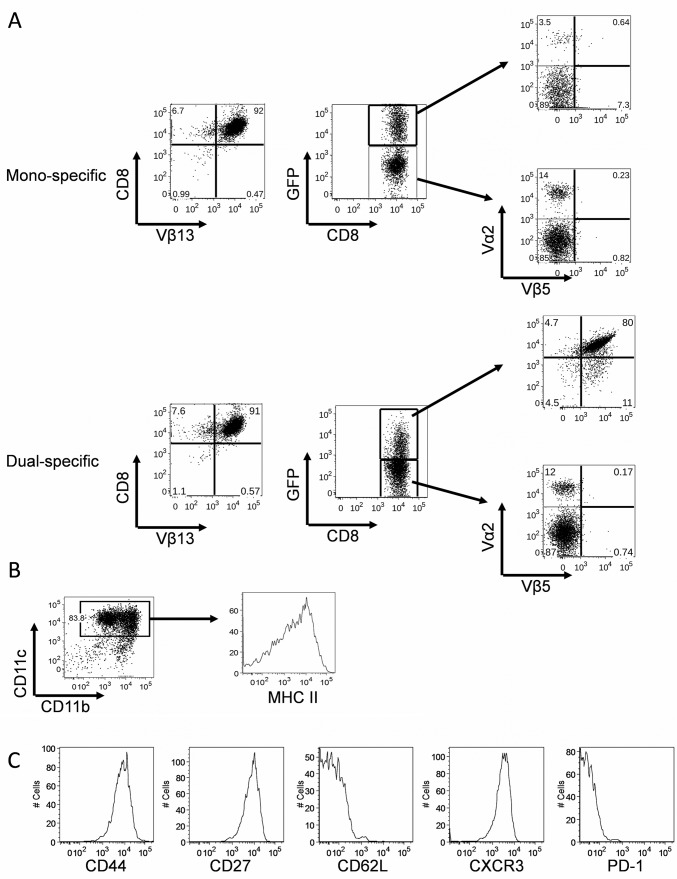
Fig. S1.
The phenotypes of bone marrow-derived dendritic cells (BMDCs) and polyclonal dual-specific CD8 T cells. (A) CD8 T cells were harvested from the transgenic mouse strain Pmel-1 and transduced with either MIG vector to generate monospecific T cells, or MIG-OT-I vector to generate dual-specific T cells. At day 5 after in vitro culture, the expression of TCR Vα2 and Vβ5 (OT-I) were examined on positively transduced (GFP+) cells and nontransduced (GFP-) cells by flow cytrometry. (B) BMDCs pulsed with tumor cell lysates were used to generate polyclonal dual-specific CD8 T cells. (C) Histograms show the expression of surface phenotypic markers on the activated dual-specific CD8 T cells.
To test the ability of transduced monospecific or dual-specific CD8 T cells to control melanoma in a therapeutic setting, a small number of cells (5 × 105 per mouse) were adoptively transferred into C57BL/6 mice with established s.c. B16-F10 melanoma tumors. Consistent with published data (14), both ACT regimens failed to prevent the tumor growth (Fig. 1C). However, when dual-specific CD8 T cells were administered in combination with a low dose of LM-OVA (ReACT), there was significant tumor regression in all mice and the majority of mice (7 of 10) had complete eradication (Fig. 1 C and D). Notably, antitumor effects required that mice were treated with both dual-specific T cells and LM-OVA as tumor growth was only slightly and transiently suppressed in mice that received monospecific CD8 T cells and LM-OVA (Fig. 1 C and D). Together, these results validate the feasibility of our approach and clearly show that ReACT leads to significantly enhanced antitumor efficacy.
The Adjuvant Effect of Listeria.
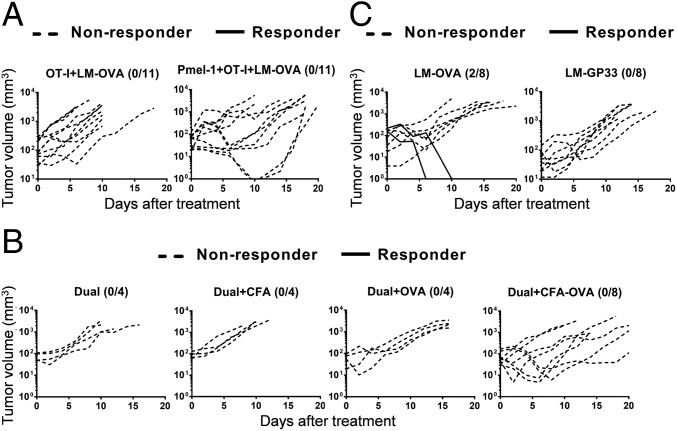
It is possible that ReACT-mediated tumor eradication was due to a bystander antibacterial effect from the dual-specific CD8 T cells. To test this possibility, we first transferred OT-I (OVA/bacteria-specific, nontransduced) CD8 T cells either alone or with Pmel-1 (tumor-specific) CD8 T cells into B16-F10 melanoma tumor bearing mice, and then intratumorally (i.t.) administrated LM-OVA. Regardless of the robust expansion of OT-I cells in response to LM-OVA infection and their migration to tumors, no obvious therapeutic benefit was seen in the OT-I+LM-OVA group compared with Fig. 1D (Fig. 2A). In the same vein, bystander OT-I response to LM-OVA only conferred transient adjuvant effects and failed to eradicate tumors even in the presence of Pmel-1 cells (Fig. 2A). These results together with the data shown in Fig. 1 demonstrated that LM-OVA infection either with monospecific T cells alone (Pmel-1 or OT-I) or mixed monospecific T cells (Pmel-1 and OT-I) was insufficient to eradicate tumors. Without expansion of adoptively transferred tumor-specific CD8 T cells, LM-OVA shows limited adjuvant effects in tumor control.
Fig. 2.
The adjuvant effect and direct antitumor effect of LM-OVA. (A) C57BL/6 mice with established B16-F10 tumors were treated with ACT of either OT-I cells (5 × 105 per mouse) alone or mixed Pmel-1 (2.5 × 105 per mouse) and OT-I cells (2.5 × 105 per mouse), followed by LM-OVA i.t. injection. The individual tumor growth curves are shown. (B) Four groups of C57BL/6 mice were inoculated with B16-F10 and received dual-specific CD8 T-cell transfer. In addition, they were treated with four different regiments including: PBS, CFA, OVA, and OVA+CFA. Tumor growth in each group was monitored over time, and individual tumor growth curves are shown. (C) C57BL/6 mice were injected i.t. with either LM-OVA or LM-GP33 1 wk after s.c. inoculation with B16-OVA tumor cells. Tumor growth in each group was monitored over time, and individual tumor growth curves are shown. In all experiments, mice that eradicated tumors were defined as responders (shown in solid lines), whereas the remaining mice were defined as nonresponders (shown in dashed lines). The number on top right represents the responder/total mice ratio. Data shown are pooled from two to three independent experiments.
In addition, to determine whether antigen alone or in combination with an adjuvant can be used for ReACT therapy in place of LM-OVA, we treated tumor-bearing mice with dual-specific CD8 T cells and administered either OVA257–264 peptide alone, Complete Freund’s Adjuvant (CFA) alone, or the combination of OVA and CFA (OVA+CFA). Consistent with previous studies (9, 15), neither antigen (OVA) nor adjuvant (CFA) alone was able to control tumor progression. Furthermore, OVA+CFA only modestly delayed tumor growth but failed to fully eradicate the tumor (Fig. 2B). These observations suggest that LM as a live intracellular pathogen, unlike CFA that is composed of inactivated mycobacteria, may infect certain immune cells such as myeloid suppressor cells in the tumor microenvironment (see below) to render more effective tumor control.
The Antitumor Effect of LM-OVA as a Cancer Vaccine.
Recombinant Listeria-expressing TAAs can serve as cancer vaccines to treat solid tumors (9). To test whether a LM-based vaccine could confer similar tumor control as seen by ReACT, we compared two recombinant stains of Listeria, LM-OVA and LM-GP33 (expressing irrelevant control GP-33 peptide derived from LCMV) in the B16-OVA melanoma tumor model. To test proof of principle and for simplicity, we used OVA257–264 as a surrogate tumor antigen as reported (16). We administrated LM-OVA and LM-GP33 i.t. to C57BL/6 mice with established B16-OVA melanoma and followed the tumor progression over time. Consistent with published work (17), LM-OVA led to enhanced tumor control and 25% eradication compared with LM-GP33–treated mice (Fig. 2C). Nonetheless, this approach did not render robust tumor eradication as seen in ReACT-treated mice (Fig. 1D). Taken together, our data suggest that combinatorial treatment with ACT and a pathogen-based cancer vaccine leads to much greater tumor control than either treatment alone.
Polyclonal ReACT Eradicates Tumor and Generates Long-Term Protection.
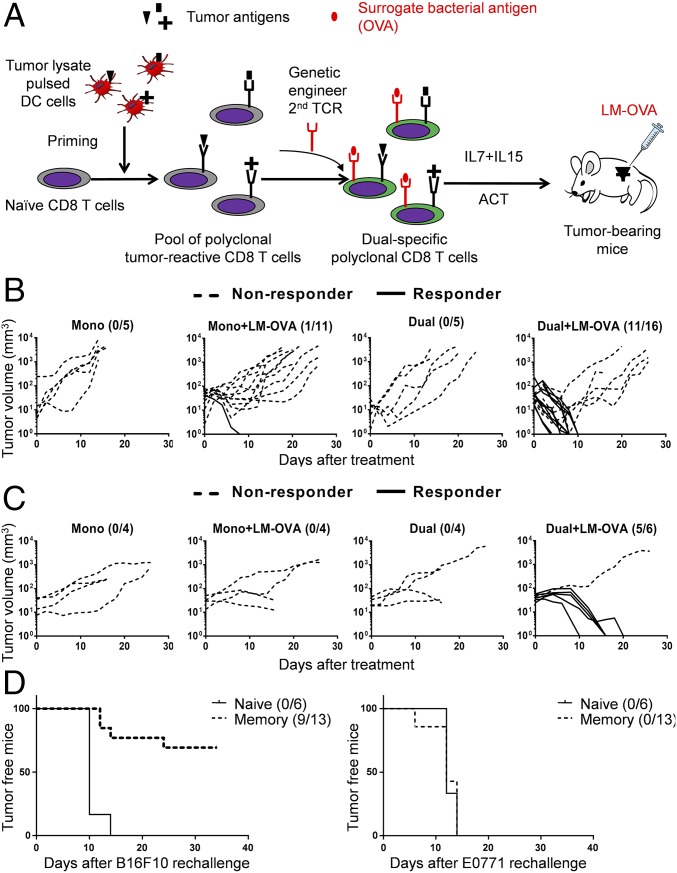
Given the lack of well-defined TAAs for most human tumors, and the advantages of using naturally occurring tumor-infiltrating lymphocytes (TILs) that recognize multiple TAAs to treat cancer patients (2), we further tested proof of principle by generating polyclonal CD8 T cells that target one bacterial antigen and multiple tumor antigens (Fig. 3A). For simplicity, we used B16-F10 cell lysate-pulsed DCs to stimulate naïve CD8 T cells to differentiate them into effector cytotoxic T cells (CTLs) that recognize various B16-F10 derived tumor antigens as shown (ref. 18 and Fig. S1 B and C). These cells were then genetically engineered to express the OT-I TCR and are referred to as polyclonal dual-specific CD8 T cells (Fig. 3A).
Fig. 3.
Polyclonal ReACT confers efficient tumor control and generates long-term protection. (A) The schematic for generating polyclonal dual-specific CD8 T cells for ReACT therapy against solid tumors. (B) Four groups of tumor-bearing mice received different treatment regimens including: polyclonal monospecific CD8 T-cell transfer (5 × 105 per mouse) with or without LM-OVA infection and polyclonal dual-specific CD8 T-cell transfer (5 × 105 per mouse) with or without LM-OVA infection. The responders (shown in solid lines) and nonresponders (shown in dashed lines) in each were defined as described in Fig. 1. The data were analyzed by Kruskai–Wallis with Dunn’s multiple comparison tests. (C) The individual growth curves of breast cancer E0771 tumors after receiving four different treatments as stated in B are shown. (D) Mice that eradicated their primary B16-F10 tumors were reinoculated with 1 × 104 B16-F10 cells (Left) and previously unencountered breast cancer cells (E0771; Right). As a reference, B16-F10 tumor growth in naive mice is also shown. The number of tumor-free mice is shown and were analyzed by Log-rank test. Data were pooled from two independent experiments.
In line with the preceding observations, transfer of neither monospecific nor dual-specific polyclonal CD8 T cells alone generated therapeutic responses against tumor growth in the absence of LM-OVA infection (Fig. 3B). The combination of polyclonal monospecific CD8 T cells with LM-OVA infection only resulted in tumor elimination in 1 of 11 mice (Fig. 3B). Strikingly, combined polyclonal dual-specific CD8 T cells and LM-OVA infection (ReACT) led to complete tumor eradication in the majority of mice (11 of 16) (Fig. 3B). Similar results were obtained in the E0771 breast cancer model (Fig. 3C), demonstrating that this therapy could potentially be applied to various types of solid tumors.
To test whether this combined therapy could generate immunological memory that protects the hosts from tumor recurrence, we challenged mice that had eradicated primary melanoma (B16-F10) tumors with a lower dose of B16-F10 cells on the left flank, and with a previously unencountered cancer line (E0771 breast cancer cells) on the right flank. The majority of these mice (7 of 10) were resistant to B16-F10, whereas none rejected the E0771 cancer cells (Fig. 3D). As expected, naïve mice did not reject either B16-F10 or E0771 tumors (Fig. 3D). These data illustrate that the polyclonal ReACT approach not only provides an enhanced immune response to eradicate primary tumor, but also establishes long-term protective immunity that prevents tumor relapse.
ReACT Increases CD8 T-Cell Expansion, Function, and Tumor-Targeted Migration.
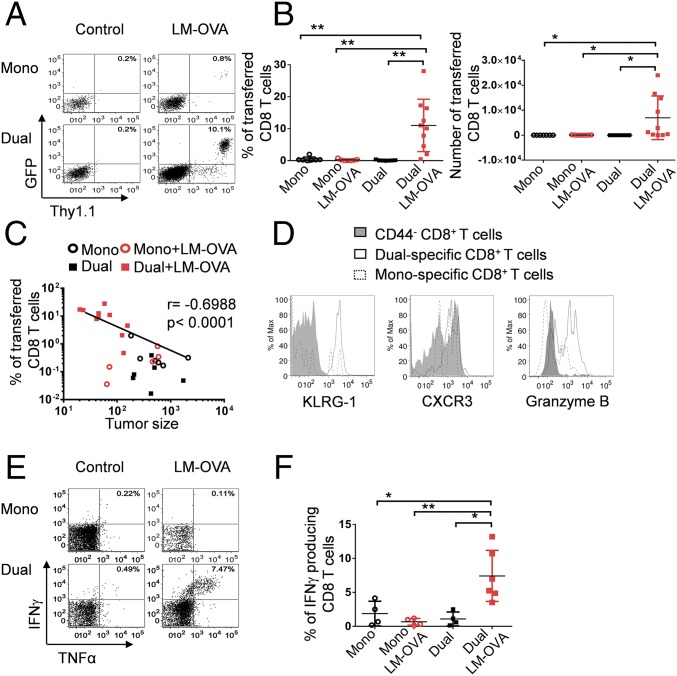
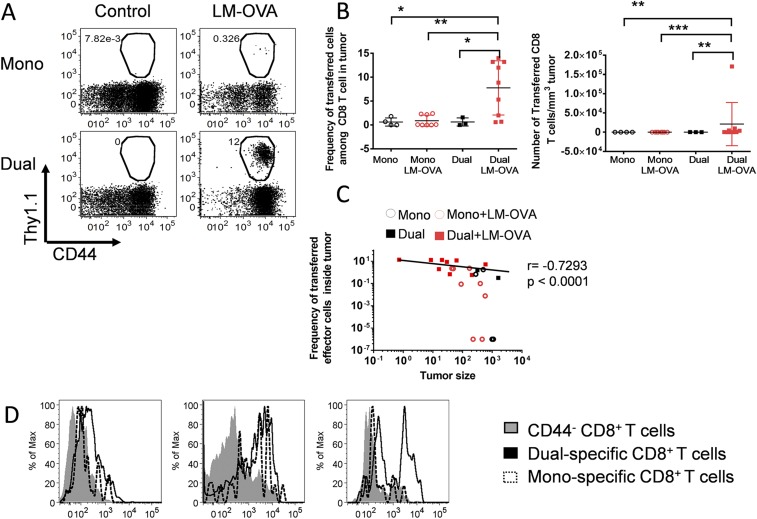
The remarkable antitumor effect of this combined strategy prompted us to study the tumor-specific CD8 T-cell responses. Without preconditioning or additional adjuvants, very low frequencies and numbers of transferred CD8 T cells were detected in the tumors from mice that only received monospecific or dual-specific CD8 T-cell transfer alone, as reported (14) (Fig. 4 A and B and Fig. S2 A and B). This observation is not surprising given that the number of transferred cells was low and in vivo expansion following ACT was lacking. Interestingly, the intratumoral LM-OVA infection slightly increased the monospecific CD8 T-cell infiltrating tumors, which is likely in response to the chemotactic inflammation. More strikingly, a significant number of transferred CD8 T cells were detected in tumors of mice that received bacterial infection combined with dual-specific CD8 T-cell adoptive transfer (Fig. 4 A and B and Fig. S2 A and B). Importantly, frequencies of CD8 T cells recruited to tumors inversely correlated with tumor size in all treatment groups (Fig. 4C and Fig. S2C). Furthermore, the dual-specific CD8 T cells displayed an activated phenotype (CD44hi, KLRG-1hi, and granzyme Bhi) (Fig. 4D and Fig. S2D), accompanied by high expression of the chemokine receptor CXCR3, which has been shown to contribute to improved T-cell migration to tumors (19). More strikingly, we observed a significant number of multipotent CD8 T cells producing both IFN-γ and TNFα in only mice receiving the combined treatment (Fig. 3 E and F). Together, these results suggest that the dual-specific CD8 T cells in response to bacterial infection robustly expand, acquire effector function, and migrate to the site of tumor, which in turn results in enhanced tumor control.
Fig. 4.
ReACT markedly increases tumor-specific CD8 T-cell expansion, function, and tumor-targeted migration. C57BL/6 mice received various combinations of treatments described in Fig. 1. Ten days later, mice were euthanized to harvest tumor-infiltrating immune cells for flow cytometric analysis. (A) The representative plots are gated on CD8 T cells, and the numbers indicate the percentage of transferred (GFP+) cells. (B) The percentage and absolute number (normalized to tumor volume) of transferred CD8 T cells were calculated and shown in the plots. (C) Correlation plot shows the relationship between tumor sizes and frequency of CD8 T cells within the tumor in each treatment group. Each data point represents an individual mouse. (D) The expression of CD44, KLRG-1, CXCR3, and granzyme B were compared in dual-specific and monospecific CD8 T cells by flow cytometry. Naïve CD8 T cells (CD44−) served as control. (E) The representative plots show the production of IFN-γ and TNFα after stimulation with gp100 peptide in vitro for 6 h. (F) The percentage of IFN-γ–producing CD8+ T cells was calculated and shown in the plot. Data shown are pooled from three independent experiments. *P < 0.05; **P < 0.01.
Fig. S2.
Polyclonal ReACT increases tumor-specific CD8 T-cell expansion and function. Four groups of B16-F10 tumor-bearing mice received different treatment regimens including: polyclonal monospecific CD8 T-cell transfer (5 × 105 per mouse) with or without LM-OVA infection and polyclonal dual-specific CD8 T-cell transfer (5 × 105 per mouse) with or without LM-OVA infection. (A) Dot plots show the frequency of adoptively transferred CD8 T cells from each group in the peripheral blood 10 d after treatments. (B) The frequency and number of adoptively transferred CD8 T cells in the tumors were enumerated and shown in scatter plots. (C) The association plot shows the relationship between tumor sizes and the frequency of CD8 T cells in the tumors. (D) The expression of PD-1, KLRG-1, and granzyme B were compared among dual-specific, monospecific, and naive CD8 T cells and shown in histograms. Results are representative of two independent experiments, with n ≥ 3 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
ReACT Reverses the Immunosuppressive TME and Recruits CD8 T Cells to the Tumor.
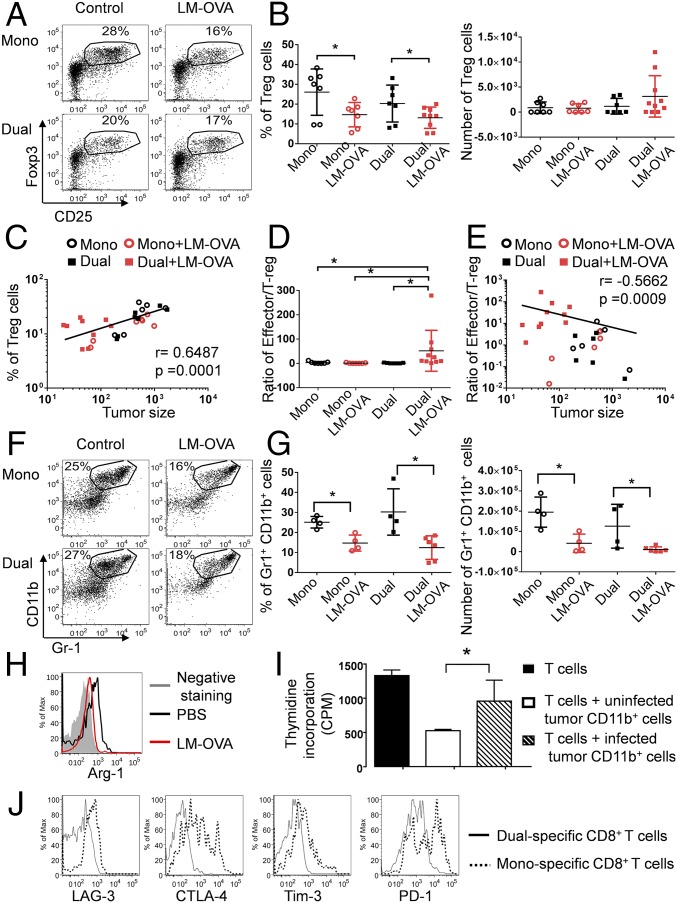
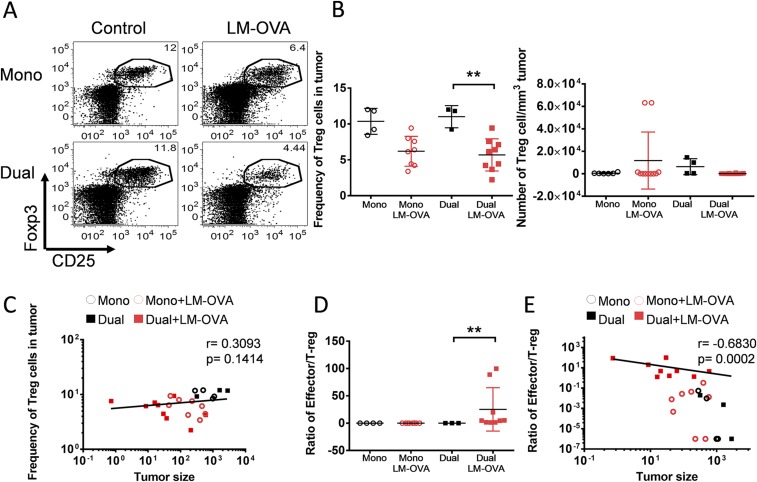
To assess whether our approach could alter the TME to synergistically improve the tumor-specific CD8 T-cell response, we examined two major immunosuppressive cells inside the tumor, Tregs and myeloid derived suppressive cells (MDSCs). The intratumoral LM-OVA infection significantly reduced the frequency of CD4+ CD25+ Foxp3+ Tregs regardless of the type of CD8 T cells transferred (monospecifc or dual-specific) (Fig. 5 A and B and Fig. S3 A and B). Notably, the frequency of Tregs in all treated mice positively correlated with tumor size (Fig. 5C and Fig. S3C). Interestingly, the effector/Treg ratio only increased in mice that received dual-specific CD8 T cells (Fig. 5D and Fig. S3D), owing to the robust expansion of effector cells as shown in Fig. 4 A and B and Fig. S2 A and B. Furthermore, the effector/Treg ratio inversely correlated with tumor size (Fig. 5E and Fig. S3E). Together, these data suggest that the ratio between effector CD8 T cells and Tregs is a critical factor that determines the final outcomes of different treatments.
Fig. 5.
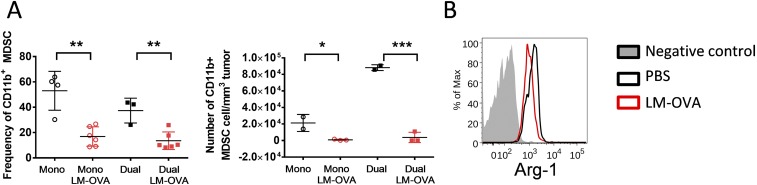
ReACT alters the tumor immunosuppressive microenvironment and tumor-specific CD8 T-cell phenotypes. (A) The frequency of CD25+ Foxp3+ Tregs inside tumors from mice that received each treatment described in Fig. 1 is shown in the dot plots. (B) The percentage and absolute number of Tregs normalized with tumor volume were calculated and plotted in the graphs. (C) Correlation plot shows the relationship between tumor sizes and frequency of Tregs inside the tumor. (D and E) The Teff/Treg ratios (D) and their correlation to tumor sizes (E) was calculated and plotted in the graphs. (F) The frequency of CD11b+Gr-1+ MDSCs in tumors is shown in the dot plots. (G) The percentage and absolute number of CD11b+Gr-1+ MDSCs normalized with tumor volume were enumerated and plotted in the graph. (H) The expression of Arg-1 was compared in CD11b+Gr-1+ cells from uninfected and infected tumor-bearing mice and shown in histograms. (I) The CD11b+ cells were sorted from B16-F10 tumors treated with either i.t. injection of LM-OVA or PBS. These cells were cocultured with activated CD8 T cells, and the proliferation of T cells was assessed by 3H-thymidine incorporation and shown in bar graphs. (J) The expression of inhibitory receptors (LAG3, CTLA4, Tim3, and PD-1) was compared between dual-specific and monospecific CD8 T cells inside tumor and plotted in histograms. Data were pooled from three independent experiments. *P < 0.05.
Fig. S3.
Polyclonal ReACT reduces Treg cells and increases effector/Treg ratios in the tumors. Tumor-bearing mice received various combinations of therapy as described in Fig. S2. (A) The intratumoral Tregs were identified as CD25+ Foxp3+ and shown in representative FACS pots gated on CD4+ cells. (B) The frequency and number of Tregs were enumerated and shown in scatter plots. (C) The correlation plot shows the relationship between the frequency of intratumoral Tregs and tumor sizes. (D and E) The CD8 Teff/Treg ratios and their association with tumor sizes were calculated and shown in graphs. Results are representative of two independent experiments, with n ≥ 3 per group. **P < 0.01.
Another important type of suppressive cell, CD11b+Gr1+ MDSCs, was also significantly reduced by LM-OVA infection (Fig. 5 F and G and Fig. S4A). This finding is consistent with previous findings that Listeria can directly infect MDSCs (20), which likely makes them susceptible to cytotoxic T-cell–mediated killing. Furthermore, Listeria infection can convert MDSCs into immune stimulatory cells (20, 21). By the same token, we observed that intratumoral Listeria infection caused reduced expression of MDSC marker Arg-1 in CD11b+Gr1+ cells (Fig. 5H and Fig. S4B). To further test whether this phenotypic change correlated with decreased immunosuppression, we isolated CD11b+ cells from LM-OVA–infected tumors and cocultured them with in vitro-activated CD8 T cells. Indeed, CD11b+ cells from LM-OVA–infected tumors were less suppressive to T-cell proliferation than CD11b+ cells from uninfected tumors (Fig. 5I), suggesting that Listeria infection diminishes the immunosuppressive function of myeloid cells and improves antitumor effector function of CD8 T cells.
Fig. S4.
Polyclonal ReACT reduces CD11b+ cells in the tumors and alters their phenotype. Tumor-bearing mice received various combinations of therapy as described in Fig. S2. (A) The frequency and absolute number of CD11b+ myeloid cells from tumors were enumerated and shown in scatter plots. (B) The Arg-1 expression in CD11b+ cells isolated from tumor-bearing mice with or without i.t. LM-OVA infection was analyzed by flow cytometry at day 3 p.i. and shown in the histogram. Results are representative of three independent experiments, with n ≥ 3 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
More intriguingly, dual-specific CD8 T cells used in ReACT expressed lower levels of several inhibitory receptors (LAG-3, CTLA-4, Tim3, and PD-1) compared with monospecific CD8 T cells (Fig. 5J), suggesting that these reenergized CD8 T cells might be bestowed with enhanced antitumor function and less exhausted phenotypes. These results collectively demonstrate that intratumoral bacterial infection can largely reverse the immunosuppression in the TME (9, 20) and recruit dual-specific CD8 T cells with greater antitumor properties to the site of tumor.
Discussion
Both adoptive cell transfer of genetically engineered T cells and pathogen-based cancer vaccines are promising strategies to treat cancer. However, adoptively transferred T cells migrate inefficiently to the tumor and readily lose effector function in the immunosuppressive TME. Pathogen-based vaccines can reverse immunosuppression in the tumor, but are less efficient at inducing tumor-specific CD8 T cells with adequate magnitude and clonal types to confer tumor eradication. In this study, we combined the strength of both approaches and developed an innovative strategy, ReACT, to treat solid tumors in a preclinical model. ReACT uses a pathogen not only to break the immunosuppressive TME, but also to drive the expansion and migration of tumor-specific T cells to the site of tumor. We have demonstrated the enhanced antitumor efficacy of this combinatorial approach in comparison with either treatment alone in primary tumor eradication. More importantly, the mice cured from ReACT also develop immunological memory that protects them from subsequent rechallenge of the same tumor.
To bridge ACT and pathogen-based cancer vaccines together, we gene engineered tumor-specific CD8 T cells with a second TCR that recognizes a pathogenic antigen to create dual-specific T cells, a technology that was first developed by Kershaw et al. (12). Several studies have shown that augmented expansion and durability of dual-specific CD8 T cells clearly increased the antitumor activity and the overall survival of tumor bearing mice. Nonetheless, tumors were not eradicated in these applications (12, 22–24). This outcome is possibly due to inefficient migration of dual-specific T cells to the tumor and unchanged immunosuppressive tumor microenvironment, given that the pathogen was either administrated systemically (24) or not used (12, 22, 23). In addition, one important distinction of dual-specific T-cell generation in ReACT is to give a pathogen-specific TCR to tumor reactive T cells. This approach is opposite from previous work that gives pathogen (EBV, CMV, and Influenza virus) reactive T cells a single tumor-specific TCR (22–24).
Our ReACT approach allows us to generate polyclonal dual-specific T cells targeting multiple TAAs to increase the ability of tumor control. This strategy could be particularly useful to improve the efficacy of TIL-based therapy. The affinity of TCRs that recognize tumor antigens is usually weak and can limit the strength of antitumor responses (6). Using a pathogen-specific TCR to drive the clonal expansion of low-affinity tumor reactive T cells, ReACT may help to overcome this issue by increasing the magnitude of the tumor reactive T-cell response.
William Coley was arguably the first to practice cancer immunotherapy a century ago. Live pathogens have been used as adjuvants (such as bacillus Calmette–Guérin) to stimulate patients’ immune systems to treat bladder cancer and melanoma for decades (10, 11). Pathogen-based immunotherapies induce potent innate immune responses that break the suppressive tumor microenvironment at least in part by targeting MDSCs and Tregs (9, 20). Our data and recently published work suggest that LM can infect and convert MDSC into immune stimulatory cells (20, 21). In addition, LM infection can also mitigate Treg-mediated immunosuppression, which likely depends on the virulence factor LLO and increased IL-12 induction in TME (9). Another study suggests that LM infection promotes potent Th1 responses, which competes for the availability of IL-2, an indispensable cytokine for Treg development and survival (25).
Despite the reduction of tumor-associated immunosuppression, with limited expansion of tumor-specific T cells both in quantity and clonal types, the antitumor effects of this approach are transient and rarely able to achieve long-lasting antitumor effects (9). New strategies that use recombinant bacteria such as Listeria-expressing tumor antigens to treat a variety of cancers have shown promising efficacy in clinical trials (9). In this study, we show greater antitumor effects when combining pathogen-based cancer vaccine with ACT of dual-specific CD8 T cells than recombinant Listeria expressing a tumor antigen. This result can be explained by a greater magnitude of clonal expansion of adoptively transferred tumor-specific CD8 T cells than that from endogenous T cells, which supports the idea that the initial T-cell–mediated killing crucially depends on sufficiently high doses of T cells within the tumor for successful eradication (26).
In summary, we developed an immunotherapy, ReACT, to treat solid tumors and validated its efficacy in proof-of-principle animal experiments. Given the broad use of both ACT and pathogen-based vaccines in cancer treatments, this combinatorial strategy holds great translational value in treating various malignancies in humans.
Methods
Tumor Cell Lines, Bacteria, and Mice.
B16-F10, B16-OVA, and E0771 were obtained from ATCC and cultured in high-glucose DMEM (Cellgro) supplemented with 10% (vol/vol) FBS. C57BL/6 mice were obtained through the National Cancer Institute grantees program (Frederick, MD). Pmel-1 TCR transgenic mice that recognize the MHC class I (H-2Db)-restricted epitope of gp100 presented on the surface of B16-F10 melanoma were purchased from Jackson Laboratories. Mice were bred and maintained in a closed breeding facility, and mouse handling conformed to the requirements of the Institutional Animal Care and Use Guidelines of Medical College of Wisconsin. Recombinant LM-expressing OVA (LM-OVA) and GP33 (LM-GP33) was developed by Hao Shen (University of Pennsylvania School of Medicine, Philadelphia) and kindly provided by Susan Kaech, Yale University, New Haven, CT.
Tumor Induction and Rechallenge.
Melanoma tumors were established by injecting 2 × 105 B16-F10 cells s.c. on one flank of the C57BL/6 mice, whereas breast tumors were established by injecting at 3 × 105 cells near the fat pad of the fourth mammary gland in the lower abdomen. Mice that eradicated their primary B16-F10 tumors were rechallenged with 1 × 105 B16-F10 cells on the one flank and 1 × 105 E0771 cells on the fat pad of the fourth mammary gland from the opposite flank. The eradication of primary tumor was assessed by no visible and palpable tumor mass at least 6–8 wk after the clearance of tumors following initial treatment. Age- and gender-matched naïve C57BL/6 mice were used as controls. Tumor growth was monitored by measuring with calipers every other day, and tumor volume was calculated as length × (width)2/2.
Retroviral Transductions To Generate Dual-Specific Tumor Reactive T Cells and Adoptive T Cells Transfer.
To produce retroviral supernatant to express OT-I ovalbumin-specific TCR in T cells, 293T cells were transfected with either MSCV-IRES-GFP (MIG) plasmid, or MIG-OT-I vector along with the pcLEco ecotropic packaging plasmid. At the same time, the splenocytes were harvested from Pmel-1 mice and seeded in 24-well plates at 5 × 106 cells per well and cultured with 10 nM gp100 (Genscript) and 10 ng/mL IL-2 (Peprotech) for 24 h, followed by spinning transduction with prepared retroviral supernatant. After the transduction, these cells were cultured in the original medium for another 2 d and washed with PBS. After an additional 3 d of culturing in T-cell media containing 10 ng/mL IL-7 and 10 ng/mL IL-15, the positively transduced cells, defined by expression GFP, were sorted for transfer. For experiments involving ACT, mice received 5 × 105 sorted Pmel-1+ monospecific or OT-I+ Pmel-1+ dual-specific CD8 T cells at least 7 d after initial tumor inoculation. At the same time, these mice were injected with either 1 × 104 colony forming unit (CFU) LM-OVA or PBS i.t.
Generation of Polyclonal Tumor Reactive CD8 T Cells.
Bone marrow cells were isolated from C57BL/6 mice and cultured in RPMI (Cellgro) medium with 10% (vol/vol) FBS and 200 ng/mL Flt3L for 1 wk. On day 7, DCs were harvested and incubated with freeze-thawed tumor lysates at a ratio of one tumor cell equivalent to one DC (i.e., 1:1) as described (18). After 18 h of incubation, DCs were harvested and maturated with LPS for 4 h. The mature DCs and purified CD8 T cells were mixed in 1:2 ratio and cultured together with low-dose IL-2 (1 ng/mL) for 24 h. Then, the activated CD8 T cells were transduced and subcultured as described above.
Immune Cell Isolation from Solid Tumors.
The dissected tumor tissues were cut into small pieces and digested with 0.7 mg/mL collagenase XI (Sigma-Aldrich) and 30 mg/mL type IV bovine pancreatic DNase (Sigma-Aldrich) for 45 min at 37 °C. The immune cells were isolated by centrifugation with Lymphocyte Cell Separation Medium (Cedarlane Labs).
MDSC Suppression Assay.
As described before (27), splenic CD8 T cells were isolated by using the Mouse T Cell Isolation Kit (Stem Cell Technology), seeded in 96-well plates at 2 × 105 cells per well, and stimulated with anti-CD3 (eBioscience) and anti-CD28 (eBioscience) antibodies. At the same time, the CD11b+ myeloid cells were sorted from tumors by fluorescence-activated cell sorting (FACS) and added to these wells at various ratios (1:16, 1:8, 1:4, and 1:2). After 48 h of incubation, 3H-Thymidine (1 μCr/well) was added and incubated for 16 h. Cells were harvested by using a Packard Filtermate Harvester 96 and counted by Microbeta counter (PerkinElmer).
Statistical Analysis.
Graphs were generated and statistical analyses performed by using GraphPad Prism version 5.02 (GraphPad Software). The overall tumor growth in Fig. 1C was analyzed by one-way ANOVA, whereas the comparison of tumor-free mice after secondary challenge was determined by Log-rank test. The Kruskal–Wallis with Dunn's multiple comparison test was used to compare the individual tumor growth curves from different treatment groups. The Spearman’s rank correlation coefficient test was used to determine the association between the tumor sizes and cell composition in mice received different treatments. For all other comparisons, t tests were used to determine the statistical significance. *P < 0.05; **P < 0.01; ***P < 0.001.
Acknowledgments
W.C. is supported by NIH Grant AI125741, the BloodCenter Research Foundation, the Wisconsin Breast Cancer Showhouse, an Ann’s Hope Melanoma Research Award, and the Women’s Health Research Program. G.X. is supported by The Elizabeth Elser Doolittle Postdoctoral Fellowship. D.M.S. is a member of the Medical Scientist Training Program at Medical College of Wisconsin, which is partially supported by a training grant from National Institute of General Medical Sciences T32-GM080202 and Grant F30DK108557 from National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614315114/-/DCSupplemental.
References
- 1.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol. 2012;24(5):633–639. doi: 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23(2):286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 8.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood LM, Paterson Y. Attenuated Listeria monocytogenes: A powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. doi: 10.3389/fcimb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol. 2014;11(3):153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 11.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist. 2011;16(1):5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002;20(12):1221–1227. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Rivière I. Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies. Cancer Gene Ther. 2015;22(2):85–94. doi: 10.1038/cgt.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hailemichael Y, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frassanito MA, et al. Identification of Meth A sarcoma-derived class I major histocompatibility complex-associated peptides recognized by a specific CD8+ cytotoxic T lymphocyte. Cancer Res. 1995;55(1):124–128. [PubMed] [Google Scholar]
- 17.Stark FC, Sad S, Krishnan L. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 2009;69(10):4327–4334. doi: 10.1158/0008-5472.CAN-08-3160. [DOI] [PubMed] [Google Scholar]
- 18.Liang X, et al. β-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8+ T cells. J Leukoc Biol. 2014;95(1):179–190. doi: 10.1189/jlb.0613330. [DOI] [PubMed] [Google Scholar]
- 19.Mikucki ME, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458. doi: 10.1038/ncomms8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 2013;108(11):2281–2290. doi: 10.1038/bjc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heemskerk MH, et al. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199(7):885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis CU, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy A, et al. Antitumor activity of dual-specific T cells and influenza virus. Cancer Gene Ther. 2007;14(5):499–508. doi: 10.1038/sj.cgt.7701034. [DOI] [PubMed] [Google Scholar]
- 25.Benson A, et al. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J Immunol. 2012;188(2):800–810. doi: 10.4049/jimmunol.1100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGray AJ, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22(1):206–218. doi: 10.1038/mt.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol. 2011;41(3):749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]